Abstract
Human cutaneous photodamage is a major medical problem that includes premature aging and fragility of the skin. Non-xenografted animal models have not been comparatively evaluated for how well they resemble the changes seen in human skin. Here, we sought to identify a suitable mouse model that recapitulates key anatomic, cellular, and molecular responses observed in human skin during acute UV exposure. Adult females from three strains of mice, C57BL/6J, SKH-1, and Balb/c, were exposed to ultraviolet-B, and then evaluated 3h or 20h after the last irradiation.
Skin from UVB-exposed C57BL/6J mice showed features resembling human photodamage, including epidermal thickening, infiltration of the dermis with inflammatory cells, induction of TNFα mRNA, accumulation of glycosaminoglycans (GAGs), particularly hyaluronan (HA) in the epidermis, and loss of collagen. Hairless SKH1 mouse skin responded similarly, but without any induction of TNFα mRNA or chondroitin sulfate (CS). Irradiated Balb/c mice were the least similar to humans. Our results in C57BL/6J mice, and to a lesser extent in SKH-1 mice, show cutaneous responses to a course of UVB-irradiation that mirror those seen in human skin. Proper choice of model is critical for investigating cellular and molecular mechanisms of photodamage and photoaging.
Introduction
UV irradiation initiates a complex sequence of molecular responses that damage skin. Inflammation is a prerequisite for development of the changes seen in photoaging (23). UVB induces cytokines such as tumor necrosis factor-α (TNFα) in human and mouse epidermal keratinocytes (28, 37) and in dermal fibroblasts (10, 34). TNFα stimulates the release of other cytokines, chemokines, and adhesion molecules, thereby contributing to the chemotaxis of inflammatory cells into skin (15, 30). Inflammatory cells release additional TNFα and proteases such as MMPs that can both inhibit Type I procollagen mRNA and damage collagen fibers (8, 26). Studies show that different strains of mice vary in the induction of TNFα after chronic UVB irradiation, suggesting variability among these strains in UVB-induced effects on cutaneous photoaging (13). Several UV-induced molecular pathways have important effects in skin, and we chose TNFα as one pathway known to have large effects, with the goal of determining the best animal model for studies of the effects of inhibitors of this pathway on photodamage.
Most pertinent features of photoaged skin are the impairment of collagen fibers, excessive deposition of abnormal elastin fibers, and increased glycosaminoglycans (9, 20). Dermal fibroblasts synthesize extracellular matrix components such as GAGs that participate in the response to UVB (5). In our acute study, we identified the molecular species of these UV-induced GAGs as mainly CS in the dermis and hyaluronic acid (HA) in the epidermis of human skin (33). Both CS and hyaluronic acid (HA) are regulated by cytokines and UV light (6, 7, 33). Evaluation of UV effects GAGs in mouse models, given these GAG findings in humans, was another goal of the current study.
The molecular mechanisms responsible for photodamage have been extensively studied using cultured cells, experimental animals, and human subjects (4, 12). Studies in animals have focused on the SKH1 mouse, largely because it is hairless. Nevertheless, it is not clear that this model mimics key features of the human response to UV. As an example, this mouse has been used to model the specific species of cutaneous GAGs in response to UV exposure (14), but published reports are inconsistent, showing increases in chondroitin sulfate (27), dermatan sulfate, HA (14), or heparan sulfate (HS) without an increase in DS (17). Previous study in hairless mice reported no change in expression of HA in the dermis after chronic UV exposure (18). Moreover, two recent studies indicate that dermal HA actually decreases in response to acute and chronic UV irradiation (1, 7).
Systematic studies comparing features of the UV response in potential mouse models have not been performed. The establishment of an animal model that mirrors the acute-term effect of UV radiation in humans is important to the study of the role of specific cytokines, inflammatory cell types, and different species of GAGs in the molecular and cellular pathogenesis of photodamage.
In the current study, we compared three commonly used strains of mice to examine key anatomic, cellular, and molecular responses that have been observed in human skin during UV-irradiation. These acute changes in mouse model may have relevance for chronic damage seen in photoaging of human skin. Our results indicate substantial overall differences amongst the strains and point to one strain as particularly similar to humans in its responses.
Materials and Methods
Animals
SKH-1 and Balb/c mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA) and C57BL/6J mice were purchased from Jackson laboratory (Bar Harbor, Maine). All mice were 6-8 weeks old female and housed in Philadelphia VAMC animal resources facility, under the supervision of certified Laboratory Animal Medicine veterinarians. These mice were treated according to a research protocol approved by the Institutional Animal Care and Use Committee.
Reagents
Chloramin-T, 4-hydroxyproline, 4(Dimethyamino)-benzyldehyde and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
UVB-irradiation
A UVB source was two FS-40 UV-B sunlamps (Light of America, Walnut, CA) with a peak irradiance of 313 nm, and a cellulose triacetate filter that removes wavelengths below 290 nm. UVB doses were measured with a UV IL-443 UVB meter (International Light, Newburyport, MA). The filtered UVB light source was measured by spectroradiometric measurement at the time of the experiments. The mice were shaved on the dorsal side to remove hair. Animal cages covered with a cellulose triacetate filter were kept under the UVB lamp. Mice were allowed to move freely in the cage during exposure. For each condition 4 mice were irradiated with UVB (100 mJ/cm2/day) for 5 days receiving a total dose of 500 mJ/cm2. They were sacrificed at either 3h or 20h after the last exposure to UVB. One group of 4 mice served as unirradiated control, and was exposed only to the room light from the covered non-UV emitting fluorescent tubes. UVB-exposed and control skin were harvested from the dorsal surface of the mouse and snap frozen in liquid nitrogen. For histologic studies, skin was fixed in 10% formalin.
Extraction of total RNA
Total RNA was extracted by Trizol (Invitrogen, CA), followed by isopropanol precipitation and 70% ethanol wash. The RNA pellets were dissolved in DEPC-treated water. The RNA was quantified and purity evaluated by measuring the optical density at 260 nm, with 260/280 (ratio >1.8).
Real-time Polymerase Chain Reaction
Total RNA (2 μg), from sham- or UVB-exposed mice, was used for cDNA synthesis. cDNA was synthesized using SUPERSCRIPT First-Strand Synthesis for RT-PCR kit with random hexamers (Invitrogen, CA). Oligonucleotide sequences and target-specific fluorescence-labeled DNA probes were purchased from Applied Biosystems. 2 μL of cDNA were amplified by PCR, in which 50 nM each of the forward and reverse gene-specific primers for mice TNFα and GAPDH were used. All PCR assays were performed in triplicates and read using an ABI PRISM 7000 sequence detection system. The cycle number was predetermined so that the products formed fell within the linear portion of the amplification curve.
Histology
Skin samples were fixed in 10% formalin buffered saline and embedded in paraffin using routine procedures. The 4-μm sections were stained with hematoxylin and eosin (H&E) stain and examined by light microscopy for inflammatory cells recruitment in the dermis of UVB-irradiated mice. For glycosaminoglycans, sections were stained for acid mucopolysaccharides using a modification of Mowry's colloidal iron stain (Hale stain) which gives a blue color to mucopolysaccharides (31).
Histochemical staining for HA, CS
Briefly, skin sections were deparaffinized, followed by rehydration in a series of aqueous alcohol solutions of decreasing alcohol content. The localization of HA was determined using the biotinylated HABP (Associates of Cape Cod, East Falmouth, MA), according to a modified, previously reported technique (21). Biotinylated HABP was used at a dilution of 1:100 and developed by the immunoperoxidase method. Negative control sections were pre-treated first with 100mM Na acetate buffer pH 5 for 15 minutes in a 37°C water bath and then with 100 TRU/ml Streptomyces hyalurolyticus hyaluronidase (Sigma, St. Louis, MO) in 100mM Na acetate buffer pH 5 prior to staining. CS was evaluated using the monoclonal mouse antibody CS-56 (Sigma, St. Louis, IL), which binds chondroitin A and chondroitin C. CS-56 antibody was used at a dilution of 1:400 to stain the skin sections. Negative controls with mouse IgM isotype control antibody substituted for anti-CS were performed concurrently (Sigma, St. Louis, IL). All antibodies were visualized with the immunoperoxidase method using the Dakocytomation LSAB+ HRP system from DAKO (Carpinteria, CA).
Quantitation of epidermal thickness, inflammatory cells and staining
Epidermal thickness was measured using Image Pro Plus version 3.0 Software (MediaCybernetics, Bethesda, MD). Three high power fields from H & E stained skin sections of each mouse were used. In each high power field, 10 different locations of epidermis were selected and values were presented as Mean ± SEM. Inflammatory cells were counted using ImageJ software (NIH, Bethesda, MD) in 10 high power fields of 3 sections for each mouse. Hale, CS and HA staining intensity was quantified using Image Pro Plus. Four high power fields per section from each mouse were analyzed for epidermal and dermal staining.
Collagen quantification by assay of hydroxyproline content
Equal amount of protein (100 μg) from each sample of skin homogenate was mixed with an equal volume of 4N NaOH and hydrolyzed by autoclave for 20 min. The autoclaved sample was neutralized by an equal volume of 2N HCl. Chloramine T (0.056M in 10% n-propanol and acetate citrate buffer) was added to oxidize the hydrolyzate for 25 min at room temperature. Ehrlich's aldehyde reagent (1M p-dimethylaminobenzaldehyde in n-propanol/perchloric acid 2:1 v/v) was freshly prepared and added to develop the chromophore by incubating the samples at 65°C for 20 min. Hydroxyproline content was read at 550 nm. The assay was carried out in duplicate for each sample. The amount of hydroxyproline was determined by comparison with a standard curve prepared from known concentrations of hydroxyproline (24).
Statistics
Comparisons of several groups simultaneously were performed by initially using analysis of variance (ANOVA). When the ANOVA indicated differences amongst the groups, pairwise comparisons of each experimental group versus the control group were performed using the Dunnett q′ statistic. Unless otherwise indicated, summary statistics are reported as Mean ± SEM, n=4. Absent error bars in graphical displays of summary statistics indicate SEM values smaller than the drawn symbols.
Results
To facilitate comparison of the three mouse strains with each other and with reported effects in humans, key anatomic, cellular and molecular responses to a course of UVB-irradiation are summarized (Table 1).
Table 1. Comparison of Key anatomic, cellular and molecular features of acute photodamage in three mouse strains with each other and with reported effects in humans.
| Anatomic, cellular and molecular features of acute photodamage | Mice Strain (Acute UVB exposure) | Human (UV exposure) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C57BL/6J | SKH-1 | Balb/c | Response | UV dose | References | |||||
| Time points after last UVB-dose | 3h | 20 h | 3 h | 20 h | 3 h | 20 h | ||||
| Epidermal thickness | ↑↑ | ↑↑ | ↑↑↑ | ↑↑↑ | ↑ | ↑ | ↑↑ | 0.5, 1, 2× MED 3 times weekly × 6 wks | Pearse et al (1987) | |
| Inflammatory cells | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑ | ↑ | ↑↑ | 2× MED UVB | Fisher et al 2001 | |
| TNF-α (mRNA) | ↑↑ | ↑↑ | ↓ | ↔ | ↔ | ↔ | ↑↑ | 2× MED UVB | Strickland et al (1997) | |
| GAG (Hale stain) in dermis | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | ↑↑ | 1× MED UVB, 5 times weekly × 4 wks | Werth et al (in press) | |
| CS Staining | Epidermis | ↔ | ↑↑ | ↔ | ↔ | ↓ | ↔ | ↔ | 1× MED UVB, 5 times weekly × 4 wks | Werth et al (in press) |
| Dermis | ↔ | ↑ | ↔ | ↔ | ↔ | ↑↑ | ↑↑ | 1× MED UVB, 5 times weekly × 4 wks | Werth et al (in press) | |
| HA Staining | Epidermis | ↑↑ | ↔ | ↑↑ | ↑↑ | ↑↑ | ↔ | ↑↑ | 1× MED UVB, 5 times weekly × 4 wks | Werth et al (in press) |
| Dermis | ↑↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | 1× MED UVB, 5 times weekly × 4 wks | Werth et al (in press) | |
| Collagen | ↓ | ↓ | ↓ | ↓ | ↔ | ↔ | ↓ | Chronic photodamage | Fligiel et al (2003) | |
↑↑↑ = very significantly increased; ↑↑ = significantly increased; ↑ = slightly increased; ↓ = significantly decreased; ↔ = no change;
Anatomic and inflammatory changes induced by UVB irradiation in the skin of C57BL/6J, SKH-1 and Balb/c
In C57BL/6L and SKH-1 mice, wrinkles and redness was apparent on the dorsal skin. In Balb/c mice there was no change in the visible appearance of the skin after UVB-irradiation. Skin thickness was determined by microscopic examination of H & E-stained sections (Figure 1a-1c). The epidermal thickness was measured by ImagePro. In both C57BL/6J and SKH-1 mice, epidermal thickness was significantly increased 3h and 20h after the last dose of the 5-day course of UVB irradiation (p<0.05) (Figure 1d). In Balb/c mice, the epidermis was not signficantly thickened after the course of UVB-irradiation, as compared to non-irradiated controls (p=ns) (Figure 1c, d).
Figure 1. UVB exposure increased the thickness of epidermis and inflammatory cells influx in the dermis.
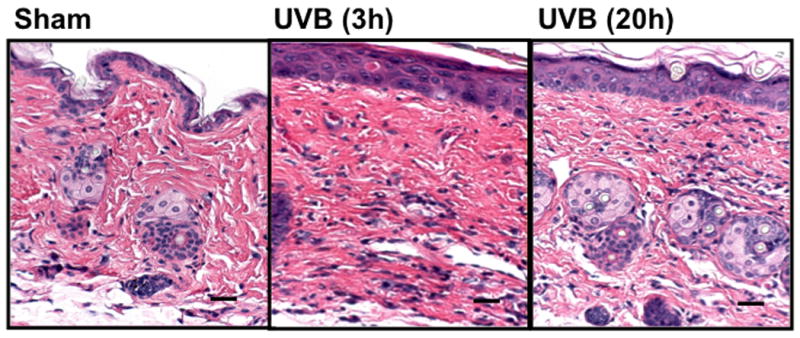
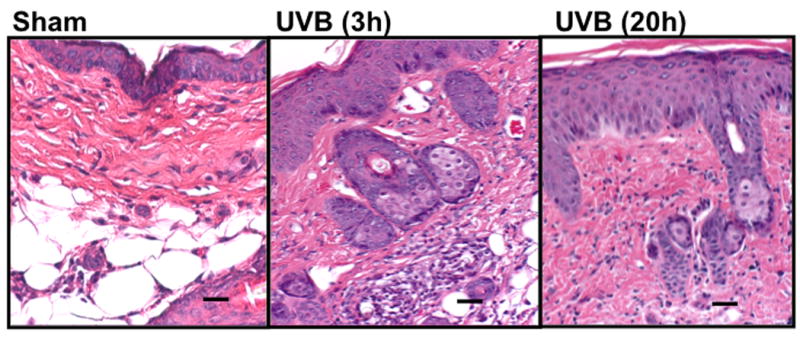
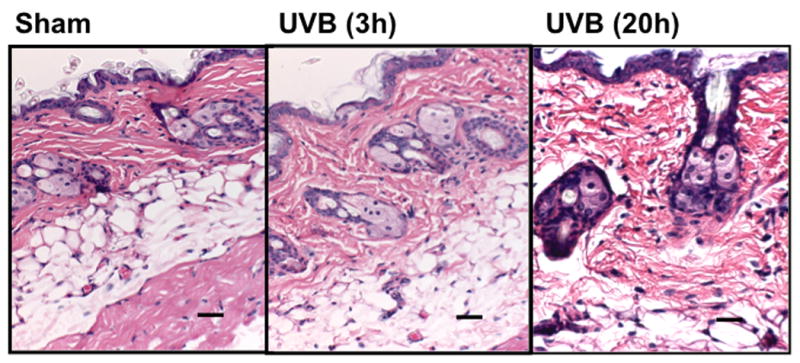
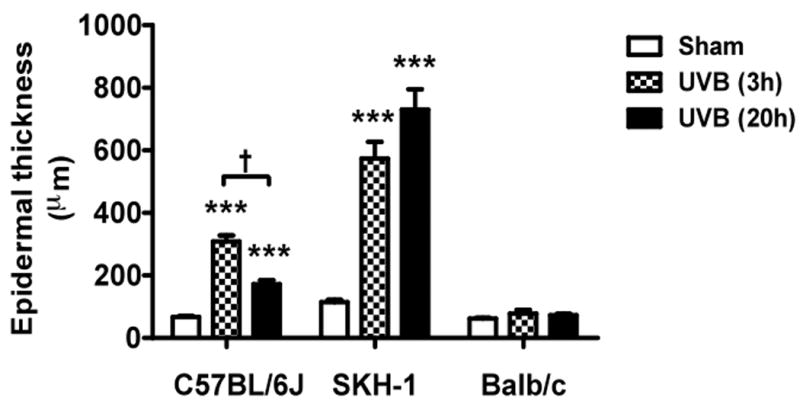
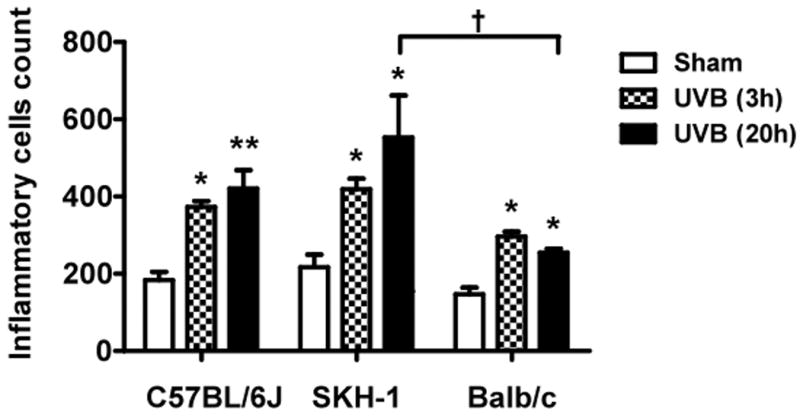
Epidermal thickness was measured in H & E stained skin sections (200×) using imagePro Plus software. (a) C57BL/6J; (b) SKH-1; (c) Balb/c; bar size = 50 μm; (d) Mean±SEM (four animals/condition); (e) Inflammatory cells were counted in 10 high power fields for each skin section. P values were obtained by analysis of variance, followed by Dunnett and Bonferroni's post-test. ***=p< 0.001, ** =p< 0.01, * =p< 0.05, †= p<0.05.
UVB exposure induced an inflammatory response in the dermis of all three mouse models at both 3h and 20h after the last dose of irradiation. A significant increase in numbers of inflammatory cells was found in the dermis of C57BL/6J, SKH-1, and Balb/c mice, as compared to non-irradiated controls (Figure 1e). When the difference in inflammatory cells response was compared among these strains 3h and 20h after UVB exposure, Balb/c mice showed significantly fewer inflammatory cells 20h after UVB compared to SKH-1 mice (p<0.05) (Figure 1e).
UVB induces TNFα mRNA expression in the skin of C57BL/6J mice, but not in irradiated SKH1 and Balb/c mice
UVB irradiation of C57BL/6J mice significantly increased cutaneous levels of TNFα mRNA over the values from non-irradiated controls, by 7-fold at 3h and by 2-fold at 20h after the last UVB dose (p<0.001). In contrast, irradiated SKH-1 mice showed a 50% decrease in cutaneous levels of TNFα mRNA levels 3h after the last exposure to UVB, compared to non-irradiated controls (p<0.01). Levels in irradiated SKH-1 skin recovered to control values by 20h after the final UVB exposure. In Balb/c mice, UVB did not alter the TNFα mRNA levels at either time point (Figure 2). Thus, C57BL/6J mice are the only ones that parallel the UVB-induced changes in TNFα expression seen in human skin.
Figure 2. Expression of TNFα mRNA in UVB-irradiated mice.
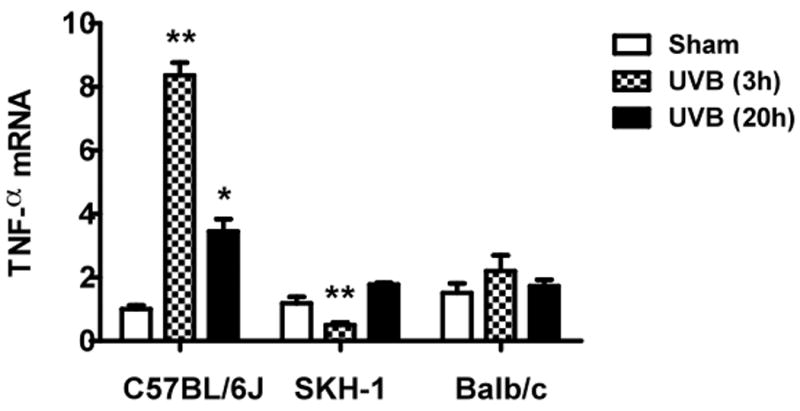
Mice were irradiated and sacrificed 3h and 20h after the last UVB exposure. RNA samples were collected at the indicated times and analyzed by real-time PCR. TNFα mRNA cycle numbers were normalized to GAPDH. Expression levels of TNFα mRNA are indicated as “fold change” compared to control animals (a) C57BL/6J; (b) SKH-1; (c) Balb/c mice. ** =p< 0.01, * =p< 0.05.
UVB irradiation of all three mouse strains increases the total GAG content of skin
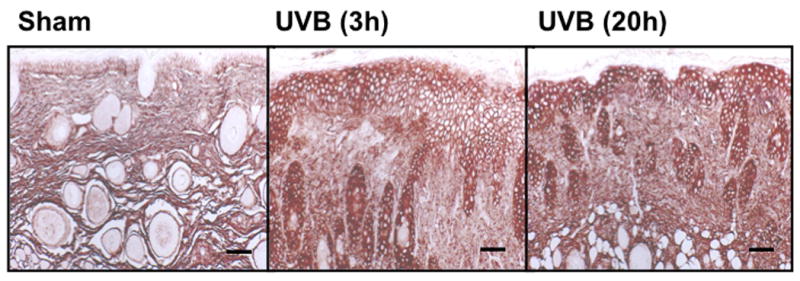
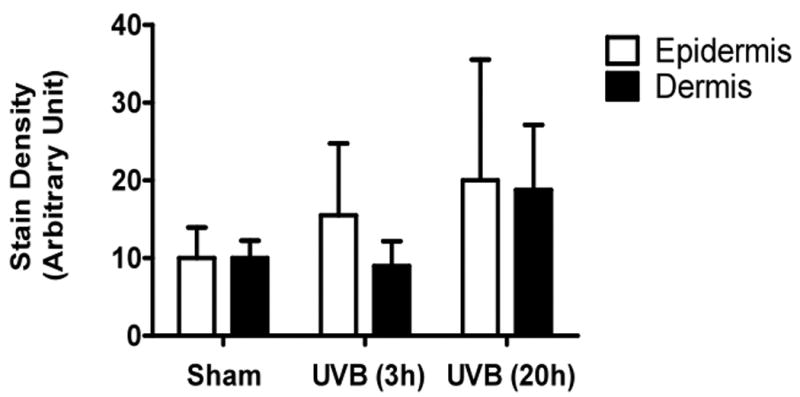
UVB exposure of mice resulted in an increase in cutaneous GAG content at 3h and 20h, as assessed by Hale stain (Figure 3, blue color). In all 3 strains of mice, the epidermis showed no real change in Hale stain intensity after UVB. Hale staining was increased in the dermis at both 3h and 20h in C57Bl/6 mice (p<0.05) (Figure 3a, b). In SKH-1 mice the dermis of UVB-irradiated skin showed an increase in Hale staining at 3h (p<0.05) relative to sham-irradiated skin (Figure 3c, d). In Balb/c mice, UVB increased the GAG expression in the dermis at 3h and 20h (p<0.05) (Figure 3e, f). Thus GAGs as measured by Hale stain increased in all 3 models in response to UVB, but there are clear differences in time course and amount of induction of GAGs.
Figure 3. UVB exposure increased the total GAGs in UVB-irradiated mice.
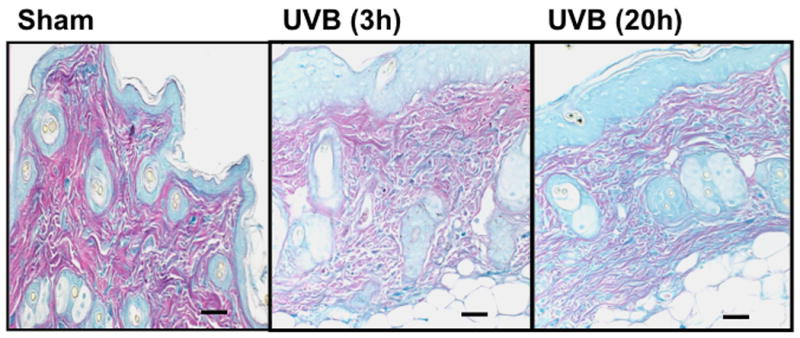
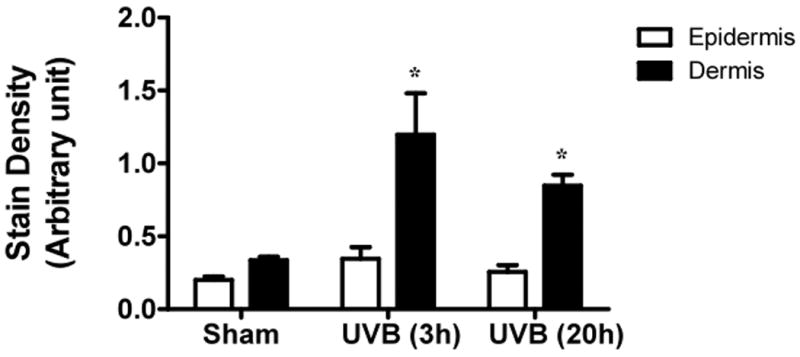
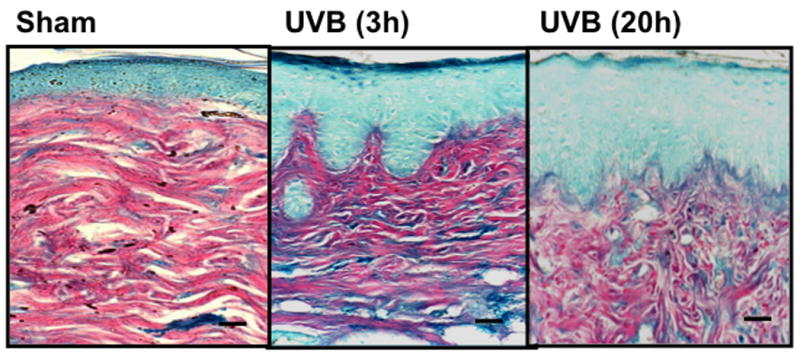
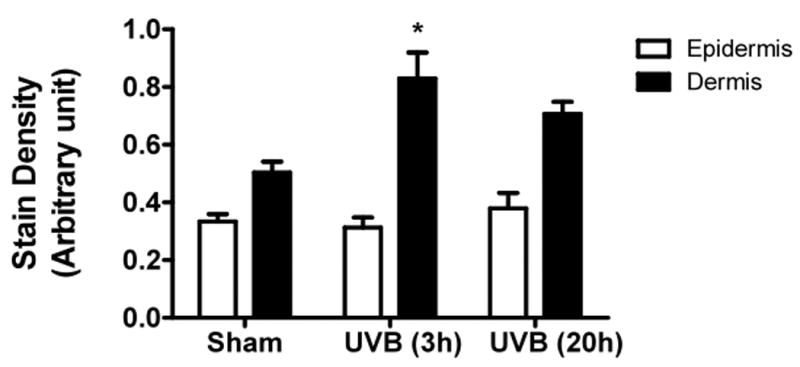
Histochemical staining of total GAGs was performed using Hale stain (200×). The blue color stains GAGs and red color stains collagen fibers. Hale staining was quantitated using ImagePro program. (a) C57BL/6J; (b) C57BL/6J Hale quantitation; (c) SKH-1; (d) SKH-1 Hale quantitation; (e) Balb/c mice; (f) Balb/c Hale quantitation; bar size = 50 μm. *= p< 0.05.
UVB irradiation significantly induces the accumulation of CS in the dermis of Balb/c mice
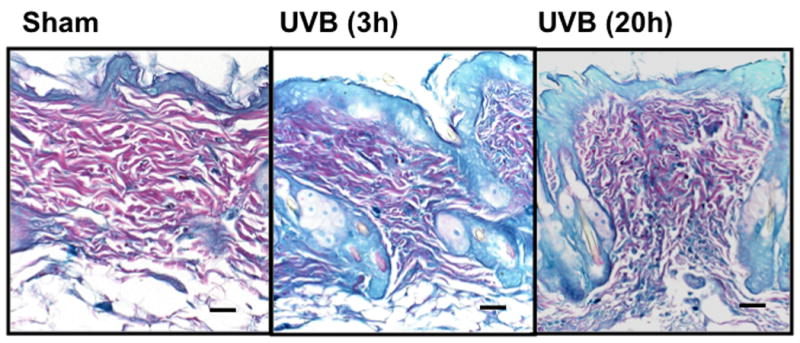
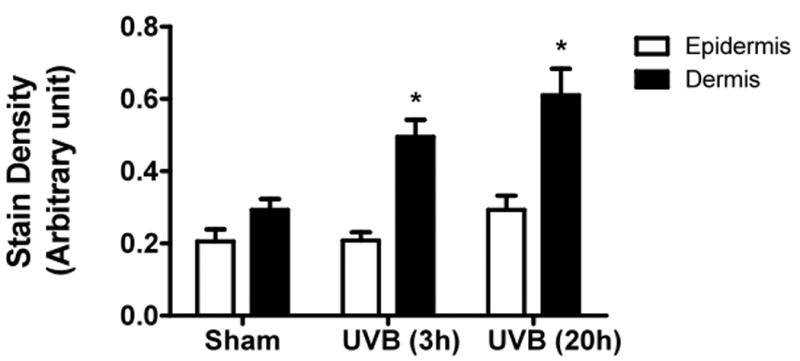
UVB irradiation of C57BL/6J mice increased only epidermal staining for CS, with a 3-fold increase at 20h after the last UVB dose, (p<0.01 compared to controls). There was a non-statistically significant increase in CS in the dermis of C57BL/6J mice (Figure 4a, b). In contrast, in SKH-1 mice, UVB-irradiation did not increase epidermal or dermal CS accumulation (Figure 4c, d). In Balb/c mice CS expression declined in the epidermis 3h after the last exposure to UVB, and returned to baseline at 20h. The CS is increased in the dermis of Balb/c mice 20h after the last exposure to UVB. The quantitation of stain intensity showed a 2-fold increase of dermal CS over control (p< 0.05) (Figure 4e, f). Therefore, given that CS increases in the dermis in human skin in response to UVB, in this regards Balb/c mice paralleled findings seen in human skin.
Figure 4. Chondroitin sulfate was increased in the dermis of Balb/c mice 20h after the last exposure to UVB, similar to humans.
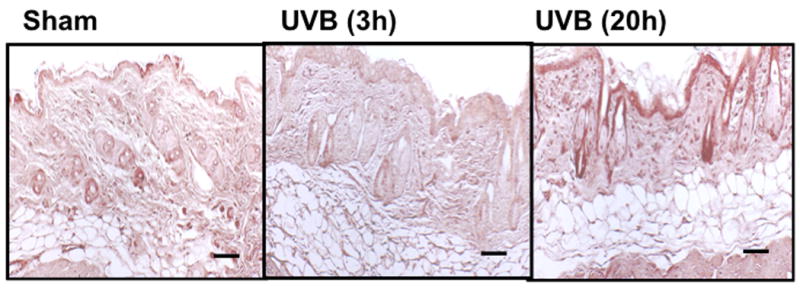
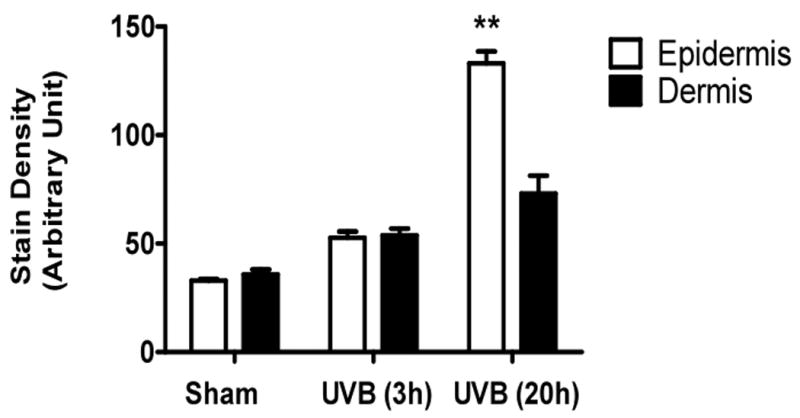
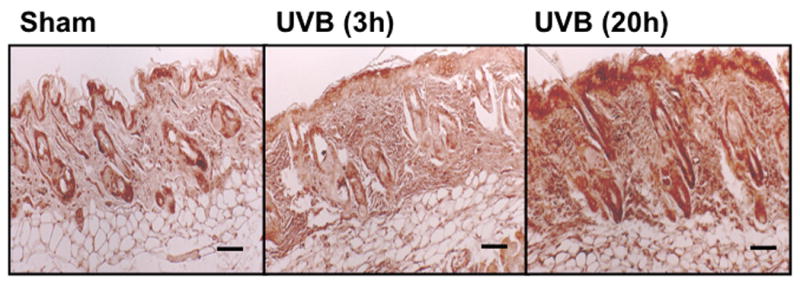
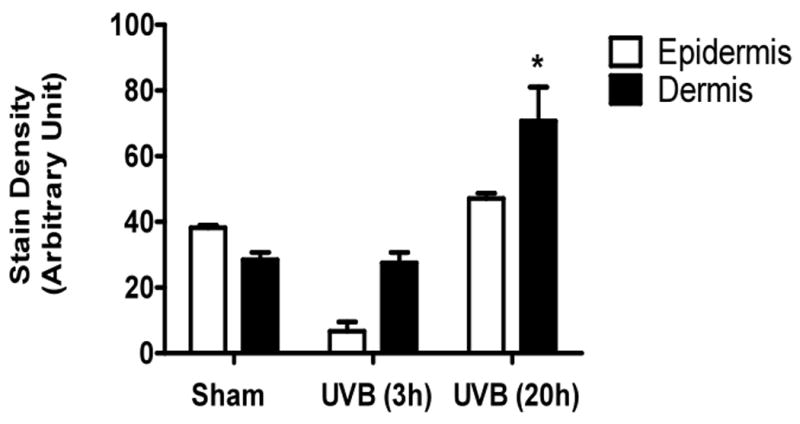
Immunohistochemical staining of CS was performed using biotinylated antibody and visualized using streptavidin-horseradish peroxidase and DAB complex (200×). Staining of CS was quantified in epidermis and dermis using ImagePro software and presented in graphical form. (a) C57BL/6J; (b) C57BL/6J CS quantitation (c) SKH-1; (d) SKH-1 CS quantitation; (e) Balb/c mice; (f) Balb/c CS quantitation. bar size = 50 μm. **= p< 0.01, *= p< 0.05.
UVB irradiation significantly increases HA content in the epidermis of all three mouse strains
UVB irradiation of all three mouse strains increased epidermal HA at 3h after the last exposure to UVB (p<0.01) (Figure 5a-f). This increase persisted at the 20h time point only in the SKH1 mice (p<0.01) (Figure 5c, d). In the dermis, UVB irradiation caused a 2-fold increase in HA content at the 3h time point in C57BL/6J mice (p<0.01), which returned to the control value by 20h (Figure 5a, b). SKH1 and Balb/c mice showed no significant changes in dermal HA at either time-point after the course of UVB irradiation (Figure 5c-f). Thus, the increase in epidermal HA in all mouse models is similar to the changes in the epidermis seen in humans. The changes in all mouse models in dermal HA at early time points do not show the decrease shown in the upper dermis in more chronically irradiated human skin.
Figure 5. UVB increased the expression of hyaluronic acid in the epidermis of all strains of mice, but only in the dermis of C57BL/6J mice 3h after the last exposure to UVB.
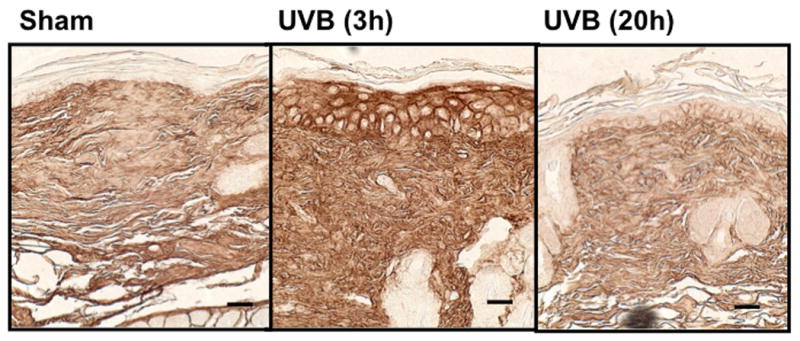
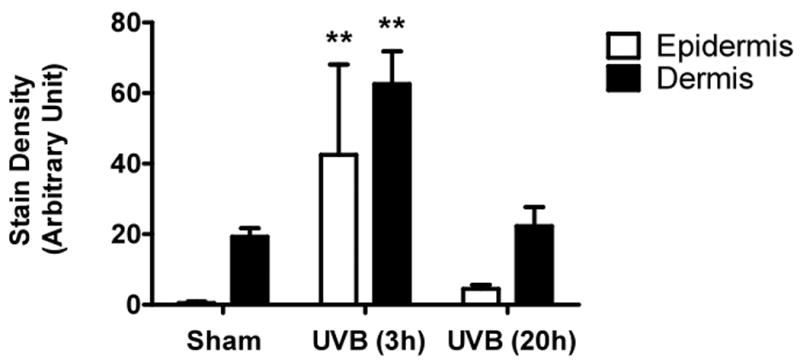
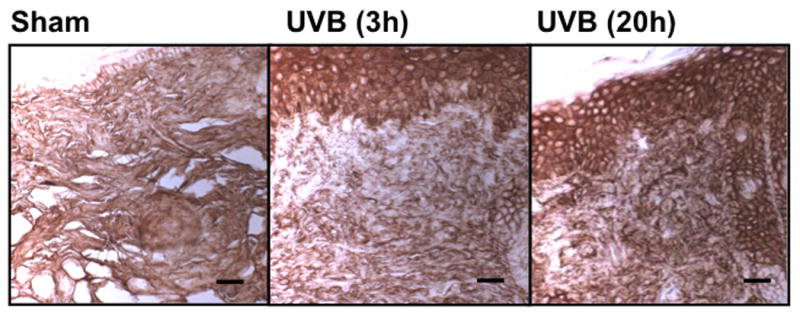
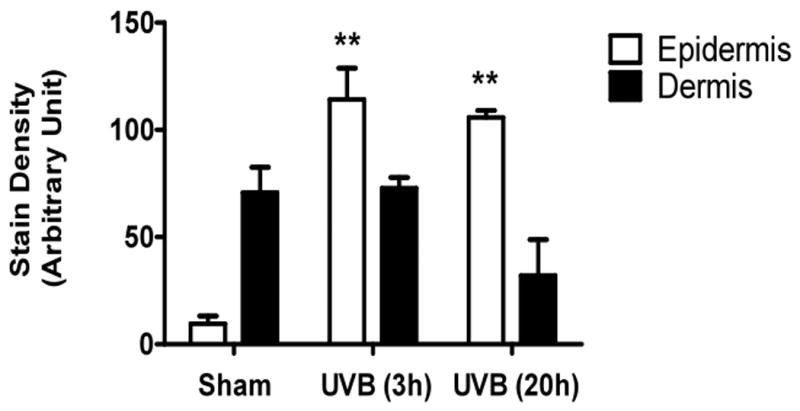
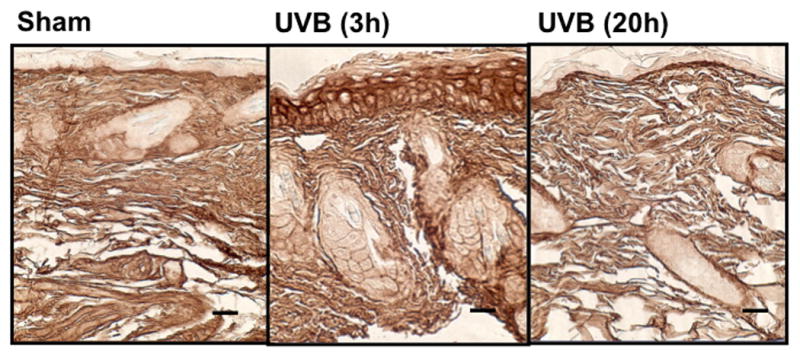
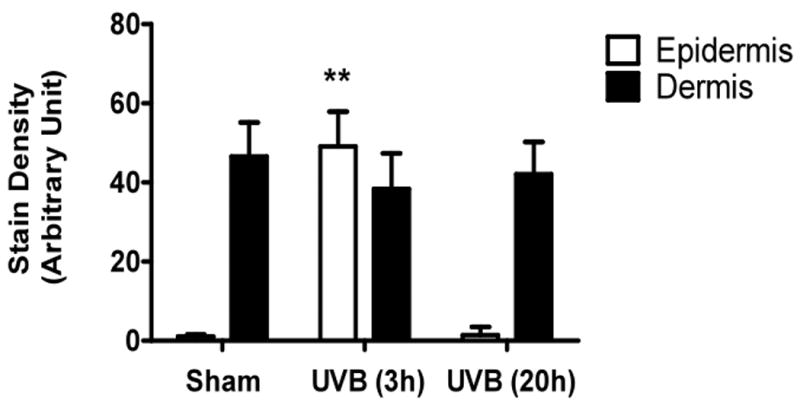
Immunohistochemical staining of HA was performed using biotinylated hyaluronan binding protein and visualized using streptavidin-horseradish peroxidase and DAB complex (200×). Staining of HA was quantified in the epidermis and dermis using ImagePro software. (a) C57BL/6J; (b) C57BL/6J HA quantitation; (c) SKH-1; (d) SKH-1 HA quantitation; (e) Balb/c mice; (f) Balb/c HA quantitation. bar size = 50 μm. ** =p< 0.01.
UVB irradiation significantly decreases dermal collagen content in C57BL/6J and SKH-1 mice
Hydroxyproline content, an indicator of the amount of collagen, was significantly decreased in the dermis of C57BL/6J mice at 3h and 20h after the last exposure to UVB compared with the unirradiated control group UVB (both P <0.05) (Figure 6). A similar UVB-induced decrease in dermal collagen was also observed in SKH-1 mice at both time points (both P <0.01). In contrast, Balb/c mice showed no change in hydroxyproline content at either time point after the course of UVB-irradiation (P = ns) (Figure 6). There was no evidence of elastosis in UV-irradiated skin from any of the three mice species, as determinined by luna stain (data not shown).
Figure 6. Collagen content decreased in C57BL/6J and SKH-1 mice, while no change was observed in Balb/c mice.
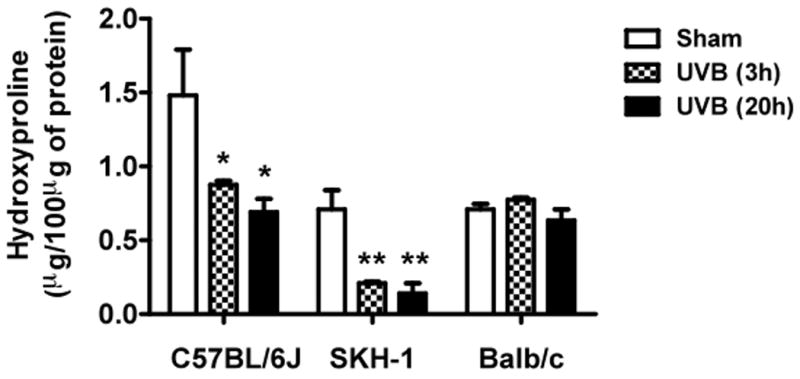
Collagen content was measured using hydroxyproline level in the skin homogenates. ** =p< 0.01, *= p< 0.05.
Discussion
In the present study, we compared three strains of mice to select a suitable animal model for acute photodamage that mirrors the cutaneous changes seen in human photoaged skin. We selected C57BL/6J black pigmented mice, assuming these mice may be the least susceptible to UVB-irradiation because of their pigmentation, SKH-1 hairless mice, the most commonly used animal model for UV-induced photoaging, and non-pigmented Balb/c mice, which might be more susceptible to UVB exposure due to lack of pigmentation in the skin. We also compared the amount of TNFα produced in response to UVB, to evaluate the potential correlation of TNFα with more severe pathogenesis of photoaged skin. Our results demonstrated that in C57BL/6J mice UVB increased the expression of TNFα after 3 and 20 h, similar to that seen in human UV-irradiated skin, suggesting these mice are closely related to humans in terms of TNFα expression in response to UVB (2). Conversely, in SKH-1 and Balb/c mice skin, UVB exposure did not induce TNFα expression, suggesting these mice are not responding similarly to that seen in human skin in response to UVB. Our results are in agreement with previous reports demonstrating that Balb/c mice do not exhibit UVB-induced TNFα (13, 28). Thus in terms of TNFα induction, C57BL/6J mice give results most similar to humans.
We next investigated other features of photodamage, including increases in epidermal thickness, inflammation, and GAGs, as well as decrease in collagen content, all changes reported in human skin in response to acute and chronic UV exposure (16, 19, 29). Previous studies have shown that chronic exposure of UV-irradiation in different strains of mice induced photoaging (4, 13). Our histological study revealed that acute UVB exposure is sufficient to increase the epidermal thickness in C57BL/6J and SKH-1 mice, consistent with human photoaged skin findings (36). Our findings are similar to previous studies that demonstrate that chronic UV exposure increases the epidermal thickness in SKH-1 mice (22). In contrast, we did not observe any significant change in epidermal thickness of Balb/c mice skin, further suggesting that these mice demonstrate fewer features of UVB effects at the doses used in these studies.
Inflammatory cell infiltrates were seen in the dermis of C57BL/6J, SKH-1 and Balb/c mice 3h and 20h post-irradiation with UVB. The numbers of inflammatory cells were overall less in Balb/c mice than that seen in the other two mice strains. In our study, the most significant increase in inflammatory cells was seen in the C57BL/6J model, which also had the largest UVB-induced increase in TNFα. It is reported that TNFα facilitates inflammatory cell migration to the site of inflammation in human skin, at least partially through upregulation of adhesion molecules (11). UV-irradiation alters the immune function and migration of Langerhans cells and dermal dendritic cells in human skin and these cells produce high level of TNFα, IL-1β, IL-6, and IL-8 (3).
Extracellular matrix in skin is comprised of elastin, collagen, and GAGs that, among other functions, provides viscosity to cell surface. Hyaluronan, CS, and dermatan sulfate are the most abundantly present GAGs in the skin. In humans, an increase in total GAG expression was observed after UV-irradiation (5). Chronic UV exposure to mice skin alters the dermal matrix components (7). In agreement with these findings, acute exposure of C57BL/6J, SKH-1, and Balb/c mice to UVB induced GAG expression in the dermis, as measured by Hale staining, suggesting their similarity to human photodamaged skin.
Furthermore, we investigated which specific GAG species contributes to an increase of total dermal GAGs. The CS expression was increased, as seen in human UVB-exposed skin, only in the Balb/c mouse dermis 20h after the last exposure to UVB (33). Many studies have demonstrated that CS suppresses the TNFα-induced inflammation in the different cells types (35). It is possible that the increase in CS in the UVB-irradiated Balb/c mice seen here suppresses the TNFα production and protects these mice from photodamage. The exact role of CS is currently unclear, and there is also the possibility of an immunostimulatory role for CS (35).
Hyaluronan, abundantly present in skin, showed an increase in the epidermal and decrease in dermal compartments of human skin after UVB-irradiation (1). The skin sections stained with HABP showed that all the three strains of mice irradiated with UVB have large increases of HA in the epidermis (SKH>Balb/c> C57BL/6J), a finding seen in human skin. C57BL/6J mice showed increased HA expression in the dermis at 3h, but not at 20h, suggesting that HA contributes to the increase of total GAGs seen in that model at 3h. The content of HA at a 3h time point in human skin is unknown. In contrast to our findings, a much longer-term study demonstrated that chronic UVB irradiation of C57BL/6J mice for 182 days for 3 times a week at a dose of 210 mJ/cm2 causes loss of hyaluronic acid from the dermis, with downregulation of hyaluronic acid synthases (7). This study is much longer than our one-week study, and thus our acute-term findings cannot be compared to their study (7).
It is well established that longer exposure to UV irradiation reduces collagen in human skin by decreasing collagen synthesis, as well as enhancing degradation. Specifically, type I collagen synthesis is blocked by the TGF-β/Smad pathway, thereby reducing type I procollagen synthesis (25). It is known that matrix metalloproteinases in skin are induced by UV and can enhance degradation of collagen. The degraded collagen may inhibit the synthesis of Type I procollagen in photoaged skin (32). Similarly to humans, chronic UVB-exposure alters the expression of collagen content in the cutaneous matrix of different strains of mice (22). Given these findings, it is intriguing that SKH-1 and C57BL/6J, but not Balb/c mice, mice showed a decrease in the collagen contents in UVB-irradiated mice skin. We did not observe elastosis in any strain of mice after UVB-irradiation. However, none of these mice showed exactly similar changes to that seen in photoaged skin of humans in terms of TNFα induction, increase in epidermal thickness, skin inflammation, GAG expression, and decrease of collagen content. The most closely related mice strain to humans in terms of these parameters is the C57BL/6J mice. These mice differ from human in the expression of HA and CS in the dermal compartment. Balb/c mice are closely related to humans in their expression of CS in the dermis. It is likely that different strains of mice vary in their photosensitivity (minimal erythema dose) to UVB. Previous reports indicate that SKH-1 mice developed erythema at 90 mJ/cm2 of UVB (Berton et al 2001). The other two strains, C57BL/6J and Balb/c mice, showed an erythematogenic dose of UVB at 36 mJ/cm2 and 200mJ/cm2 respectively (Sumiyoshi and Kimura 2010, Goettsch et al 1999). This suggests that Balb/c mice require a higher dose of UVB to develop erythema and the changes seen in the dermal connective tissue. Further study is required to determine the molecular mechanisms for these differential effects of UVB in various mouse models of acute photodamage, and to correlate them with findings in humans to optimize choice of model for future studies.
Acknowledgments
This material is based upon work supported by a Merit Review Grant from the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
References
- 1.Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. The Journal of investigative dermatology. 2007;127:687–97. doi: 10.1038/sj.jid.5700614. [DOI] [PubMed] [Google Scholar]
- 2.Barr RM, Walker SL, Tsang W, Harrison GI, Ettehadi P, Greaves MW, Young AR. Suppressed alloantigen presentation, increased TNF-alpha, IL-1, IL-1Ra, IL-10, and modulation of TNF-R in UV-irradiated human skin. The Journal of investigative dermatology. 1999;112:692–8. doi: 10.1046/j.1523-1747.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 3.Bechetoille N, Dezutter-Dambuyant C, Damour O, Andre V, Orly I, Perrier E. Effects of solar ultraviolet radiation on engineered human skin equivalent containing both Langerhans cells and dermal dendritic cells. Tissue engineering. 2007;13:2667–79. doi: 10.1089/ten.2006.0405. [DOI] [PubMed] [Google Scholar]
- 4.Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. Journal of dermatological science. 2009;53:10–8. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein EF, Underhill CB, Hahn PJ, Brown DB, Uitto J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. The British journal of dermatology. 1996;135:255–62. doi: 10.1111/j.1365-2133.1996.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 6.Campo GM, Avenoso A, Campo S, Angela D, Ferlazzo AM, Calatroni A. TNF-alpha, IFN-gamma, and IL-1beta modulate hyaluronan synthase expression in human skin fibroblasts: synergistic effect by concomital treatment with FeSO4 plus ascorbate. Mol Cell Biochem. 2006;292:169–78. doi: 10.1007/s11010-006-9230-7. [DOI] [PubMed] [Google Scholar]
- 7.Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H, Homey B, Krutmann J, Reifenberger J, Fischer JW. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. The American journal of pathology. 2007;171:1451–61. doi: 10.2353/ajpath.2007.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 9.Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. The Journal of investigative dermatology. 2003;120:842–8. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa H, Wang B, Kondo S, Shivji GM, Sauder DN. Costimulation with ultraviolet B and interleukin-1 alpha dramatically increase tumor necrosis factor-alpha production in human dermal fibroblasts. J Interferon Cytokine Res. 1997;17:307–13. doi: 10.1089/jir.1997.17.307. [DOI] [PubMed] [Google Scholar]
- 11.Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. The British journal of dermatology. 1995;132:345–52. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 12.Hachiya A, Sriwiriyanont P, Fujimura T, Ohuchi A, Kitahara T, Takema Y, Kitzmiller WJ, Visscher MO, Tsuboi R, Boissy RE. Mechanistic effects of long-term ultraviolet B irradiation induce epidermal and dermal changes in human skin xenografts. The American journal of pathology. 2009;174:401–13. doi: 10.2353/ajpath.2009.070500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochevar IE, Moran M, Granstein RD. Experimental photoaging in C3H/HeN, C3H/HeJ, and Balb/c mice: comparison of changes in extracellular matrix components and mast cell numbers. The Journal of investigative dermatology. 1994;103:797–800. doi: 10.1111/1523-1747.ep12413286. [DOI] [PubMed] [Google Scholar]
- 14.Koshiishi I, Horikoshi E, Mitani H, Imanari T. Quantitative alterations of hyaluronan and dermatan sulfate in the hairless mouse dorsal skin exposed to chronic UV irradiation. Biochim Biophys Acta. 1999;1428:327–33. doi: 10.1016/s0304-4165(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 15.Krutmann J, Kock A, Schauer E, Parlow F, Moller A, Kapp A, Forster E, Schopf E, Luger TA. Tumor necrosis factor beta and ultraviolet radiation are potent regulators of human keratinocyte ICAM-1 expression. The Journal of investigative dermatology. 1990;95:127–31. doi: 10.1111/1523-1747.ep12477839. [DOI] [PubMed] [Google Scholar]
- 16.Kutting B, Drexler H. Evaluation of skin-protective means against acute and chronic effects of ultraviolet radiation from sunlight. Current problems in dermatology. 2007;34:87–97. doi: 10.1159/000099606. [DOI] [PubMed] [Google Scholar]
- 17.Margelin D, Fourtanier A, Thevenin T, Medaisko C, Breton M, Picard J. Alterations of proteoglycans in ultraviolet-irradiated skin. Photochem Photobiol. 1993;58:211–218. doi: 10.1111/j.1751-1097.1993.tb09551.x. [DOI] [PubMed] [Google Scholar]
- 18.Margelin D, Medaisko C, Lombard D, Picard J, Fourtanier A. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. The Journal of investigative dermatology. 1996;106:505–9. doi: 10.1111/1523-1747.ep12343819. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura Y, Ananthaswamy HN. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expert reviews in molecular medicine. 2002;4:1–22. doi: 10.1017/S146239940200532X. [DOI] [PubMed] [Google Scholar]
- 20.Menter JM, Pearl WS, Yadven MW, Willis I. UV-induced elevation of skin glycosaminoglycan levels I: rapid measurement of skin glycosaminoglycans by titration with acridine orange. Photochem Photobiol. 1983;38:481–6. doi: 10.1111/j.1751-1097.1983.tb03371.x. [DOI] [PubMed] [Google Scholar]
- 21.Meyer LJ, Stern R. Age-dependent changes of hyaluronan in human skin. The Journal of investigative dermatology. 1994;102:385–9. doi: 10.1111/1523-1747.ep12371800. [DOI] [PubMed] [Google Scholar]
- 22.Moloney SJ, Edmonds SH, Giddens LD, Learn DB. The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem Photobiol. 1992;56:505–11. doi: 10.1111/j.1751-1097.1992.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. International journal of cosmetic science. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 24.Prockop DJ, Udenfriend S. A specific method for the analysis of hydroxyproline in tissues and urine. Analytical biochemistry. 1960;1:228–39. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- 25.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. The American journal of pathology. 2004;165:741–51. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–4. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz E. Connective tissue alterations in the skin of ultraviolet irradiated hairless mice. The Journal of investigative dermatology. 1988;91:158–61. doi: 10.1111/1523-1747.ep12464405. [DOI] [PubMed] [Google Scholar]
- 28.Scordi IA, Vincek V. Timecourse study of UVB-induced cytokine induction in whole mouse skin. Photodermatol Photoimmunol Photomed. 2000;16:67–73. doi: 10.1034/j.1600-0781.2000.d01-6.x. [DOI] [PubMed] [Google Scholar]
- 29.Seite S, Fourtanier A, Moyal D, Young AR. Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review. The British journal of dermatology. 163:903–14. doi: 10.1111/j.1365-2133.2010.10018.x. [DOI] [PubMed] [Google Scholar]
- 30.Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. The Journal of investigative dermatology. 1997;108:763–8. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- 31.Tickoo SK, Amin MB, Zarbo RJ. Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and patterns of staining. The American journal of surgical pathology. 1998;22:419–24. doi: 10.1097/00000478-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. The American journal of pathology. 2001;158:931–42. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werth B, Bashir M, Chang L, Werth VP. Ultraviolet irradiation induces the accumulation of chondroitin sulfate, but not other glycosaminoglycans, in human skin. PLoS One. 2010 doi: 10.1371/journal.pone.0014830. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werth VP, Zhang W. Wavelength-specific synergy between ultraviolet radiation and interleukin-1 alpha in the regulation of matrix-related genes: mechanistic role for tumor necrosis factor-alpha. The Journal of investigative dermatology. 1999;113:196–201. doi: 10.1046/j.1523-1747.1999.00681.x. [DOI] [PubMed] [Google Scholar]
- 35.Xu CX, Jin H, Chung YS, Shin JY, Woo MA, Lee KH, Palmos GN, Choi BD, Cho MH. Chondroitin sulfate extracted from the Styela clava tunic suppresses TNF-alpha-induced expression of inflammatory factors, VCAM-1 and iNOS by blocking Akt/NF-kappaB signal in JB6 cells. Cancer letters. 2008;264:93–100. doi: 10.1016/j.canlet.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. The British journal of dermatology. 2007;157:874–87. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, Kimura H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32:1405–11. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]


