Summary of recent advances
Endocannabinoids (eCB) function as retrograde messengers at both excitatory and inhibitory synapses, and control various forms of synaptic plasticity in the adult brain. The molecular machinery required for specific eCB functions during synaptic plasticity is well established. However, eCB signaling plays surprisingly fundamental roles in controlling the acquisition of neuronal identity during CNS development. Recent work suggests that selective recruitment of regulatory signaling networks to CB1 cannabinoid receptors dictates neuronal state-change decisions. In addition, the spatial localization and temporal precision of eCB actions emerges as a novel organizer in developing neuronal networks. Current challenges include fitting novel molecular candidates into regulatory eCB signaling pathways, and defining the temporal dynamics of context-dependent signaling mechanisms underpinning particular neuronal specification events.
Introduction
Synaptic communication in complex neuronal networks relies on the coincident activity of integrated feed-back mechanisms allowing optimal temporal refinement of activity-dependent synaptic connectivity [1]. Generation and maintenance of the structural and functional coherence of neuronal circuits is the basic cellular principle of higher brain functions. Thus, multiple mechanisms have evolved during brain development to allow the temporal refinement of neuronal excitability. Although redundancy at the level of co-existent signaling networks with partially overlapping functions ensures the continuous adaptation of pre- and postsynaptic components between connected neurons, retrograde synaptic signaling emerges as a uniquely powerful means to tune the temporal and spatial efficacy of synaptic information transfer.
During the past decade, several retrograde messengers have been identified (Figure 1) that exhibit robust differences in their speed of affecting synaptic integration, temporal flexibility and efficiency, and spatial precision [2–7]. Endocannabinoid (eCB) signaling represents a key retrograde signaling pathway [8] for tuning both homosynaptic and heterosynaptic plasticity [9] in the postnatal brain. The general molecular paradigm is that eCBs are synthesized postsynaptically in an activity-dependent manner and engage presynaptic CB1 cannabinoid receptors (CB1Rs) on both excitatory and inhibitory afferents thus decreasing neurotransmitter release. Four basic forms of eCB-mediated synaptic plasticity have been described (Figure 1) [8–10]: (i) in depolarization induced suppression of inhibition (DSI), depolarization of a postsynaptic neuron stimulates eCB production that activates presynaptic CB1Rs at GABAergic interneuron terminals, leading to a decrease in inhibitory neurotransmission. eCBs produced in the same fashion acting on CB1 receptors on excitatory neurons evoke depolarization induced suppression of excitation (DSE). (ii) In metabotropic suppression of inhibition (MSI), activation of postsynaptic Gq/11-linked receptors (typically M1 or M3 muscarinic acetylcholine receptors or group I metabotropic glutamate receptors [mGluRs]) leads to production of eCBs activating presynaptic CB1Rs thus decreasing inhibitory neurotransmission. As before, when eCBs produced in this fashion activate CB1Rs on excitatory terminals, the process is termed metabotropic suppression of excitation (MSE). (iii) eCB-mediated long-term depression (LTD) is evoked during sustained stimulation of group I mGluRs, as might happen during prolonged low frequency stimulation of excitatory pathways. LTD can affect either the stimulated pathway (homosynaptic LTD) or a neighboring pathway (heterosynaptic LTD), if the terminals of the neighboring pathway express CB1Rs. (iv) In slow-self inhibition (SSI), eCBs are produced following the repetitive depolarization of a neuron and activate CB1Rs on the same neuron, opening inwardly rectifying potassium channels and causing sustained hyperpolarization of the neuron [11]. SSI is remarkable in that in this form of eCB-mediated plasticity, eCBs are both produced and act in the same cell, as opposed to being retrograde messengers in the other forms. It should be noted that since eCBs can inhibit both excitatory and inhibitory neurotransmission, their net effect at the circuit level, also influenced by the coincident presence of other factors, can be either inhibitory or stimulatory.
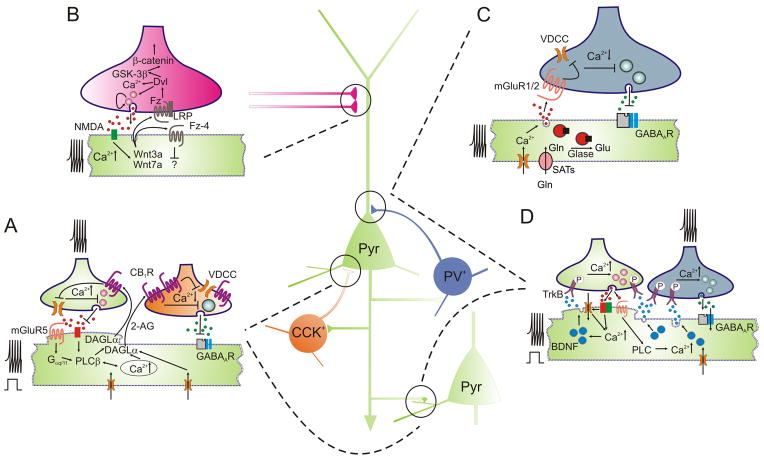
Fig. 1. Overview of signaling mechanisms underscoring synaptogenesis in the embryonic CNS with continued control of retrograde neurotransmitter release at mature synapses.
(A) Activity-dependent eCB release inhibits neurotransmitter release from synapses of both pyramidal cells (Pyr) and cholecystokinin (CCK)+ interneurons [2]. (B) Wnt signaling has been implicated in the control of presynaptic assembly and neurotransmitter release at excitatory afferents [4,48]. (C) Dendritic release of glutamate provides negative feed-back at perisomatic terminals of parvalbumin (PV)+ basket cells [7]. (D) In contrast, dendritic BDNF release enhances the efficacy of synaptic communication at select cortical synapses [5,24]. Abbreviations: 2-AG, 2-arachidonoylglycerol; AMPAR, AMPA receptor; CB1R, CB1 cannabinoid receptor; DAGLα/β, sn-1-diacylglycerol lipase α/β isoforms; Dvl, dishevelled; Fz, frizzled; GABAAR, GABAA receptor; Glu, glutamate; Gln, glutamine; Glase, glutaminase; GSK-3β, glycogen synthase kinase-3β; LRP, low-density lipoprotein receptor; mGluR, metabotropic glutamate receptor; NMDAR, N-methyl-D-aspartate receptor; PLC(β), phospholipase C (β isoform); SATs, system A amino acid transporters; TrkB, tyrosine kinase B receptor; VDCC, voltage-dependent Ca2+ channel; Wnt, Wingless-Int family of ligands.
Molecular determinants of eCB-mediated retrograde signaling at central synapses appear to be developmentally organized such that they can feed-back to control the earliest events of presynaptic neurotransmitter release during the transition from synaptogenesis to synaptic communication in developing neuronal circuits [12,13]. This leads to the question whether molecular underpinnings of eCB signaling loops acting so efficiently in the postnatal brain subserve particular physiological functions during brain development. The answer appears to be yes, but we are far from understanding the molecular logic and temporal dynamics of eCB signaling networks in the embryonic brain, and how their specific neurodevelopmental functions relate to and define their retrograde control of neurotransmitter release at mature synapses. Important open questions include: where and when eCBs are produced in the developing brain; the molecular identity of eCBs and whether they represent ‘active’ signals; whether respective receptors and intracellular signal transduction cascades differ from those in the postnatal brain; how eCB signaling integrates with other regulatory systems; and how the relative power of this newly-emerging signaling entity contributes to the defining of neurodevelopmental processes. In this review, we focus on recent discoveries establishing eCB-driven cellular identification events in the developing cerebrum, and define a unifying concept of how eCB signaling provides positional signals for excitatory and inhibitory afferents along the dendritic tree of cortical neurons, thus shaping the complexity of cortical connectivity.
Molecular logic of endocannabinoid signaling sculpted by developmental principles
2-Arachidonoylglycerol (2-AG) and anandamide (AEA), members of the eCB family of neuroactive lipids, are primarily synthesized by sn-1-diacylglycerol lipase α/β (DAGLα/β) [14] and α/β-hydrolase 4/glycerophosphodiesterase 1 (ABDH4/GDE-1) [15] and bind to cannabinoid receptors in the brain (Figure 1) and at the periphery. 2-AG and AEA promiscuously activate CB1, CB2 (CB1/2R), and other cannabinoid receptors including GPR55 [16]. However, the identity of eCBs and bioactive lipids stimulating GPR55 remains ambiguous: AEA and lysophosphatidylinositol, but not 2-AG, palmitoylethanolamine or virodhamine were shown to activate GPR55 [17–20]. CB1R and GPR55 are expressed on neurons, whereas CB2Rs are primarily found on microglia in the adult [16,17]. CB1R is one of the most abundantly expressed G protein-coupled receptors (GPCRs) by neurons and is selectively targeted to axons and synapses. In contrast, GPR55 expression appears significantly regionalized [18] with the identity and subcellular distribution of this receptor being unknown. Enzymatic inactivation of 2-AG and AEA primarily involves monoglyceride lipase [21] and fatty-acid amide hydrolase [22], respectively. eCB signaling in the cerebrum is principally restricted to excitatory synapses between pyramidal cells and inhibitory synapses of cholecystokinin (CCK)-containing GABAergic interneurons [3,23]. The spatial confinement of eCB signaling to particular synapse populations supports the concept that neurons may simultaneously express molecular determinants of multiple retrograde signaling systems and possess the capacity for domain-specific recruitment of particular signaling machineries to subsynaptic microterritories along their dendrites. Accordingly, several spatially-segregated retrograde feed-back mechanisms have recently been proposed (Figure 1): dendritic release of Wnt family ligands regulates neurotransmitter release at cerebellar mossy fiber-granule cell synapses [4]; quantal glutamate release suppresses inhibitory inputs originating from parvalbumin-containing GABAergic basket cells on cortical pyramidal cells [7]; while brain-derived neurotrophic factor (BDNF) released from secretory granules upon afferent stimulation enhances synaptic plasticity of both excitatory and inhibitory cortical synapses [5,6,24]. A striking similarity amongst the Wnt, glutamate and BDNF/TrkB signaling systems is that they play crucial roles during brain development through coordinated control of cellular positioning, identification, and presynaptic and postsynaptic differentiation [4,25–29]. Thus, we argue that it is not a mere coincidence that three signaling systems critical for axon remodeling and presynaptic development during early organization of neuronal networks also play key roles in regulating synaptic activity at mature synapses. This suggests that the eCB system, like the other signaling cassettes, might have a dual function and play unexpectedly fundamental roles in both the wiring and firing of the nervous system.
Expression dictates function: context-dependent signaling at CB1 cannabinoid receptors
The existence of eCB ligands and CB1Rs in the developing rodent and human brain triggered an initial wave of interest when CB1Rs were unequivocally identified as the targets of Δ9-tetrahydrocannabinol (THC) from cannabis [30]. It took almost another decade for the cellular specificity, functions, and interacting partners of eCB signaling networks affecting CNS patterning to emerge. In fact, both 2-AG and AEA are present in the developing CNS from very early stages of differentiation, though 2-AG levels are characteristically a magnitude higher. Although absolute extracellular eCB levels may not reflect true signaling competence, we postulate that 2-AG is the prime eCB in the developing brain, nonetheless, AEA might play a role in regulating 2-AG bioavailability by controlling its levels and physiological efficacy [31].
Moderate CB1R levels in neural progenitors persist throughout pre- and postnatal life. Likely eCB actions on neural stem and progenitor cells are underscored by ample DAGL expression in neurogenic telencephalic niches [32,33], CB1R/CB2R and metabolic enzyme expression in neurospheres, and by the sensitivity of neural stem cells to pharmacological and genetic disruption of eCB signaling [32–35] (Figure 2). A robust increase in CB1R expression in immature post-mitotic neurons becomes evident as soon as neuronal lineage-commitment occurs [36–38]. The presence of an eCB-rich transient territory marked by DAGL-expressing cells at the outermost border of the subventricular zone (SVZ) facing the first layer of CB1R-positive post-mitotic neurons (corresponding to cells entering the intermediate zone in the cerebrum) [33] suggests that a propulsive eCB tone exists in the developing neocortex that facilitates radial migration of immature pyramidal cells and GABAergic interneurons [25] from the SVZ and deep migratory stream, respectively (Figure 2). Neurons undergoing axonal polarization and morphogenesis selectively target CB1Rs to their elongating axon [39]. CB1R expression levels peak as synaptic connectivity is established by cortical pyramidal cells (embryonic day 14–16 in mouse [33]) and GABAergic interneurons (> embryonic day 17 [12]). Once synaptogenesis terminates, CB1R expression adjusts such that ideal tuning of synaptic efficacy can occur. eCB-driven neuronal specification however relies on the temporal control of eCB synthesis and inactivation such that eCB actions are defined by (i) the identity of endogenous ligands momentarily available to initiate signaling events; (ii) the chronospecificity of available receptors underpinning specific neuronal identification events; (iii) selective recruitment of signal transduction machineries to activated receptors such that complex cellular regulatory networks required for cellular state-change decisions are activated [40]; (iv) the coherence of signaling events at the genomic level such that temporally compartmentalized transcriptional regulation of neuronal differentiation is precisely executed [40].
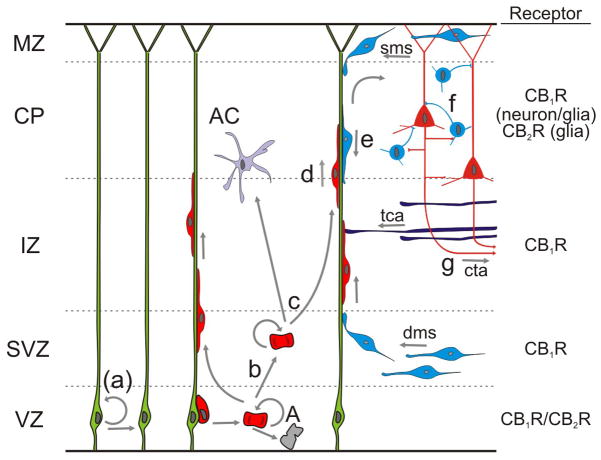
Fig. 2. Neuronal fate decision controlled by endocannabinoids.
Studies with CB1/2R agonists and antagonists on cultured neurospheres [49] and in adult mice [32,34] have provided evidence that eCB signaling can affect neural stem cell fate and proliferation [(a)]. During development, eCB signaling through CB1Rs affects neural progenitor proliferation [33] [b] and lineage commitment [35] [c] in the cortical subventricular zone (SVZ). Notably, CB1R expression is minimal on neuronal progenitors, whereas robust upregulation of CB1R expression coincides with neuronal commitment [33,36]. Thus, eCBs have the potential to exert powerful control on both radially migrating post-mitotic pyramidal cells [d; red] [33] and tangentially migrating immature GABAergic interneurons [e; blue] [25] populating the cortical plate (CP). Upon final positioning, eCB signaling contributes to the control of cell type-specific neuronal identification [f] and both intracortical and long-range axon patterning [g] [12,33,37]. The sequence of cellular specification events was adopted from Rakic [50]. Abbreviations: A, apoptosis; AC, astrocyte; CB1R, type 1 cannabinoid receptor; CB2R, type 2 cannabinoid receptor; cta, corticothalamic axon; dms/sms, deep/superficial migratory stream; IZ, intermediate zone; MZ, marginal zone; tca, thalamocortical axon; VZ, ventricular zone.
From birth to functional integration in complex circuitries, neuronal development is regulated by a continuum of cellular state-specific differentiation factors, whose concerted, ordered (inter-)actions ultimately define neuronal fate. Multimodal interplay amongst co-existing signaling systems will define the relative power of eCB actions whilst shaping the central nervous system. An increasingly accepted rule is that receptor homo- or heterodimers form functional units of GPCR signaling. The ability of opioid [41], A2A adenosine [42], and D2 dopamine receptors [43] to heteromerize with CB1Rs provides a mechanism to generate differential G-protein coupling at the CB1R, thus directly modulating physiological output. Alternatively, eCB signaling at CB1Rs can act as either upstream regulator (transactivation, BDNF/TrkB [25]) or downstream signal effector (N-cadherin/FGF-2 at FGF receptor [38,44]) thereby providing differential control of axonal growth and guidance. The physiological significance of multimer receptor mosaics as signaling ‘hubs’ is being recognized as a primary level of differential regulation of neuronal function. However, added levels of signaling complexity exists with regards to eCBs: (i) the 2-AG precursor diacylglycerol (DAG) is abundantly generated upon activation of, among others, Gαi and Gαq-coupled GPCRs by phospholipase C cleavage of polyphosphoinositide. Thus, coincident signaling through several GPCRs may affect 2-AG production by regulating DAG precursor bioavailability. (ii) AEA can modulate the activity of various ion channels [45] thus affecting neuronal excitability. Promiscuous AEA actions in the developing CNS may amplify primary eCB actions on CB1Rs. (iii) Agonist stimulation of both CB1R and GPR55 activates distinct signaling pathways. When both receptors are present, the prevailing cellular response has been proposed to depend on the activation state of integrins. When CB1R is linked to unclustered integrins (β1), AEA stimulation induces Gi/o signaling and downstream activation of spleen tyrosine kinase (Syk)-mediated activation of Erk1/2, nuclear translocation of NF-κB and concomitant inhibition of phosphoinositide 3-kinase (PI3K) [20]. Since GPR55 activation mobilizes Ca2+ release from intracellular store through PI3K [17], inhibition of this enzyme has the potential to uncouple some aspects of GPR55 signaling. Once heteromultimeric integrins become clustered by e.g. activation of Mn2+ or diminished extracellular [Ca2+], CB1R is released from the β1 integrin subunit and Syk-mediated PI3K inactivation ceases. Consequently, GPR55 signaling is freed from CB1R-induced repression resulting in intracellular Ca2+ mobilization and differential transcription factor activation. Overall, these data promote the concept that the cellular signaling context drives differential engagement of cannabinoid receptors and selective recruitment of signal transduction cascades upon agonist stimulation. The cumulative impact of cannabinoid receptor activation thus clearly exceeds that of each individual receptor type.
Endocannabinoid define synapse positioning
Establishment of long-range excitatory axons by pyramidal cells precedes the neurochemical specification and synapse patterning of GABAergic interneurons during corticogenesis. Prominent DAGLα/β localization to pyramidal cell axons is required for axonal elongation through cell-autonomous signaling [33,37] (Figure 3). De novo synthesized 2-AG can exert either autocrine regulation via CB1Rs distributed along the longitudinal axis of the growing axon, or paracrine signaling amongst neighboring axons, thus maintaining the integrity of an eCB-rich axonal trajectory. The lack of CB1Rs in glutamatergic growth cones suggests that directional steering decisions are independent of CB1Rs. Fasciculating excitatory axons define an ‘eCB protomap’ by demarcating eCB-rich hot-spots and eCB-sparse corridors (Figure 3A). This eCB-based spatial matrix provides permissive and repulsive niches for GABAergic axons navigating to their postsynaptic targets. Local GABAergic axons lack 2-AG synthesis capacity and use target-derived 2-AG as navigational cues. Consequently, when an inhibitory afferent approaches an eCB-rich brain microdomain of excitatory axons, agonist stimulation of CB1Rs will activate RhoA GTPases, initiating a repulsive response by collapsing the side of the growth cone facing the 2-AG gradient (Figure 3B). Thus, spatial navigation of GABAergic growth cones will be restricted to eCB-sparse microenvironments. Through 2-AG release, pre-existing excitatory axons will thus determine the spatial distribution of inhibitory afferents along the dendritic arbors of pyramidal neurons. This novel mechanism of eCB-driven synapse segregation is further supported by the fact that CB1R and DAGL levels peak in cortical neurons throughout the period of axonal elongation with progressively reduced CB1R expression and increased somatodendritic DAGL targeting as synaptogenesis terminates [12,14,33,36,37]. Another striking feature of our proposed model of neuronal network formation is that the ratio of CB1R and DAGL levels between glutamatergic and GABAergic neurons remains steady throughout neuronal specification and postnatal function. Lastly, the selective recruitment of DAGLα to dendritic spines of adult neurons [3] reflect postsynaptic arrangements (see also Figure 1A) corresponding to eCB-rich hot-spots during corticogenesis.
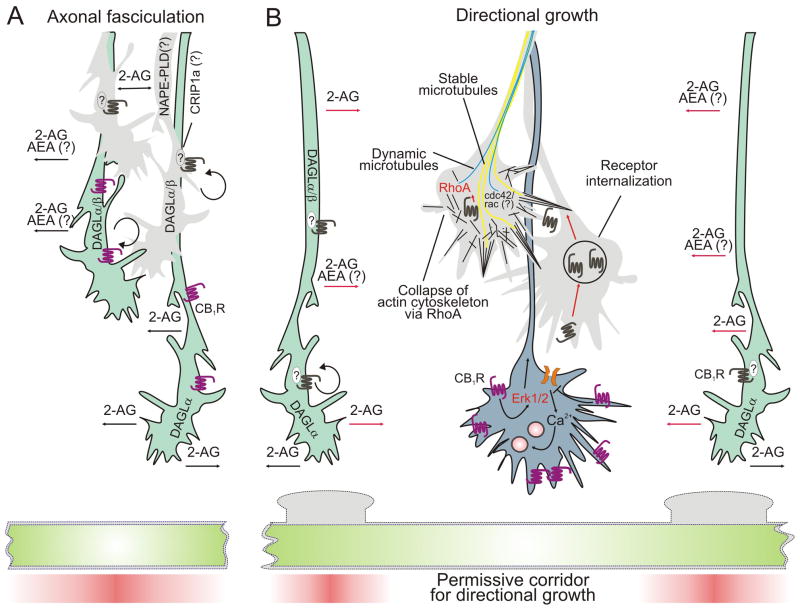
Fig. 3. Unifying concept defines endocannabinoid-driven spatial segregation of inhibitory and excitatory synapses.
(A) Pyramidal cells emit the first axons in cerebral circuits. They exhibit moderate CB1R expression with these receptors distributed all along the axis of the elongating axon. Sn-1-diacylglycerol lipases (DAGLα/β) are co-expressed in excitatory axons permitting cell-autonomous endocannabinoid (eCB) signaling that drives axonal elongation. Target-derived 2-arachidonoylglycerol (2-AG) might act as an additional attractive force. (B) Later-arriving GABAergic axons do not express DAGLs in their growth cones, but do express high levels of CB1Rs that sense a microenvironment that contains hotspots of 2-AG emanating from excitatory afferents (red shading). In these neurons, upon eCB stimulation, CB1Rs are removed from motile filopodia and translocated to the central growth cone domain where they activate the extracellular signal-regulated kinase 1/2 (Erk1/2) pathway, and RhoA GTPases. RhoA activation leads to a collapsing response on the side of the growth cone facing the eCB gradient thus contributing to growth cone steering decisions. Consequently, GABAergic axons target specific dendritic domains of postsynaptic neurons whose subcellular distribution and size are defined by excitatory afferents. Abbreviations: AEA, 2-arachidonoylethaloamine/anandamide; CB1R, CB1 cannabinoid receptor; CRIP1a, cannabinoid receptor interacting protein 1a; NAPE-PLD, N-acyl-phosphatidylethanolamine-selective phospholipase D; RhoA/Cdc42/Rac, members of the Rho family of small GTPases
Therapeutic implications
The neurodevelopmental impact of THC exposure during pregnancy has recently gained considerable attention given the long-lasting effects of prenatal cannabis use on emotional control, social behaviors, and cognition in affected offspring [46]. THC may be deleterious acting as an agonist or antagonist. Since THC is an intermediate potency, low efficacy CB1R agonist, THC can potentially antagonize the actions of the more efficacious 2-AG in vivo. Thus, in additional to activating CB1Rs, THC effects may be antagonistic when efficacious eCBs are present [10,47]. Physiological situations favoring antagonistic THC actions include low receptor density (e.g., pyramidal cells) or limiting downstream signaling molecules (e.g., during early specification events). Intriguingly, high-affinity antagonists at CB1Rs including, among other compounds, AM251, rimonabant, and taranabant, appear to exert even more powerful effects on developing glutamatergic neurons [33,37] by inhibiting axon fasciculation and postsynaptic target selection. A cell-autonomous eCB tone provided by DAGL activity in developing axons may define the cellular basis for undesired effects of CB1R antagonists, and account for the cortical delamination and perturbed long-range axon patterning induced by AM251 and rimonabant [33,37]. Alternatively, deleterious drug effects may be due to inverse agonism at CB1Rs. We conclude that eCBs can reach very high local concentrations but are secreted in a spatially and temporally precisely coordinated fashion. However, cannabinoid receptor antagonists engage their cognate receptors indiscriminately thus impacting the chronodynamics of eCB signaling in the developing brain.
Conclusions
The eCB system is emerging as a key regulatory signaling network fundamental to the wiring of the brain during development with an array of functions ranging from lineage segregation of stem cells to refinement of synaptic functions in complex neuronal networks. We have recently experienced a quantum leap in understanding the molecular hierarchy and signaling principles selectively governed by eCB signals in the embryonic brain. Nevertheless, the key unresolved question remains to define the exact importance of eCB signaling and its interactions with other developmentally-regulated signaling entities. The recent identification of robust developmental phenotypes in mice with cell-type specific conditional deletion of CB1Rs [12,33,37] challenged our prevailing concept on the limited significance of eCB signaling in the developing brain. Instead, these findings argue that promiscuity at the level of ligands, receptors, and signal transduction pathways provides an essential redundancy allowing exceptional cellular adaptation of eCB signaling: losing a single component will bear modest effects on neurodevelopment. However, losing more than one may be catastrophic.
Acknowledgments
This work was supported by the Swedish Medical Research Council (T.H.), Scottish Universities Life Science Alliance (T.H.), Alzheimer’s Association (K.M., T.H.), European Molecular Biology Organization Young Investigator Programme (T.H.), European Commission (HEALTH-F2-2007-201159; T.H.), National Institutes of Health grants DA023214 (T.H.), DA11322 (K.M.), DA15916 (K.M.), DA21969 (K.M.), and BBSRC (P.D.).
Footnotes
Conflict of interest statement
The authors have no conflict of interest with any aspect of data or concepts presented in this review.
Contributor Information
Tibor HARKANY, Email: Tibor.Harkany@ki.se, t.harkany@abdn.ac.uk.
Ken MACKIE, Email: kmackie@indiana.edu.
Patrick DOHERTY, Email: Patrick.Doherty@kcl.ac.uk.
References
- 1.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 2.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •3.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. Using correlated light and electron microscopy, the authors elucidate the subcellular localization of DAGLα and CB1 cannabinoid receptors at cerebellar and hippocampal synapses. This is the first study that clearly defines the molecular machinery underpinning eCB-mediated retrograde signaling and identifies dendritic spines as sites of DAGLs accumulation in adult brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •4.Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. This study explores the roles of Wnt ligands in retrograde signaling at mossy fiber-granule cell synapses. Using loss of function models, the authors demonstrate that Wnt signaling is required for the assembly of presynaptic terminals and neurotransmitter release in postnatal mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harkany T, Holmgren C, Hartig W, Qureshi T, Chaudhry FA, Storm-Mathisen J, Dobszay MB, Berghuis P, Schulte G, Sousa KM, Fremeau RT, Edwards RH, Mackie K, Ernfors P, Zilberter Y. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: Involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 9.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 10.Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol. 2007;578:773–785. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- ••12.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. Using growth cone turning assays, neuroanatomy, and transgenic analysis, this study identifies eCBs as target-derived axon guidance cues, and demonstrates that eCB signaling is required for adequate postsynaptic target selection of GABAergic interneurons expressing CB1 cannabinoid receptors. [DOI] [PubMed] [Google Scholar]
- 13.Bernard C, Milh M, Morozov YM, Ben Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci USA. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••14.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di MV, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. This study establishes DAGLs as 2-AG synthesis enzymes and reveals a striking translocation of DAGLs from the axonal to the somatodendritic compartment of neurons in parallels with functional maturation of synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- •17.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0711278105. This study shows that GPR55 is a cannabinoid receptor with signaling properties clearly distinct from CB1 or CB2 cannabinoid receptors. GPR55 was found to increase intracellular calcium via mechanisms involving Gq, G12, RhoA, actin, phospholipase C, and calcium release from IP3R-gated stores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 20.Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, Malli R, Graier WF. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 22.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 23.Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, Zieglgansberger W, Lutz B, Rammes G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki T, Begum T, Reza F, Horibe S, Inaba M, Yoshimura Y, Komatsu Y. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci Res. 2008;61:192–200. doi: 10.1016/j.neures.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci USA. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B, Ben-Ari Y. Early development of neuronal activity in the primate hippocampus in utero. J Neurosci. 2001;21:9770–9781. doi: 10.1523/JNEUROSCI.21-24-09770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 29.Manent JB, Jorquera I, Ben Ari Y, Aniksztejn L, Represa A. Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J Neurosci. 2006;26:5901–5909. doi: 10.1523/JNEUROSCI.1033-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- ••31.Maccarrone M, Rossi S, Bari M, De CV, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. This study identifies an essential interplay between AEA and 2-AG: elevation in AEA levels reduced the levels, metabolism and physiological effects of 2-AG in striatal slices. Since AEA is an endovanilloid substance, the authors were able to resolve that this regulatory mechanism is mediated by transient receptor potential vanilloid 1 (TRPV1) channels through a previously unknown glutathione-dependent pathway. [DOI] [PubMed] [Google Scholar]
- 32.Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, Zentar MP, Pollard S, Yanez-Munoz RJ, Williams G, Walsh FS, Pangalos MN, Doherty P. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- ••33.Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di MV, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. This study demonstrates that pyramidal cell specification relies on the temporal and spatial coherence of eCB signaling and that both autocrine and paracrine eCB signaling contributes to the adequate neurochemical identification and connectivity patterning of this cell type. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- •35.Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. The authors demonstrate using in vitro and in vivo genetic analyses that eCB signaling contributes to the fate decision of neural progenitors by promoting commitment towards the glial lineage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••36.Begbie J, Doherty P, Graham A. Cannabinoid receptor, CB1, expression follows neuronal differentiation in the early chick embryo. J Anat. 2004;205:213–218. doi: 10.1111/j.0021-8782.2004.00325.x. This study reveals a robust up-regulation of CB1 cannabinoid receptor expression in post-mitotic neurons first born at both the central and peripheral nervous systems. As development proceeds, the number of CB1 cannabinoid receptor-expressing neurons expands reflecting the importance of eCB signaling during neuronal specification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson S, Chambers D, Hobbs C, Doherty P, Graham A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol Cell Neurosci. 2008;38:89–97. doi: 10.1016/j.mcn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald NA, Henstridge CM, Connolly CN, Irving AJ. An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol Pharmacol. 2007;71:976–984. doi: 10.1124/mol.106.029348. [DOI] [PubMed] [Google Scholar]
- ••40.Bromberg KD, Ma’ayan A, Neves SR, Iyengar R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. Using global transcription factor profiling, the authors identified a signaling network controlling CB1 cannabinoid receptor-mediated neuronal outgrowth. In silico prediction identified novel roles for breast cancer 1 protein BRCA1 in neuronal differentiation, and a new pathway from CB1 cannabinoid receptors through phosphoinositol 3-kinase to the transcription factor paired box 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Muller C, Woods AS, Hope BT, Ciruela F, Casado V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferre S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- 43.Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Molecular Pharmacology. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 44.Brittis PA, Silver J, Walsh FS, Doherty P. Fibroblast growth factor receptor function is required for the orderly projection of ganglion cell axons in the developing mammalian retina. Mol Cell Neurosci. 1996;8:120–128. doi: 10.1006/mcne.1996.0051. [DOI] [PubMed] [Google Scholar]
- 45.van der Stelt M, Di Marzo V. Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat. 2005;77:111–122. doi: 10.1016/j.prostaglandins.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16 (Suppl 1):i3–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]