Abstract
Background & Aims
Premature activation of trypsinogen activation can cause pancreatic injury and has been associated with chronic pancreatitis (CP). Mice that lack intra-acinar activation of trypsinogen, such as trypsinogen-7–null (T−/−) and cathepsin B-null (CB−/−) mice, have been used to study trypsin-independent processes of CP development. We compared histological features and inflammatory response of pancreatic tissues from these mice to those from wild-type after development of CP.
Methods
CP was induced in wild-type, T−/−, and CB−/− mice by twice-weekly induction of acute pancreatitis for 10 weeks; acute pancreatitis was induced by hourly intraperitoneal injections of caerulein (50 μg/kg ×6). Pancreatic samples were collected and evaluated by histologic and immunohistochemical analyses. Normal human pancreas samples, obtained from the islet transplant program at the University of Minnesota, were used as controls and CP samples were obtained from surgical resections.
Results
Compared with pancreatic tissues from wild-type mice, those from T−/− and CB−/− mice had similar levels of atrophy, histo-morphologic features of CP, and chronic inflammation. All samples had comparable intra-acinar activation of nuclear factor (NF)-κB, a transcription factor that regulates the inflammatory response, immediately after injection of caerulein. Pancreatic tissues samples from patients CP had increased activation of NF-B (based on nuclear translocation p65 in acinar cells) compared with controls.
Conclusion
Induction of CP in mice by caerulein injection does not require intra-acinar activation of trypsinogen. Pancreatic acinar cells of patients with CP have increased levels of NF-κB activation, compared with controls; regulation of the inflammatory response by this transcription factor might be involved in pathogenesis of CP.
Keywords: mouse model, inflammation, digestive enzyme, PRSS1
The pancreas synthesizes and secretes enzymes responsible for the digestion of food. The digestive proteases are released as inactive proenzymes called zymogens. Trypsinogen, a zymogen, is activated to trypsin in the duodenum by duodenal enterokinase during digestion. Other digestive proenzymes are then activated by trypsin. This physiological mechanism of extra-pancreatic activation of zymogens (1) prevents autodigestion of the pancreas.
In 1896, Chiari postulated that zymogens are inappropriately activated within the pancreas, leading to autodigestion during acute pancreatitis (2). Intra-acinar trypsinogen activation, the initial step in digestive proteases activation, has been considered the primary mechanism of pancreatic injury ever since (3, 4). Decades of research have confirmed premature intra-acinar activation of trypsinogen during pancreatic injury (3, 5-7). However, demonstration of premature trypsinogen activation does not establish that it is causally responsible for the pathogenesis of pancreatitis; such causality has so far been assumed without definitive evidence (8).
Exploring the causality of intra-acinar trypsinogen activation and the pathogenesis of pancreatitis has, only now, become possible with the development of new genetically modified mouse models (9-11). Our group has generated a knock-out mouse model lacking the trypsinogen-7 gene (T−/−), which does not demonstrate pathologic (caerulein-induced) intra-acinar trypsinogen activation (9). This model allows us, for the first time, to clarify the role of intra-acinar trypsinogen activation by studying pancreatic injury in its absence, and investigate potential trypsin-independent pathways and their role in pancreatic injury. Using the T−/− mice, we have recently shown that pathologic trypsinogen activation is important in initial pancreatic injury during acute pancreatitis (9).
Analogous to acute pancreatitis, the popular belief is that intra-pancreatic trypsinogen activation is responsible for the pathogenesis of chronic pancreatitis (CP) (12-17). The identification of a trypsinogen (PRSS1) mutation in hereditary pancreatitis (an uncommon form of CP) in 1996 (18) has served to further consolidate support by many experts of this paradigm (12-15). However, no evidence exists to convincingly establish this paradigm of trypsin-based pancreatic injury as the pathogenic mechanism of CP. Moreover, despite the tendency to lump acute and chronic pancreatitis together, any assumptions for CP based on acute pancreatitis are premature because the relationship between acute and chronic pancreatitis is not clear (19, 20).
In this study, we have explored the currently accepted paradigm of trypsin-based pancreatic injury as the pathogenic mechanism of CP. We have used novel models that lack pathologic (caerulein induced) trypsinogen activation (9, 11). Using these models, we specifically aimed to 1) examine the role of intra-acinar trypsinogen activation in the pathogenesis of CP, and 2) explore/identify pathogenic mechanisms in CP that are independent of intra-acinar trypsinogen activation.
Material and methods
All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Minnesota. CP was induced by repeated episodes (twice a week ×10) of acute pancreatitis with caerulein hyperstimulation (50μg/kg i.p. every hour ×6). Animals were sacrificed 8 days after the last cycle of injections. Two independent experiments, each with N=8-10 animals per group, were conducted. For further experimental and methodological details, see supplementary material and methods section.
Trypsinogen-7 knock-out (T−/−) mice
These mice were generated in C57BL/6 background by targeted deletion of trypsinogen-7 gene as we described previously (9).
Cathepsin B knock-out (CB−/−) mice
These mice in C57BL/6 background (previously characterized (11, 21)) were obtained from Dr. G.J. Gores, Mayo Clinic, Rochester, MN.
Human pancreas samples
Sections of pancreas received from the islet transplant program at the University of Minnesota transplant facility from healthy donors were used as controls. Sections of CP were obtained from surgical resection specimens. The protocol for acquisition and use of all human pancreatic samples was approved by University of Minnesota Institutional Review Board.
Statistical analysis
JMP 9.0 (SAS institute, Cary, NC) was used for all statistical analyses. Normal variables are expressed as means ± SEM and analyzed using ANOVA with Tukey-Kramer post-hoc test unless specified otherwise. Non-parametric variables are reported as medians, depicted with box-whisker plots with outliers, and analyzed with Wilcoxon test. The central horizontal line in the box represents median, the vertical edges of the box represent quantiles, and the whiskers denote range (JMP 9.0). For all analyses, α=0.05 and two-tailed p-values are reported.
Results
Trypsinogen-7 knock-out mice (T−/−)
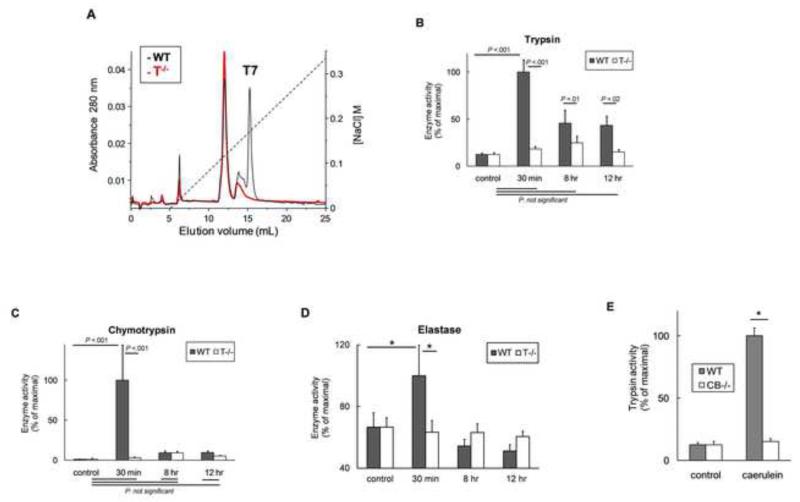
Details of the generation and characterization of T−/− mice along with studies pertaining to acute pancreatitis have been published separately (9). In addition to PCR and western blot data in our previous paper (9), additional evidence confirming the absence of trypsinogen-7 and specificity of the absent isotype in T−/− is reported here. Analysis by FPLC (Figure 1A) confirmed the deletion of trypsinogen-7 gene in T−/− mice which showed complete absence of trypsinogen-7 peak compared with WT. The identity of the trypsinogen-7 peak was confirmed by N-terminal sequencing.
Figure 1. (A) Characterization of Trypsinogen-7 knock-out (T−/−) mice.
FPLC analysis of pancreatic homogenates showed absence of trypsinogen-7 peak in T−/− mice. The specificity of this peak was confirmed by N-terminal sequencing. (B) T−/− mice lack pathologic trypsinogen activation. Trypsin activity was measured at indicated time-points in caerulein treated groups (compared to respective saline treated controls) as described in methods. N =10-16/group, pooled from 3 independent experiments. (C, D) Caerulein-induced activation of chymotrypsinogen (C) and elastase (D) in WT and T−/− mice. Significant activation of chymotrypsin and elastase was seen in WT mice at 30 minute time-point which was not observed in T−/− mice. P>.9 for chymotrypsin activity in control vs 8-hr or 12-hr time-points both for WT and T−/− mice. N=10-15/group for chymotrypsin activity. N=4-6 for controls and 30 minute groups, 10-12 for 8 hr and 12 hr groups for elastase activity. WT and T−/− control mice showed similar elastase activities (11±2 vs 12±1 slope/mg protein, P>.5) suggesting lack of compensatory changes in elastase expression in T−/− mice. (E) Cathepsin B knock-out (CB−/−) mice as a parallel tool. These mice have been previously characterized to lack significant pathologic trypsinogen activation which was confirmed in our laboratory. Trypsin activity was measured 30 minutes after caerulein injection (50μg/kg i.p.). N≥6 per group. *=statistically significant. Enzyme activities reported in B-E were measured in pancreatic homogenates, are normalized to respective controls and expressed as percent of maximal activity which was 90.4±12 for trypsin, 116.4±51 for chymotrypsin and 16.4±3 for elastase activities (slope/mg protein).
For ease of reference, we summarize the key features of T−/− mice. These mice demonstrated a healthy phenotype. Trypsinogen-7 accounted for 60% of expressed mouse trypsinogens, which was absent in T−/− mice (9). Expression of other trypsinogen isotypes was unaltered, which was sufficient for physiological function, in T−/− mice (9) suggesting a large pancreatic physiological reserve.
Intra-acinar trypsinogen activation is known to peak at 30 minutes after caerulein injection (5, 22), which was observed as expected in WT mice but not in T−/− mice (9) (Figure 1B a smaller peak of trypsinogen activation known to occur in later stages of pancreatitis, thought to be a consequence of inflammation and cellular damage, was seen in WT mice but no significant activation was observed in T−/− mice (Figure 1 B). In addition, absence of subsequent digestive enzyme activation in T−/− mice was demonstrated by lack of activation of chymotrypsinogen (Figure 1C) and elastase (Figure 1D).
Cathepsin B knock-out mice (CB−/−)
Cathepsin B is a lysosomal protease. During early pancreatic injury, lysosomal and zymogen compartments colocalize and cathepsin B activates trypsinogen (6, 11). Mice lacking cathepsin B (CB−/−) have been generated and characterized previously (11, 21). These mice (CB−/−) also lack significant trypsinogen activation (Figure 1E) (11) and were used as a parallel model
Experimental groups
Baseline weight of mice in all experimental groups was similar (average weight 26±0.4g). After 10 weeks, the controls averaged at 40±1g, while the caerulein-injected mice (CP groups) weighed 27±0.5g.
Pancreatic atrophy
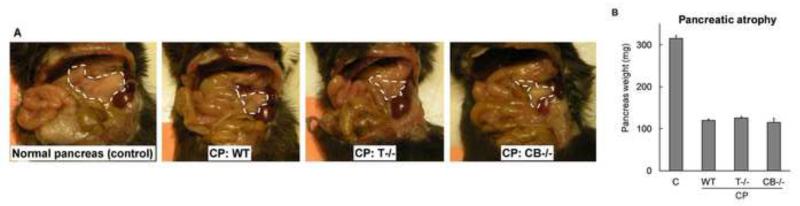
One of the hallmarks of chronic pancreatitis is pancreatic atrophy. In our study, a remarkable atrophy of the pancreas was seen in caerulein-injected WT, T−/− and CB−/− CP groups (Figure 2A, B).
Figure 2. Atrophy of pancreas in WT, T−/− and CB−/− CP groups was comparable.
(A)Representative pictures demonstrating pancreas atrophy. (B) Comparison of whole pancreas weight. N= 16-20/group, P<.0001 pair wise for each CP group vs control, not significant pair wise among CP groups.
Histo-morphologic features of chronic pancreatitis
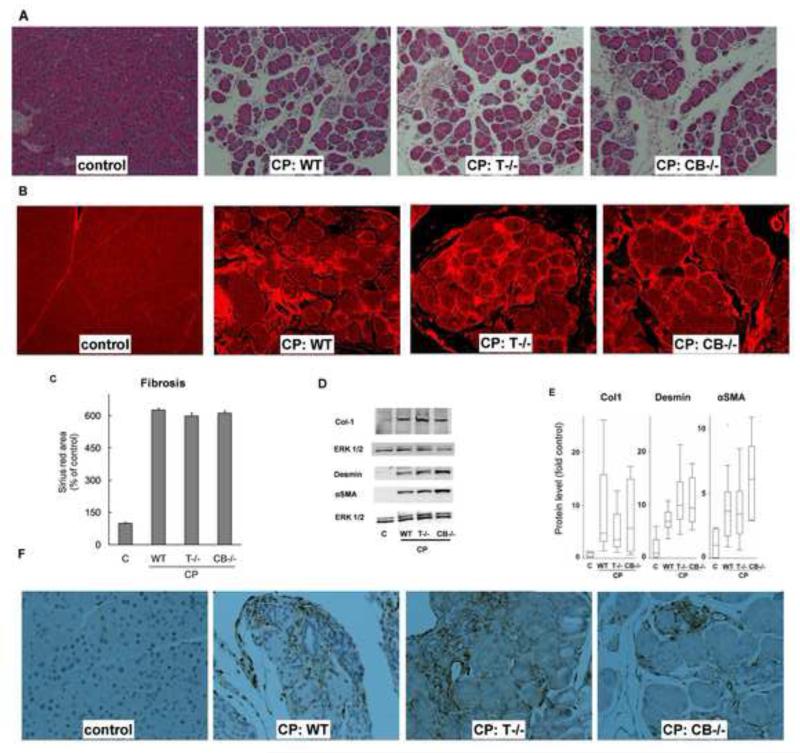
Histology Histologic features of CP groups are shown in Figure 3A. The prominent features of CP, including moderate acinar loss, fibrosis, microscopic duct dilatations, tubular complexes and inflammatory infiltrate were observed to a similar extent in WT, T−/− and CB−/− CP groups. These histologic changes are known to be characteristic of CP in humans (23) and were also observed in sections of human CP used in our study (see below).
Fibrosis and stellate cells Marked fibrosis was seen in WT, T−/− and CB−/− CP groups compared with controls (Figure 3B). The extent of fibrosis, measured objectively by increase in Sirius red-stained area (Figure 3C) and overexpression of collagen-1 (Figure 3D,E), was comparable in all CP groups. Activated pancreatic stellate cells are fibrogenic and are known to proliferate in CP (23). Comparable proliferation (measured by overexpression of desmin) and activation (measured by overexpression of α-SMA) (Figure 3D,E) of stellate cells was seen in CP groups. Marked increase in stellate cell population in CP groups was confirmed by staining for vimentin, a stellate cell marker (Figure 3F).
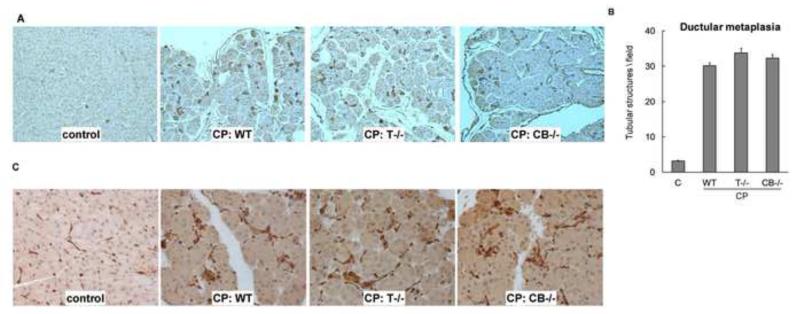
Duct metaplasia and duct function Appearance of new duct/tubular structures is an important feature of CP. Cytokeratin-19 stain (an epithelial marker known to stain ducts) revealed similar degrees of ductular metaplasia in all CP groups (Figure 4A). The degree of ductular metaplasia was quantified by measuring the number of tubular complexes (Figure 4B). Recently, compensatory overexpression of aquaporin-1 as a result of impaired duct function (reduced water secretion) was demonstrated in autoimmune pancreatitis, a form of CP (24). We observed comparable overexpression of aquaporin-1 (Figure 4C) in all CP groups.
Blood glucose levels (2 hour after 2mg/g intra-peritoneal glucose injection) in controls and CP groups were similar (140±3 mg/dl). No gross or microscopic steatorrhea was seen in any group. These are consistent with absence of frank pancreatic insufficiency in this model of chronic pancreatitis with 10 weeks of repeated caerulein injections (25, 26).
Figure 3. (A) Histologic features characteristic of chronic pancreatitis were similar in all experimental CP groups.
Representative hematoxylin and eosin-stained sections (100×) showing moderate acinar loss, fibrosis, microscopic duct dilatations, tubular complexes and inflammatory infiltrate WT, T−/− and CB−/− CP groups (N=16-20/group). (B) Fibrosis: Sirius red-stained sections (100×) under fluorescence showing marked fibrosis in CP groups. Quantification (C) confirmed comparable fibrosis in WT, T−/− and CB−/− CP groups (P<.0001 pair wise for each CP group vs control, not significant pair wise among CP groups, N=16-20/group). (D,E) Comparable overexpression of collagen-1, desmin and αSMA in CP groups. Representative western blots are shown in (D) and their quantification is shown as box-whisker plots with outliers in (E). N=5-8/group for each protein. Control vs WT, T−/−, CB−/− CP groups respectively: P= .0008, .001, .04 for collagen-1; .001, .001, .009 for desmin and .001, .001, .009 αSMA. P-values not significant for all pair wise comparisons among CP groups. Vimentin staining confirmed marked increase in stellate cell population in the CP groups (F). Representative sections (200×) stained with vimentin are shown.
Figure 4. (A) Ductular metaplasia in CP groups.
Representative cytokeratin-19 stained sections (100×) from controls and CP groups showing increase in ductular/tubular complexes. These structures were counted in high power (100×) fields (B). Tubular complexes (per field) increased to 30±1, 34±1, 32±1 in WT, T−/−, CB−/− CP groups from 3±0.2 in controls (P<.0001 for each CP group vs controls, N=10/group). (C) Aquaporin-1 (AQP1) overexpression in CP: Dysfunction of ducts in CP is believed to cause compensatory overexpression of water channels Aquaporin-1 (see text). Comparable overexpression of AQP1 was seen in WT, T−/− and CB−/− groups with CP as compared to controls. Representative AQP-1 stained sections (200×) are shown (N=10/group).
Inflammation in chronic pancreatitis is independent of trypsinogen activation
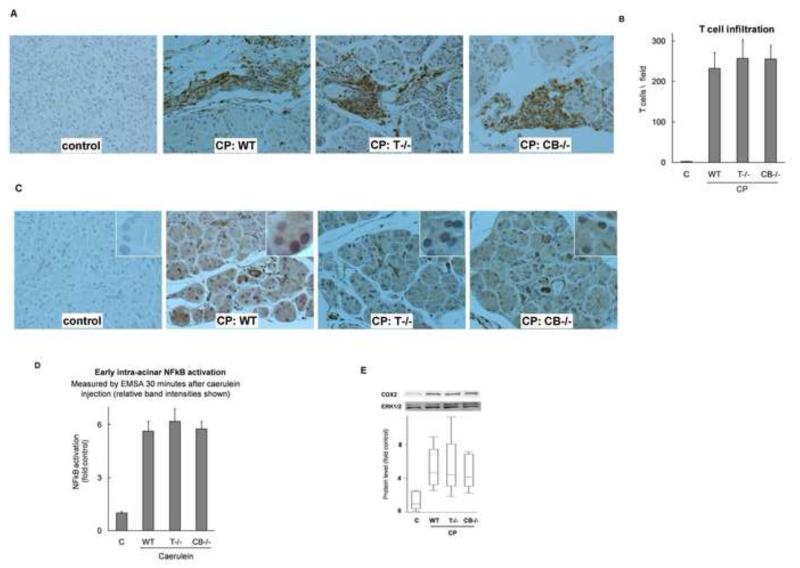
Inflammatory infiltration was analyzed by staining the tissue sections for CD3, a T lymphocyte marker. As shown in Figure 5A and B, T-cell infiltration was comparable in WT, T−/− and CB−/− CP groups. Additionally, overexpression of TGFβ, a chronic inflammatory mediator known to play a crucial role in stellate cell activation and fibrogenesis (27), was similar in CP groups (data not shown). These results, together with data presented in the next section (Figure 5 C-F), establish that pathogenesis of inflammation in CP is independent of intra-acinar trypsinogen activation.
Figure 5. (A, B) Inflammatory infiltrate in CP groups.
Representative sections (200×) stained for CD3 (T-Cell marker) shown in (A), and quantified in (B). Comparable in WT, T−/−, CB−/− CP groups (P=.03, .01, .01 respectively vs control, not significant pair wise among CP groups, N=10/group). (C) Sustained NFκB activation in chronic pancreatitis: NFκB p65 immunostaining demonstrates its nuclear localization in acinar cells. Representative sections (200×) from controls and WT, T−/−, and CB−/− CP groups (insets are zoomed-in views showing closer details). (D) NFκB activation occurs independent of trypsinogen activation in immediate response to pathologic stimulus: NFκB activation in the pancreas 30 minutes after caerulein injection (50μg/kg) was assessed by EMSA and relative band intensities have been plotted. p<0.0001 for each group vs controls, not significant for other pairwise comparisons; N=3-5/group. Controls include N=3 each of WT, T−/− and CB−/− mice which were similar. Intra-acinar localization of NFκB was confirmed by in-vitro experiments on isolated acinar cells (see text). (E) Cyclooxygenase-2 (COX2): Overexpression of COX2, which is regulated by NFκB, was comparable in WT, T−/−, CB−/− CP groups (P= .0008, .001, .014 respectively vs controls, not significant pair wise among CP groups; N=5-8/group).
NFκB activation occurs early during pancreatic injury and is independent of trypsinogen activation
NFκB activation occurs as early as 30 minutes after caerulein injection in this model of pancreatic injury, a time-course parallel to trypsinogen activation (5, 22). We have recently demonstrated activation of NFκB in the caerulein model of acute pancreatitis in T−/− and CB−/− mice along with WT mice (9). Here we confirm with quantitative analysis that the extent of NFκB activation in WT, T−/− and CB−/− groups is similar (Figure 5 D). Intra-acinar localization of NFκB activation is further confirmed in-vitro in isolated acinar cells subjected to supramaximal caerulein stimulation (6.8 ±1.6, 7.0±1.7, 4.5±0.5 fold NFκB activation in WT, T−/− and CB−/− acini respectively, p<0.01 each group vs control, n=3-5/group).
Persistent NFκB activation in chronic pancreatitis experimental groups
Upon activation, the p65 subunit of NFκB translocates to the nucleus. Thus, nuclear staining for NFκB p65 suggests activation of NFκB in WT, T−/− and CB−/− CP groups (Figure 5C). While NFκB activation occurred (Figure 5D) during each acute episode in the CP model, any residual effects of the last acute episode should have resolved as the CP samples were collected 8 days after the last acute episode. Thus, p65 nuclear translocation in CP groups indicates sustained NFκB activation in CP. Additionally, comparable degrees of COX2 overexpression (Figure 5E) were seen in all CP groups. COX2 is regulated by NFκB and is known to be a key mediator of chronic inflammation (28, 29). Overexpression of COX2 provides quantifiable evidence of the translational aspect of persistent NFκB activation and also points to its pathogenic role in CP.
Sustained NFκB activation occurs in human chronic pancreatitis
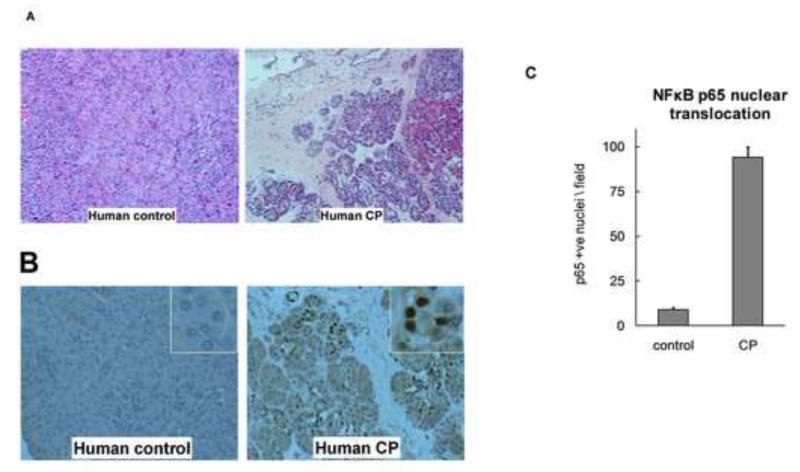
Pancreas sections from CP patients (N=7, age 35±6, all females, 5 idiopathic, 1 hereditary, 1 obstructive) demonstrated characteristic histo-morphologic features as compared to controls (N=7, age 31±4, 5 males) (Figure 6 A). Staining for p65 in these sections from CP patients (Figure 6 B) demonstrated p65 nuclear translocation in acinar cells. Quantification of nuclear p65 positivity in acinar cells is shown in Figure 6 C. These data establish that sustained NFκB activation occurs in CP.
Figure 6. NFκB activation in human chronic pancreatitis.
A) Representative hematoxylin and eosin-stained sections (100×) of pancreas from healthy controls and CP patients demonstrating histo-morphologic features of CP. Similar features were seen in the mouse CP groups. B and C) Persistent NFκB activation occurs in human chronic pancreatitis. NFκB component protein p65 immunostaining demonstrates its nuclear localization in acinar cells (B) in human chronic pancreatitis sections (200×) (insets are zoomed-in views showing closer details). Acinar cells whose nuclei stained positive for p65 were counted in each field (200×) and are shown in (C). Pancreas sections from seven CP patients and seven controls were analyzed (P<.001).
Discussion
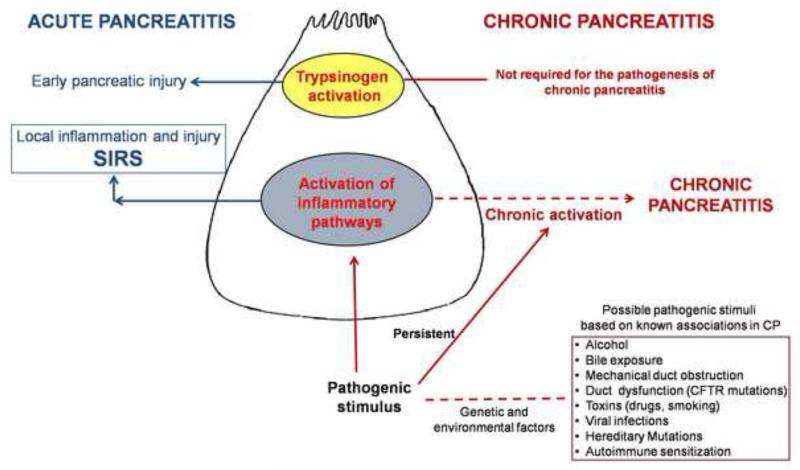
We have established that the pathogenesis of caerulein-induced chronic pancreatitis (CP) does not require intra-acinar trypsinogen activation. Further, independent of intra-acinar trypsinogen activation, sustained activation of NFκB pathway occurs in CP which may be an important pathogenic mechanism of CP. These novel data mark a paradigm shift in our current understanding of pancreatic injury (Figure 7).
Figure 7. A simplistic schematic summarizing novel findings in the pathogenesis of pancreatic injury.
The section on acute pancreatitis (in navy blue) is based on our previous study (9) and is being mentioned here for completeness. The left side of the schematic (dark red) describes the proposed paradigm of chronic pancreatitis stating that sustained activation of inflammatory pathways (NFκB pathway is thought to be the most important) in acinar cells results in chronic pancreatitis. Chronicity of pathogenic stimulus (which may be any of the recognized etiologic associations) may drive the sustained NFκB response. A complex interplay of genetic predispositions as well as environmental factors may influence the effect of pathogenic stimuli. In this schematic, solid lines depict links with experimental proof while hypothesized links are shown by dotted lines.
In line with our study, it has been previously shown that acinar expression of constitutively active trypsin was not sufficient to induce CP (10). While reduced acinar necrosis was seen in acute pancreatitis in T−/− (9) and CB−/− mice, CP induced by repeated episodes of acute pancreatitis in these mice was similar to WT mice. Further, induction of acinar cell death by expression of active trypsin failed to produce chronic inflammation or fibrosis (10). These together indicate that necrosis-fibrosis sequence (16, 17) may not be necessary in CP.
Hereditary pancreatitis, an uncommon form of CP, is associated with mutations in cationic trypsinogen (12-15, 18, 30). These are thought to lead to CP due to increased intra-acinar trypsin activity (13, 30, 31) based on in vitro data primarily on the R122H and N29I mutants (30). However, incomplete penetrance, intermittent nature of the disease, and lack of progression to CP in some despite recurrent episodes of acute pancreatitis remain unexplained (32, 33). Notably, a mouse model expressing R122H mutation in mouse trypsinogen was reported in 2006 (34) but no follow-up of that model has been reported since.
Despite the popular belief, no confirmatory evidence exists thus far to show that increased intraacinar trypsin activation per se is the disease causing mechanism of these mutations associated with hereditary pancreatitis. In this study using an experimental model, we show that intra-pancreatic trypsinogen activation is not required for pathogenesis of CP. It can certainly be argued that experimental models may not accurately reflect human disease. Although some of the observed mutations could be incidental (35, 36), the association of hereditary pancreatitis with mutations in the trypsinogen system cannot be discounted. Recently, trypsinogen gene loci (PRSS1-PRSS2) variants were recognized to modulate the risk of sporadic and alcoholic-related pancreatitis (37). It is possible that alternate disease mechanisms operating independent of activation of trypsinogen may exist. Pancreatic acinar cells are essentially secretory cells with likely well-developed protective mechanisms to maintain its secretory machinery. Could mutations in the trypsinogen genes cause misfolding and disruption of the efficient secretory machinery of acinar cells leading to chronic cellular stress response (such as ER stress, autophagy response) and inflammation? Recent studies have started to explore such possibilities (17, 38-42).
Many experts are linking emerging evidence of mutations/polymorphisms in SPINK (43-45), PSTI (46), cathepsin B (47), CFTR (13, 46) and other genes (48) in alcoholic and non-hereditary forms of CP to increased trypsinogen activation (13-15, 49). While certainly a possibility, these associations are much weaker than that for cationic trypsinogen and hereditary pancreatitis, and are likely modulating factors rather that causative factors in non-hereditary forms of pancreatitis (13, 15). Further, alternate mechanisms other than trypsinogen activation may be responsible for these associations. For example, PSTI and SPINK proteins have been shown to be important in cell death regulation (50), autophagy (51), growth (50, 52) and inflammation (52, 53). Such effects may explain amelioration of pancreatitis with PSTI overexpression (54, 55).
Activation of NFκB pathway has been previously recognized during early pancreatic injury in experimental models (22, 56). However, its relationship with trypsinogen activation has been a matter of heated debate with conflicting results complicated by limitations of existing models (22, 53, 57, 58). Using T−/− and CB−/− mice, we have established that intra-acinar NFκB activation occurs early during pancreatitis independent of trypsinogen activation (9). We further show persistence of NFκB activation in CP occurring independent of trypsinogen activation. Importantly, persistent activation of NFκB was seen in human CP, which confirms the experimental findings. To our knowledge, this is the first report of sustained intra-acinar NFκB activation in human CP.
The NFκB pathway is a ubiquitous, precisely regulated cellular mechanism controlling diverse processes (28, 59). Dysregulation of NFκB leading to chronic NFκB activation is important in several cancers (60) and chronic inflammatory diseases (29). Our findings suggest that sustained activation of NFκB may be crucial pathogenic mechanism in CP. Its downstream targets, including COX2 and IL1β, are known to be key drivers of chronic inflammation (28, 29). In fact, mice overexpressing IL1β in the pancreatic acini (26) develop severe chronic pancreatitis. However, the diversity of NFκB-regulated processes (59) lend great complexity to experimental models utilizing manipulation of NFκB proteins. Constitutive NFκB ablation led to increased severity of acute (61) and chronic pancreatitis (62) in one model and reduced severity in another (63). Conditional inhibition of NFκB resulted in reduced severity (64) while conditional activation led to full spectrum of pancreatitis, including systemic inflammatory response (64-67), and CP in a recent study where caerulein injections were required for NFκB activation in a p65 transgenic mice (67). Disruption of NFκB regulated physiological processes in the constitutive models may account for the apparent discrepancies.
We propose that sustained activation of inflammatory pathways such as NFκB results in chronic pancreatitis (Figure 7). NFκB seems to be the important inflammatory pathway in pancreatic injury (68). However, other inflammatory pathways such as AP-1 (69) and stress kinases may also have some role in CP, which will require further studies. Chronicity of the pathologic stimulus (which may be any of the recognized etiologic associations, Figure 7) may drive persistent activation of the inflammatory pathways. Conversely, withdrawal of the stimulus may lead to disease reversal which has recently been demonstrated in alcohol-related pancreatic injury (70). A complex interplay of genetic predispositions as well as environmental factors may influence the effects of the pathogenic stimuli. Contrary to the sentinel acute pancreatitis episode (SAPE) theory (12), a sentinel episode of acute pancreatitis may not be necessary, although sensitization resulting in unchecked inflammation may be important in some forms of CP like autoimmune pancreatitis.
The caerulein based model used in this study is an excellent histo-morphologic model (23, 25) and mimics the well-described recurrent acute pancreatitis to CP sequence in humans (12, 20). Such a histo-morphologic model is useful despite lacking a direct etiologic correlation as the histo-morphologic features are largely indistinguishable among different CP etiologies (71). Unfortunately, there are no CP models based on common etiologies. Models using alcohol develop CP with repeated caerulein exposure but not with alcohol alone (72, 73). Rat models based on chemical injury (74, 75), which are rather artificial models, have not been reproduced in mice. Thus, no alternate mouse model is available currently. Use of a single model of CP is an inherent limitation of our study.
In conclusion, our results establish that intra-acinar trypsinogen activation is not required for caerulein-induced chronic pancreatitis. Independent of trypsinogen activation, persistent activation of NFκB pathway occurs in CP and may be important in its pathogenesis.
Supplementary Material
Acknowledgments
Grant support: Supported in part from NIH grants (AKS) DK093047, DK058694 and DK092145 and intramural support from Department of Surgery, University of Minnesota
Abbreviations
- CP
Chronic Pancreatitis
- AP
Acute pancreatitis
- NFκB
Nuclear Factor kappa B
- WT
Wild Type
- T−/−
Trypsinogen-7 knock-out mice
- CB−/−
Cathepsin B knock-out mice
- PRSS1
Protease Serine 1
- PRSS2
Protease Serine 2
- PSTI
Pancreas Secretory Trypsin Inhibitor
- SPINK
Serine Protease Inhibitor Kazal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None to disclose.
Authors Contributions
RS: Was involved in acquisition, analysis, interpretation of the data and drafting of the manuscript.
VD: Was involved in generation of T7 knockout mice, study concepts and revision of the manuscript
RD: Was involved in the acquisition, analysis and interpretation of the data, drafting of the manuscript, generation of T7 knockout mice.
AS: Study concept and design, interpretation of data, drafting of the manuscript, obtained funding and study supervision. PI of the project.
References
- 1.Barrett KE, et al. Barrett KE, Barman SM, Boitano S, Brooks H, editors. Protein Digestion. Ganong’s Review of Medical Physiology. (23 ed) 2010 p. http://www.accessmedicine.com/content.aspx?aID=5242356.
- 2.Chiari H. ÜberdieSelbstverdauung des menschlichenPankreas. ZeitschriftfürHeilkunde. 1896;17:69–96. [Google Scholar]
- 3.Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27(5):444–51. doi: 10.1097/MOG.0b013e328349e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saluja AK, Lerch MM, et al. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–69. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 5.Hofbauer B, Saluja AK, et al. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275(2 Pt 1):G352–62. doi: 10.1152/ajpgi.1998.275.2.G352. [DOI] [PubMed] [Google Scholar]
- 6.Saluja A, Saluja M, et al. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest. 1989;84(4):1260–6. doi: 10.1172/JCI114293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloppel G, Dreyer T, et al. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol. 1986;409(6):791–803. doi: 10.1007/BF00710764. [DOI] [PubMed] [Google Scholar]
- 8.Sah RP, Saluja AK. Trypsinogen activation in acute and chronic pancreatitis: is it a prerequisite? Gut. 2011;60(10):1305–7. doi: 10.1136/gut.2011.241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawra R, Sah RP, et al. Intra-acinar Trypsinogen Activation Mediates Early Stages of Pancreatic Injury but Not Inflammation in Mice With Acute Pancreatitis. Gastroenterology. 2011;141(6):2210–7. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaiser S, Daniluk J, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011 doi: 10.1136/gut.2010.226175. gut.2010.226175 [pii]10.1136/gut.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halangk W, Lerch MM, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106(6):773–81. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 13.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med. 2010;61:413–24. doi: 10.1146/annurev.med.041608.121416. [DOI] [PubMed] [Google Scholar]
- 14.Chen JM, Ferec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2009;10:63–87. doi: 10.1146/annurev-genom-082908-150009. [DOI] [PubMed] [Google Scholar]
- 15.LaRusch J, Whitcomb DC. Genetics of pancreatitis. Curr Opin Gastroenterol. 2011;27(5):467–74. doi: 10.1097/MOG.0b013e328349e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witt H, Apte MV, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132(4):1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Stevens T, Conwell DL, et al. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99(11):2256–70. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 18.Whitcomb DC, Gorry MC, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14(2):141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 19.Yadav D, O’Connell M, et al. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107(7):1096–103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 20.Guda NM, Romagnuolo J, et al. Recurrent and relapsing pancreatitis. Curr Gastroenterol Rep. 2011;13(2):140–9. doi: 10.1007/s11894-011-0176-x. [DOI] [PubMed] [Google Scholar]
- 21.Canbay A, Guicciardi ME, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112(2):152–9. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hietaranta AJ, Saluja AK, et al. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280(1):388–95. doi: 10.1006/bbrc.2000.4120. [DOI] [PubMed] [Google Scholar]
- 23.Kloppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20(Suppl 1):S113–31. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 24.Ko SB, Mizuno N, et al. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology. 2010;138(5):1988–96. doi: 10.1053/j.gastro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuschwander-Tetri BA, Burton FR, et al. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45(4):665–74. doi: 10.1023/a:1005423122127. [DOI] [PubMed] [Google Scholar]
- 26.Marrache F, Tu SP, et al. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology. 2008;135(4):1277–87. doi: 10.1053/j.gastro.2008.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menke A, Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int J Gastrointest Cancer. 2002;31(1-3):41–6. doi: 10.1385/IJGC:31:1-3:41. [DOI] [PubMed] [Google Scholar]
- 28.Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2009;116(6):451–65. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- 29.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 30.Teich N, Rosendahl J, et al. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Hum Mutat. 2006;27(8):721–30. doi: 10.1002/humu.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin-Toth M. Biochemical models of hereditary pancreatitis. Endocrinol Metab Clin North Am. 2006;35(2):303–12. ix.. doi: 10.1016/j.ecl.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebours V, Boutron-Ruault MC, et al. The natural history of hereditary pancreatitis: a national series. Gut. 2009;58(1):97–103. doi: 10.1136/gut.2008.149179. [DOI] [PubMed] [Google Scholar]
- 33.Howes N, Lerch MM, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2004;2(3):252–61. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 34.Archer H, Jura N, et al. A mouse model of hereditary pancreatitis generated by transgenic expression of R122H trypsinogen. Gastroenterology. 2006;131(6):1844–55. doi: 10.1053/j.gastro.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Szmola R, Sahin-Toth M. Uncertainties in the classification of human cationic trypsinogen (PRSS1) variants as hereditary pancreatitis-associated mutations. J Med Genet. 2010;47(5):348–50. doi: 10.1136/jmg.2009.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosendahl J, Teich N, et al. Complete analysis of the human mesotrypsinogen gene (PRSS3) in patients with chronic pancreatitis. Pancreatology. 2010;10(2-3):243–9. doi: 10.1159/000243769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitcomb DC, Larusch J, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nature genetics. 2012;44(12):1349–54. doi: 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kereszturi E, Sahin-Toth M. Intracellular autoactivation of human cationic trypsinogen mutants causes reduced trypsinogen secretion and acinar cell death. J Biol Chem. 2009;284(48):33392–9. doi: 10.1074/jbc.M109.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kereszturi E, Szmola R, et al. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat. 2009;30(4):575–82. doi: 10.1002/humu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szmola R, Sahin-Toth M. Pancreatitis-associated chymotrypsinogen C (CTRC) mutant elicits endoplasmic reticulum stress in pancreatic acinar cells. Gut. 2009;59(3):365–72. doi: 10.1136/gut.2009.198903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lugea A, Tischler D, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140(3):987–97. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1804–12. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- 43.Bhatia E, Choudhuri G, et al. Tropical calcific pancreatitis: strong association with SPINK1 trypsin inhibitor mutations. Gastroenterology. 2002;123(4):1020–5. doi: 10.1053/gast.2002.36028. [DOI] [PubMed] [Google Scholar]
- 44.Chandak GR, Idris MM, et al. Absence of PRSS1 mutations and association of SPINK1 trypsin inhibitor mutations in hereditary and non-hereditary chronic pancreatitis. Gut. 2004;53(5):723–8. doi: 10.1136/gut.2003.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drenth JP, te Morsche R, et al. Mutations in serine protease inhibitor Kazal type 1 are strongly associated with chronic pancreatitis. Gut. 2002;50(5):687–92. doi: 10.1136/gut.50.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keiles S, Kammesheidt A. Identification of CFTR, PRSS1, and SPINK1 mutations in 381 patients with pancreatitis. Pancreas. 2006;33(3):221–7. doi: 10.1097/01.mpa.0000232014.94974.75. [DOI] [PubMed] [Google Scholar]
- 47.Mahurkar S, Idris MM, et al. Association of cathepsin B gene polymorphisms with tropical calcific pancreatitis. Gut. 2006;55(9):1270–5. doi: 10.1136/gut.2005.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paliwal S, Bhaskar S, et al. Comprehensive screening of chymotrypsin C (CTRC) gene in tropical calcific pancreatitis identifies novel variants. Gut. 2012 doi: 10.1136/gutjnl-2012-302448. [DOI] [PubMed] [Google Scholar]
- 49.Apte MV, Pirola RC, et al. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol. 2010;25(12):1816–26. doi: 10.1111/j.1440-1746.2010.06445.x. [DOI] [PubMed] [Google Scholar]
- 50.Ohmuraya M, Sugano A, et al. Role of Intrapancreatic SPINK1/Spink3 Expression in the Development of Pancreatitis. Front Physiol. 2012;3:126. doi: 10.3389/fphys.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohmuraya M, Hirota M, et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129(2):696–705. doi: 10.1016/j.gastro.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 52.Ohmuraya M, Yamamura K. Roles of serine protease inhibitor Kazal type 1 (SPINK1) in pancreatic diseases. Exp Anim. 2011;60(5):433–44. doi: 10.1538/expanim.60.433. [DOI] [PubMed] [Google Scholar]
- 53.Tando Y, Algul H, et al. Induction of IkappaB-kinase by cholecystokinin is mediated by trypsinogen activation in rat pancreatic lobules. Digestion. 2002;66(4):237–45. doi: 10.1159/000068364. [DOI] [PubMed] [Google Scholar]
- 54.Nathan JD, Romac J, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128(3):717–27. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 55.Nathan JD, Romac J, et al. Protection against chronic pancreatitis and pancreatic fibrosis in mice overexpressing pancreatic secretory trypsin inhibitor. Pancreas. 2010;39(1):e24–30. doi: 10.1097/MPA.0b013e3181bc45e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gukovsky I, Gukovskaya AS, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275(6 Pt 1):G1402–14. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 57.Han B, Ji B, et al. CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280(3):C465–72. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 58.Ji B, Gaiser S, et al. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem. 2009;284(26):17488–98. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 60.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18(49):6938–47. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 61.Algul H, Treiber M, et al. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J Clin Invest. 2007;117(6):1490–501. doi: 10.1172/JCI29882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treiber M, Neuhofer P, et al. Myeloid, but not pancreatic, RelA/p65 is required for fibrosis in a mouse model of chronic pancreatitis. Gastroenterology. 2011;141(4):1473–85. 85 e1–7. doi: 10.1053/j.gastro.2011.06.087. [DOI] [PubMed] [Google Scholar]
- 63.Altavilla D, Famulari C, et al. Attenuated cerulein-induced pancreatitis in nuclear factor-kappaB-deficient mice. Lab Invest. 2003;83(12):1723–32. doi: 10.1097/01.lab.0000101734.82054.be. [DOI] [PubMed] [Google Scholar]
- 64.Baumann B, Wagner M, et al. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest. 2007;117(6):1502–13. doi: 10.1172/JCI30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aleksic T, Baumann B, et al. Cellular immune reaction in the pancreas is induced by constitutively active IkappaB kinase-2. Gut. 2007;56(2):227–36. doi: 10.1136/gut.2005.084665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Ji B, et al. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122(2):448–57. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 67.Huang H, Liu Y, et al. Activation of Nuclear Factor-kappaB in Acinar Cells Increases the Severity of Pancreatitis in Mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakonczay Z, Jr., Hegyi P, et al. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57(2):259–67. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 69.Adcock IM. Transcription factors as activators of gene transcription: AP-1 and NF-kappa B. Monaldi Arch Chest Dis. 1997;52(2):178–86. [PubMed] [Google Scholar]
- 70.Vonlaufen A, Phillips PA, et al. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut. 2011;60(2):238–46. doi: 10.1136/gut.2010.211250. [DOI] [PubMed] [Google Scholar]
- 71.Shrikhande SV, Martignoni ME, et al. Comparison of histological features and inflammatory cell reaction in alcoholic, idiopathic and tropical chronic pancreatitis. Br J Surg. 2003;90(12):1565–72. doi: 10.1002/bjs.4353. [DOI] [PubMed] [Google Scholar]
- 72.Perides G, Tao X, et al. A mouse model of ethanol dependent pancreatic fibrosis. Gut. 2005;54(10):1461–7. doi: 10.1136/gut.2004.062919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Guo M, et al. Does chronic ethanol intake cause chronic pancreatitis?: evidence and mechanism. Pancreas. 2008;37(2):189–95. doi: 10.1097/MPA.0b013e31816459b7. [DOI] [PubMed] [Google Scholar]
- 74.Sparmann G, Merkord J, et al. Pancreatic fibrosis in experimental pancreatitis induced by dibutyltin dichloride. Gastroenterology. 1997;112(5):1664–72. doi: 10.1016/s0016-5085(97)70049-0. [DOI] [PubMed] [Google Scholar]
- 75.Puig-Divi V, Molero X, et al. Induction of chronic pancreatic disease by trinitrobenzene sulfonic acid infusion into rat pancreatic ducts. Pancreas. 1996;13(4):417–24. doi: 10.1097/00006676-199611000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.