Abstract
BACKGROUND
Maximal hyperemia is the critical prerequisite for fractional flow reserve (FFR) assessment. Despite intravenous (IV) adenosine currently being the recommended approach, intracoronary (IC) administration of adenosine constitutes a valuable alternative in everyday practice. However, it is surprisingly unclear which IC strategy allows the achievement of FFR values that are comparable to IV adenosine.
OBJECTIVES
This study sought to compare increasing doses of IC adenosine versus IV adenosine for FFR.
METHODS
30 intermediate coronary stenoses undergoing FFR measurement were prospectively and consecutively enrolled. Hyperemia was sequentially induced by bolus of IC adenosine (ADN; 150 μg) followed by IV adenosine (IVADN) infusion over 3 minutes at dose of (140 μg/kg/min). FFR values, symptoms, and development of atrioventricular block were recorded.
RESULTS
150 μg doses of IC adenosine were well tolerated and associated with fewer symptoms than IV adenosine. Intracoronary adenosine doses induced a significant decrease of FFR compared with baseline levels (P < 0.01). Among the 6 patients with FFR values less than 0.80 identified by IVADN, 4 were correctly identified also by 150 μg bolus IC adenosine. Larger randomized studies with cross-over design are necessary to verify the results.
CONCLUSIONS
This small pilot study suggests that IC adenosine might be an alternative to IV adenosine. Larger randomized studies with a cross-over design are necessary.
Keywords: AVB: atrioventricular- nodal block, CAD: Coronary artery disease, FAME: FFR versus angiography for multi vessel evaluation, FFR: Fractional flow reserve, IC: Intracoronary, ICADN Intracoronary Adenosine, IV: Intravenous, IVADN: intravenous Adenosine, PCI: percutaneous coronary intervention, QCA: quantitative coronary Angiography, CBF: Coronary blood flow
Introduction
Ischemic heart disease remains a leading cause of morbidity and mortality worldwide.1 Borderline coronary artery lesions are responsible for about 80% of acute coronary syndromes (ACS).2 It is not uncommon during the performance of coronary angiography to identify borderline coronary stenosis (>40 and <80%) in the absence of more severe lesions.3
Percutaneous coronary intervention (PCI) of an intermediate stenosis without evidence of ischemia is often performed, but its benefit is unproven. Coronary pressure-derived fractional flow reserve (FFR) is an index used to identify a stenosis responsible for reversible ischemia,4 which profits from revascularization.5
Uncertainty about the significance of these lesions may be augmented by the error associated with visual estimation of the severity of the stenosis. Thus, in a patient with a borderline lesion the results of an FFR can be very useful to evaluate the functional significance of coronary lesions.3
The measurement of fractional flow reserve (FFR) is commonly used in clinical practice to assess the hemodynamic significance of epicardial coronary stenosis and an FFR value of 0.75 is accepted as a physiologically significant stenosis. However, an FFR between 0.75 and 0.80 is considered to be a borderline and represents a gray zone of functional significance for coronary stenosis.6
Achievement of maximal hyperemia of coronary microcirculation is the prerequisite for the exact assessment of FFR in order to minimize the effect of microvascular resistance. With suboptimal coronary hyperemia, FFR will be artificially high, resulting in a potentially significant underestimation of the functional severity of coronary stenosis.7
To achieve maximal hyperemia, IV adenosine is considered the standard method, but its use in the catheterization laboratory is time consuming and expensive compared with intracoronary adenosine.8 Therefore, this study compared intracoronary adenosine for the potential to achieve a maximal hyperemia equivalent to the standard intravenous route.
Patients and Methods
This study was conducted at the Cardiology Department at Ain Shams University hospital. The study sample was comprised of 22 patients who underwent elective coronary angiography and were found to suffer from 30 borderline lesions in the period from January 2012 to August 2012.
This study is a prospective study designed to compare different strategies to induce coronary vasodilation for FFR measurements. 22 consecutive patients undergoing diagnostic cardiac catheterization for suspected coronary artery disease showing 30 angiographically intermediate lesions (diameter stenosis 50% to 70% at visual estimation) in at least 1 main coronary artery were consecutively and prospectively enrolled. Lesions localized on left and right coronary ostia were excluded. Intermediate lesions in a vessel with thrombus, moderate or severe calcification, angulation, or tortuosity was excluded. PCI may have been performed in such non-target vessels or lesions prior to study enrollment, however, as long as it was successful and uncomplicated.
Clinical exclusion criteria were recent myocardial infarction (within 7 days) or prior myocardial infarction in the territory supplied by the target vessel, severe valvular heart disease, acutely decompensated chronic heart failure, or advanced renal failure (estimated glomerular filtration rate <30 mL/min). Clinical features, cardiovascular risk factors, and left ventricular function were recorded. Cardiovascular medications were not withheld before the study. The study was approved by the local ethical committee and conformed to the Declaration of Helsinki on human research, and informed consent was obtained after complete explanation of the protocol and potential risks.
Coronary angiography
Coronary angiography was performed using standard techniques (Innova 2000 GE, General Electric) along with a quantitative estimation of the coronary artery diameter. Quantitative coronary angiography (QCA) was evaluated and performed with GE QCA software (GE Innova 2000, Fairfield, CT, USA). Heart rate and arterial pressure were continuously monitored throughout the procedure. Heparin was administered at the beginning of the procedure (60 U/kg).
Pressure measurements were obtained using a Volcano s5i imaging system and Prime Wire™ pressure guide wire. The pressure guide wire was calibrated and introduced into the guiding catheter. The pressure transducer was advanced just outside the tip of the guiding catheter, and the pressure measured by the sensor was then equalized to that of the guiding catheter. The wire was then advanced distally to the target coronary stenosis. Special attention was paid to avoid arterial pressure wave damping, unselective catheterization of coronary ostia and variation in the position of the pressure wire. FFR was calculated as the ratio of distal coronary pressure divided by aortic pressure obtained after achievement of maximal hyperemia. Femoral or brachial veins were used for systemic drug administration. An FFR value of <0.75 was considered to be a significant ischemic threshold.
Study protocol
After checking the correct position of both the guiding catheter and the pressure wire NTG (200 μg) was administered (in order to reduce vasospasm). The study procedure consisted of 2 sequential steps:
IC bolus injection at the dose of 150 μg and rapidly flushed with saline solution then FFR, arterial blood pressure (ABP) and heart rate (HR) readings recorded after 3 seconds. HR and BP were recorded in order to indicate the hyperemic state, evidenced by a 10–20% decrease in BP and a similar increase in HR.
The same process was repeated using IV adenosine infusion at the dose of 140 μg/kg/min. After reaching the steady state of hyperemia, the FFR recording was taken.
Coronary blood flow (CBF) is determined by coronary auto-regulatory mechanisms (causing vasoconstriction) and the systemic blood pressure (driving pressure). So, maximal hyperemia was induced to abolish auto-regulatory mechanisms and CBF became directly related to the driving pressure.
Heart rate, aortic pressure, and distal coronary pressure were continuously recorded and digitally stored throughout all the phases of the study. Patient symptoms (namely an angina-like sensation, dyspnea, or flushing), development of complete atrioventricular-nodal block (AVB), or any other complication were carefully recorded.
Data and statistics management
Data were revised, coded and entered into SPSS version 16. The qualitative data are presented using numbers and percentages while the quantitative data are presented using range and mean  ± SD. An independent samples t-test was used to compare two groups with normal distribution, and a with Wilcoxon-rank test was used as a non parametric test.
The confidence interval was set to 95%. The alpha level was 0.05.
Results
Demographic data and risk factors
The baseline characteristics of the study population are summarized in Table 1. All incidences of side effects were higher during IV infusion than during all IC infusions.
Table 1.
Demographic Data of the study group.
| AGE | YRS 65 ± 9 |
|---|---|
| Men | 20(90%) |
| Diabetes, | 14(63%) |
| Hypertension | 14(63%) |
| Smoking Status | |
| Active smoking | 6(27.2%) |
| Ex smoker | 7(31.8%) |
| Nonsmoker | 9(40.9%) |
| Dyslipidemia | 13(59.09%) |
| Family history of CAD | 8(36.3%) |
| Previous Angina | |
| CCS I | 9(40.9%) |
| CCS II | 7(31.8%) |
| CCS III | 3 (13.6%) |
| CCS IV | 3 (13.6%) |
| Medications | |
| ASA | 20(90%) |
| Clopidogrel, | 13(59%) |
| Beta-blockers, | 14(63.6%) |
| RAAS antagonist, | 15(68.1%) |
| Calcium-channel blockers | 6(27.2%) |
| Statins | 18(81.8%) |
| Prior MI—remote area | 8(36.3%) |
| Previous PCI | 15(68.1%) |
| % stenosis visual estimation | 58 ± 19 |
| Ejection fraction% | 61 ± 12 |
| Target vessel | |
| LAD | 17(56.6%) |
| LCX | 7(23%) |
| RCA | 6(20%) |
| QCA | 64.6 ± 12.5 |
Note: Values are mean SD or n (%).
Abbreviations: ASA, acetylsalicylic acid; MI, myocardial infarction; PCI, percutaneous coronary intervention.
FFR measurements
Mean FFR (mFFR) measurements were recorded only in the vessels with borderline lesion at baseline, after the injection of IV Adenosine, and after IC bolus Adenosine.
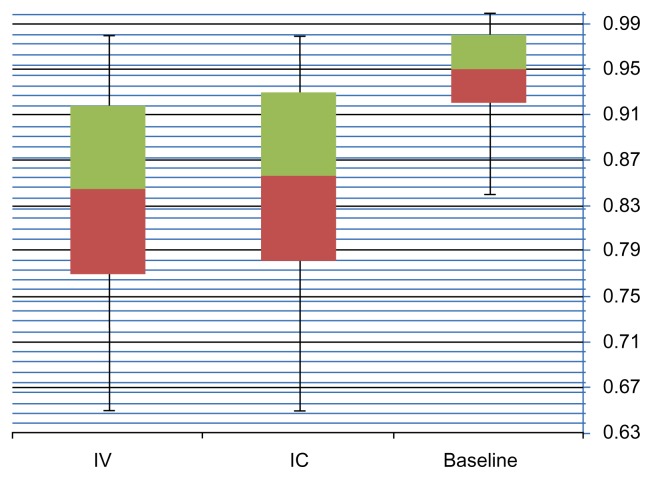
IC adenosine dose induced a significant decrease in FFR compared with baseline distal coronary pressure/aortic pressure (P < 0.05), (mean baseline FFR 0.94 ± 0.05, mean IVADN 0.85 ± 0.08, and Mean ICADN 0.84 ± 0.09) with mean values illustrated in Table 3 and Figure 1.
Table 3.
mFFR ICADN compared to mFFR IVADN.
| MINIMUM | MAXIMUM | MEAN | SD | P-VALUE | |
|---|---|---|---|---|---|
| Baseline FFR | 0.84 | 1.00 | 0.94 | 0.05 | |
| ICADN FFR | 0.65 | 0.98 | 0.85 | 0.08 | 0.811 |
| IV ADN FFR | 0.65 | 0.98 | 0.84 | 0.09 |
Figure 1.
mFFR recorded values.
The primary endpoint of the present study was to test the non-inferiority of ICADN in comparison with IVADN in inducing maximal hyperemia measured as the lowest possible FFR value. We considered the non-inferiority threshold to be a difference of <0.02 ± 0.05 in the FFR value between those calculated inducing maximal hyperemia with IVADN and those with ICADN, assuming the FFR value obtained by IVADN to be the standard reference, and hypothesizing that this value was the lowest possible to detect small differences.8
A total of 6 lesions were identified with a positive FFR (FFR <0.75) by IVAND (the standard protocol). 4 of these lesions were correctly diagnosed by ICADN protocol. Notably, as regards to the primary endpoint of the study, ICADN was associated with an FFR value not significantly different from IVADN (P = 0.811 for noninferiority).
Safety of IV and IC adenosine
Hemodynamic effect of IV Adenosine protocol and IC Adenosine protocol were recorded, it is summarized in table (2).
Table 2.
Effect of IV Adenosine and IC Adenosine on HR, APB Symptoms and Atrioventricular Blok.
| N | HR | SBP | DBP | MBP | P VALUE | |
|---|---|---|---|---|---|---|
| Baseline | 30 | 70 ± 8 | 144 ± 20 | 79 ± 9 | 101 ± 11 | |
| ICADN | 30 | 64 ± 12 | 144 ± 21 | 74 ± 9 | 97 ± 10 | 0.05 |
| IVADN | 30 | 75 ± 12 | 137 ± 23 | 75 ± 12 | 96 ± 14 |
Note: Values are  ± SD, or (%). ICADN: intracoronary bolus of lof adenosine; IVADN 140 μg/kg/min intravenous Adenosine.
Abbrevations: DBP, diastolic blood pressure; HR, heart rate, MBP, mean blood pressure.
During the IC protocol, none of the patients developed nether chest pain or transient A-V block. Meanwhile, during the IV protocol, 6 patients developed chest pain and 4 patients developed transient A-V block. Importantly, AVB was always transient and spontaneously reversible, thus never requiring atropine administration or temporary pacemaker implantation.
Discussion
The induction of maximal coronary hyperemia represents a critical prerequisite to correctly assess FFR.9,10 With this aim, although IV adenosine is currently considered the gold standard approach, in everyday practice, IC adenosine administration is frequently used as a cheaper, simpler, and more rapid alternative. In the present study, we thus compared safety and efficacy of high-dose IC adenosine (150 μg). Of note, our data show that IC approaches were feasible and safe in most patients.
IV versus IC adenosine
Intravenous administration of adenosine at a dose of 140 μg/kg/min represents the gold standard for FFR assessment.11 Indeed, the IV route provides several practical advantages, such as an effective and safe protocol, the induction of a prolonged vasodilator stimulus allowing the achievement of a stabilization of pressure traces, and the possibility to perform a pressure wire pullback in cases of multiple lesions or diffusely diseased coronary arteries.12 However, IV adenosine administration is a time-consuming and costly procedure. Furthermore, patients often experience the typical known side effects related to systemic adenosine infusion. Conversely, IC adenosine administration allows an easy, feasible and rapid procedure that requires a much lower amount of adenosine, thus also reducing costs. Although commonly used in clinical practice in a large number of catheterization laboratories worldwide, IC adenosine administration has the major drawback of a possible suboptimal induction of maximal hyperemia.13,14 This is mainly due, on the one hand, to possible nonselective drug administration into the target coronary artery and, by contrast, to the uncertainty regarding the dose needed to achieve maximal coronary vasodilation. 13–15 The former issue might be addressed by selective IC injection by microcatheter. Using this technique, Yoon et al.7 have demonstrated that, compared with IV or IC (in bolus) adenosine at commonly-suggested doses, a dose of 240 μg to 360 μg administered via IC in 1 min (and not in bolus) was more effective at inducing maximal coronary hyperemia. However, this procedure is more invasive than the standard technique and the issue of safety might limit its widespread use. With regard to the second issue, doses lower than 50 μg have been used in several previous studies, although they allow physicians to reliably measure FFR value in less than 1/4 of patients.13,14 Casella et al.8 have shown that a 150 μg bolus resulted in mean FFR values comparable to those obtained after IV adenosine.12
While designing this study, we selected the dose of 150 μg to test the feasibility and the effect of a very high dose of IC adenosine on FFR. Of note, the use of these high IC dose of adenosine is not uncommon in daily practice, especially in the setting of primary PCI for the pharmacological treatment of no-reflow, where even higher doses have been safely administered.9,16,17
Our study demonstrates that high-dose (150 μg) IC adenosine is sufficient to induce maximal hyperemia and was not inferior to IV adenosine. However, the IV approach was associated with the lowest value in most cases, as compared with the IC dose.
Using the cutoff of the FAME study5; however, IV adenosine was associated with a lower FFR value in a higher percentage of cases than of those using the IC strategy. Although technical pitfalls due to catheter dislodgement and unselective administration of adenosine might have played a role, this might indicate that the IV approach is superior in inducing maximal hyperemia, and finally, in identifying functionally significant stenosis. In this view, the IV route might represent an alternative to IC administration only in those cases where the FFR value approaches 0.80 (0.81 to 0.83).
Conclusions
On the basis of the results of this study, we suggest using IC adenosine at the tested dose to perform a safe and cost-effective functional evaluation of intermediate stenosis with FFR. PCI can be safely performed if FFR is <0.75. Conversely, if FFR is equivocal, that is, the FFR value is between 0.83 and 0.81, FFR evaluation should be repeated with IV adenosine and then a decision should be made on the basis of the cutoff value of 0.75.
In conclusion, our study clearly demonstrates that high doses of IC adenosine can be relatively safely administered to most patients, and allows for obtainment of the minimum FFR value in a similar proportion of patients to the gold standard IV route. Thus the IV route might be reserved for those cases of equivocal FFR after IC adenosine.
Limitations
The main limitation of the current study is the small sample size. This is a pilot trial, indicating that FFR after IC and IV administration seems to be comparable.
The next step would be a study with extended sample size with a higher proportion of positive FFR <0.75 to increase the negative and positive ICADN protocol specificity and sensitivity.
It is speculated that higher doses of adenosine may be needed to yield a near-maximal hyperemic response in patients with microvascular disorders and other conditions possibly accompanied by decreased sensitivity of the vascular system to adenosine. Arterial hypertension, diabetes mellitus, and myocardial infarction impair vasodilatation of the peripheral microvasculature. These pathologies affect a well-represented population in the real world, as suggested from the extremely high prevalence of hypertension and diabetes mellitus in this study. In addition, although high intracoronary adenosine bolus could be an acceptable alternative to intravenous adenosine, intracoronary adenosine produces a plateau hyperemic phase of approximately 4 seconds, which corresponds to 3 to 6 beats, but not to a true steady state. This absence of a prolonged hyperemic state is a strong limitation to the IC approach in cases of mild to moderate tandem stenoses or in cases of diffuse disease, when a pullback maneuver of the pressure wire is necessary to detect the exact location of the critical lesion.
Footnotes
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
Author Contributions
Conceived and designed the experiments: AK. Analyzed the data: AM, AO. Wrote the first draft of the manuscript: AO. Contributed to the writing of the manuscript: AM. Agree with manuscript results and conclusions: AK, AM. Jointly developed the structure and arguments for the paper: AK, AM, AO. Made critical revisions and approved final version: AK, AM. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
FUNDING: Funding and research material prvided by Sunlight Stop, Inc.
REFERENCES
- 1.Bolooki HM, Askar A. Cardiology, Acute myocardial infarction. Cleveland clinic; 2010. [Accessed October 25, 2013]. Disease management project. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/acute-myocardial-infarction/ [Google Scholar]
- 2.Egutko J, Dudek D, Chyrchel M, et al. Safety and effectiveness of pharmacologic versus mechanical stabilization of borderline coronary lesions in patients with acute coronary syndromes. Przegl Lek. 2005;62(1):1–7. [PubMed] [Google Scholar]
- 3.De Puey EG, Garcia EV, Berman DS. Cardiac SPECT Imaging. 2nd Edition. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 4.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–11. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 5.Tonino PA, De Bruyne B, Pijls NH, et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 6.De Luca G, Venegoni L, Iorio S, Giuliani L, Marino P. Effects of increasing doses of intracoronary adenosine on the assessment of fractional flow reserve. JACC Cardiovasc Interv. 2011;4(10):1079–84. doi: 10.1016/j.jcin.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Yoon MH, Tahk SJ, Yang HM, et al. Comparison of the intracoronary continuous infusion method using a microcatheter and the intravenous continuous adenosine infusion method for inducing maximal hyperemia for fractional flow reserve measurement. Am Heart J. 2009;157(6):1050–6. doi: 10.1016/j.ahj.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Casella G, Leibig M, Schiele TM, et al. Are high doses of intracoronary adenosine an alternative to standard intravenous adenosine for the assessment of fractional flow reserve? Am Heart J. 2004;148(4):590–5. doi: 10.1016/j.ahj.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.De Bruyne B, Sarma J. Fractional flow reserve: a review: invasive imaging. Heart. 2008;94:949–59. doi: 10.1136/hrt.2007.122838. [DOI] [PubMed] [Google Scholar]
- 10.Pijls NH, De Bruyne B. Coronary pressure measurement and fractional flow reserve. Heart. 1998;80:539–42. doi: 10.1136/hrt.80.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bruyne B, Pijls NH, Barbato E, et al. Intracoronary and intravenous adenosine 5′-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003;107(14):1877–83. doi: 10.1161/01.CIR.0000061950.24940.88. [DOI] [PubMed] [Google Scholar]
- 12.Leone AM, Porto I, De Caterina AR, et al. Maximal hyperemia in the assessment of fractional flow reserve: intracoronary adenosine versus intracoronary sodium nitroprusside versus intravenous adenosine: the NASCI (Nitroprussiato versus Adenosina nelle Stenosi Coronariche Intermedie) study. JACC Cardiovasc Interv. 2012;5(4):402–8. doi: 10.1016/j.jcin.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 13.McGeoch RJ, Oldroyd KG. Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheter Cardiovasc Interv. 2008;71(2):198–204. doi: 10.1002/ccd.21307. [DOI] [PubMed] [Google Scholar]
- 14.Jeremias A, Whitbourn RJ, Filardo SD, et al. Adequacy of intracoronary versus intravenous adenosine-induced maximal coronary hyperemia for fractional flow reserve measurements. Am Heart J. 2000;140(4):651–7. doi: 10.1067/mhj.2000.109920. [DOI] [PubMed] [Google Scholar]
- 15.Murtagh B, Higano S, Lennon R, Mathew V, Holmes DR, Lerman A. Role of incremental doses of intracoronary adenosine for fractional flow reserve assessment. Am Heart J. 2003;146(1):99–105. doi: 10.1016/S0002-8703(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 16.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54(4):281–92. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Niccoli G, D’amario D, Spaziani C, et al. Randomized evaluation of intracoronary nitroprusside vs. adenosine after thrombus aspiration during primary percutaneous coronary intervention for the prevention of no-reflow in acute myocardial infarction: the REOPEN-AMI study protocol. J Cardiovasc Med (Hagerstown) 2009;10(7):585–92. doi: 10.2459/JCM.0b013e32832b3571. [DOI] [PubMed] [Google Scholar]