Abstract
In vitro cytotoxicity of tafluprost, which is the most recently developed anti-glaucoma prostaglandin (PG) analog, in ocular surface cells is addressed in comparison with other PG analogs. Irrespective of cell lines and models, the cytotoxicity of anti-glaucoma PG eyedrops was primarily related to the concentration of benzalkonium chloride (BAK) contained in the eyedrops as a preservative. Accordingly, preservative-free tafluprost was apparently less cytotoxic than BAK-preserved PG analogs. Furthermore, our study for cytotoxicity assays on ocular cells, conducted by comprehensive investigations covering a variety of concentrations and treatment times, which is termed the cell viability score (CVS) system, demonstrated that 0.001% BAK-preserved tafluprost was not cytotoxic, and suggested that tafluprost may even reduce the cytotoxic effect of BAK.
It has been reported that adverse reactions associated with tafluprost in healthy human volunteers and patients with glaucoma include conjunctival hyperemia, eyelid pigmentation, eyelash bristles, and deepening of upper eyelid sulcus. Nonetheless, most clinical studies have demonstrated that not only preservative-free tafluprost but also BAK-preserved tafluprost is well tolerated and safe in patients with glaucoma and ocular hypertension.
Keywords: tafluprost, prostaglandin analog, cytotoxicity, ocular surface cells, safety evaluation
Introduction
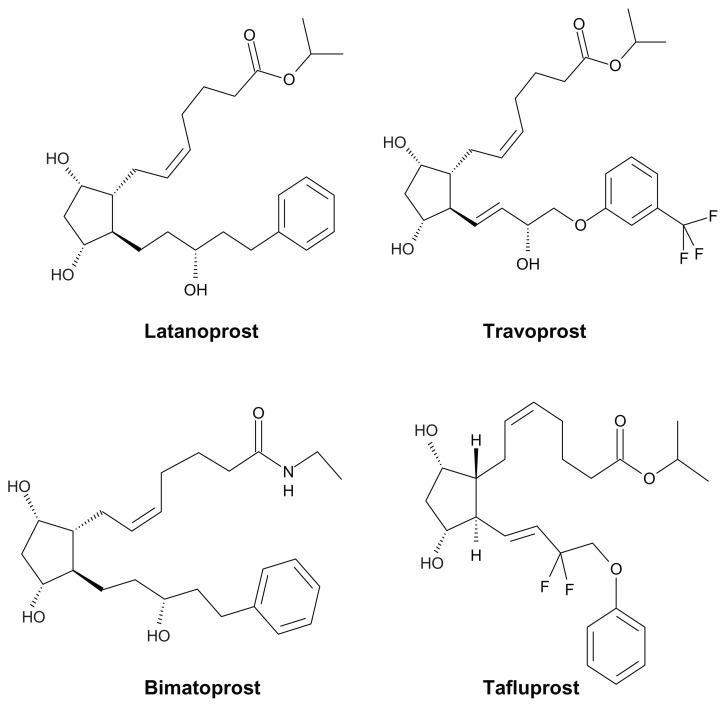
It has been considered that elevated intraocular pressure (IOP) is a key risk factor for the progression of glaucoma.1,2 Prostaglandin (PG) derivatives exert ocular-hypotensive or IOP-lowering effects through stimulation of prostanoid receptors, a process which possibly activates signal-transduction systems such as intracellular Ca2+ and cyclic AMP.3–5 Latanoprost, a selective prostanoid FP-receptor agonist, is one of the most potent PGF2α derivatives for reducing IOP, and thus was successfully developed as an anti-glaucoma agent.6 Following latanoprost, travoprost and then bimatoprost were launched in the United States and other countries.7,8 More recently, tafluprost was developed by screening for prostanoid FP-receptor agonists that might be more potent at inducing IOP reduction while causing fewer or weaker side effects, such as eye color change, which when it occurs is most likely due to an increased amount of melanin within iris stroma melanocytes.9 Chemical structures of latanoprost, travoprost, bimatoprost, and tafluprost are shown in Figure 1.
Figure 1.
Chemical structures of anti-glaucoma prostaglandin analogs.
Since PG analogs have shown greater IOP-lowering efficacy than β-adrenergic blockers,10 they are commonly used as first-line therapy against glaucoma.11 Among them, tafluprost 0.0015% (Taflotan®; Santen Oy, Tampere, Finland) is the most recently released PG analog, being approved in Europe in 2008, then in the US in 2012 for the treatment of elevated IOP in patients with open-angle glaucoma, in addition to ocular hypertension. Tafluprost is also the first preservative-free PG analog commercially available in the US.
The aim of this paper is to give an overview of the preclinical toxicological profiles of tafluprost for ophthalmic use, including the application of the cell viability score (CVS) system developed by our research group to the cytotoxic evaluation of tafluprost on ocular surface cells. Safety evaluation of the clinically used tafluprost in healthy human volunteers and patients with glaucoma is also reviewed in the paper.
New Fluoroprostaglandin F2α Derivative, Tafluprost
The accumulated findings relating to latanoprost and other PGF2α derivatives have indicated that prostanoid FP-receptor agonists are among the most promising ocular-hypotensive agents. Nakajima et al. tried to find new prostanoid FP-receptor agonists possessing potent ocular-hypotensive effects with minimal side effects by evaluating the agonistic activities of newly synthesized PGF2α derivatives for the prostanoid FP-receptor both in vitro and in vivo.9 They examined the iris constrictions induced by the derivatives and their effects on melanin content by using cat isolated iris sphincters and cultured B16 melanoma cells, respectively, and also evaluated the effects of derivative ester forms on miosis and IOP in cats and cyno-molgus monkeys, respectively. Based on these examinations, they found that 15,15-difluoroprostaglandin F2α derivatives, especially tafluprost, have more potent prostanoid FP-receptor agonistic activities than latanoprost. Then Takagi et al. further evaluated the pharmacological characteristics of tafluprost by examining its receptor-binding affinities, IOP-lowering effect, effects on aqueous humor dynamics, and stimulating effect on melanogenesis.12 They found that tafluprost has a high affinity for the prostanoid FP receptor, has potent IOP-lowering effects in both ocular normotensive and hypertensive monkeys that exceed those of latanoprost, and has less stimulating effect on melanogenesis in melanoma cells. Ota et al. evaluated the effect of tafluprost on mouse IOP, in comparison with three clinically available PG analogs, latanoprost, travoprost, and unoprostone, considering the effect of variations in IOP during 24 h.13 They demonstrated that tafluprost 0.005% lowered normal mouse IOP more effectively than did latanoprost 0.005%.
In vitro Cytotoxicity of Tafluprost in Ocular Surface Cells in Comparison with that of Other PG Analogs
Table 1 summarizes in vitro cytotoxicity studies for PG analog ophthalmic solutions in ocular surface cells.14–20 Irrespective of cell lines and models, the cytotoxicity of anti-glaucoma PG eyedrops was primarily related to the concentration of benzalkonium chloride (BAK) contained in the eyedrops as a preservative. For instance, Liang et al. demonstrated that cytotoxicity evaluated in a three-dimensional-reconstituted corneal epithelium system was in the order of 0.02% BAK-latanoprost >0.015% BAK-travoprost >0.005% BAK-bimatoprost, in which 0.02% BAK-latanoprost showed the highest cytotoxicity. 17 BAK-bimatoprost and preservative-free (PF) tafluprost did not induce any obvious cytotoxicity. Similarly, Pellinen et al reported that the order of decreasing cytotoxicity in human corneal and conjunctival epithelium was 0.02% BAK-latanoprost ≥0.015% BAK-travoprost >0.005% BAK-bimatoprost ≥PF tafluprost.18 In these studies, tafluprost showed the lowest cytotoxicity among the PG analog eyedrops tested, presumably due to the lack of BAK. Indeed, when BAK-preserved tafluprost was assayed, the cytotoxicity of tafluprost in ocular surface cells was comparable to that of BAK-preserved PG analogs including latanoprost and travoprost.15,16 Furthermore, when preservatives other than BAK were used, the degree of cytotoxicity was apparently reduced. For instance, sofZia- or polyquaternium-1-preserved travoprost showed cytotoxicity weaker than that of BAK-preserved travaprost,15,16 and sodium benzoate-preserved latanoprost showed cytotoxicity weaker than BAK-preserved latonoprost.19 The optimal concentration of preservatives is still to be determined from the point of view of ocular surface safety and preservative efficacy, so that we cannot say at the moment whether sofZia, polyquaternium-1 and sodium benzoate are better than BAK as a preservative. Nonetheless, the cytotoxicity of anti-glaucoma PG eyedrops currently available apparently depends on the BAK concentrations.
Table 1.
Summary of in vitro cytotoxicity of prostaglandin analog ophthalmic solutions in ocular surface cells.
| CELL LINE OR MODEL | PROSTAGLANIDN ANALOGUE OPHTHALMIC SOLLUTION | PRESERVATIVE | ASSSAY | CYTOTOXICITY | AUTHOR (YEAR) |
|---|---|---|---|---|---|
| Human conjunctival EC | Latanoprost 0.005% | 0.02% BAK | Microplate cytofluorometry | latonoprost (BAK) >travoprost (BAK) |
Brasnu et al. (2008) |
| Travoprost 0.004% | 0.015% BAK | Neutral red fluorescence stain test | >bimatoprost (BAK) >tafluprost (PF) |
||
| Bimatoprost 0.03% | 0.005% BAK | Apoptosis | |||
| Tafluprost 0.0015% | free | DNA content | |||
| Transformed human | Tafluprost 0.0015% | 0.01% BAK | LIVE/DEAD viability/cytotoxicity |
latonoprost (BAK) ≒tafluprost (BAK) |
Kahook et al. (2010) |
| corneal EC | Travoprost 0.004% | 0.015% BAK | >travoprost (BAK) >travoprost (sofZia) |
||
| Travoprost 0.004% | sofZia | ||||
| Latanoprost 0.005% | 0.02% BAK | ||||
| Transformed human | Tafluprost 0.0015% | 0.01% BAK | LIVE/DEAD viability/cytotoxicity |
latonoprost (BAK) >tafluprost (BAK) |
Ammer et al. (2010) |
| corneal EC | Travoprost 0.004% | 0.015% BAK | >travoprost (BAK) >travoprost (sofZia) |
||
| Travoprost 0.004% | 0.001% PQ | >travoprost (PQ) | |||
| Human conjunctival EC | Travoprost 0.004% | sofZia | |||
| Latanoprost 0.005% | 0.02% BAK | ||||
| 3D-human corneal | Latanoprost 0.005% | 0.02% BAK | MTT | latonoprost (BAK) >travoprost (BAK) |
Liang (2011) |
| EC model | Travoprost 0.004% | 0.015% BAK | (3-(4,5-di-methylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide) | >bimatoprost (BAK) >tafluprost (PF) |
|
| Bimatoprost 0.03% | 0.005% BAK | ||||
| Tafluprost 0.0015% | free | ||||
| Human corneal EC | Tafluprost 0.0015% | free | WAST-1 | latanoprost(BAK) ≥travoprost (BAK) |
Pellenen (2012) |
| Latanoprost 0.005% | 0.02% BAK | (4-[3-(4-iodophenyl)- 2-(4-nitrophenyl)-2H-5- tetrazolio]-1,3-benzene disulphonate) | >bimatoprost (BAK) ≥tafluprost (PF) |
||
| Human conjunctival EC | Travoprost 0.004% | 0.015% BAK | |||
| Bimatoprost 0.03% | 0.005% BAK | ||||
| Stratified human corneal | Latanoprost 0.005% | free | Carboxyfluorescein permeability | latonoprost (BAK) >latonoprost (SB) |
Nakagawa et al. (2012) |
| epithelial sheet | Latanoprost 0.005% | SB | (barrier function) | ≒latonoprost (PF) >travoprost (sofZia) |
|
| Travoprost 0.004% | sofZia | ≒tafluprost (BAK) | |||
| Tafluprost 0.0015% | 0.01% BAK | ||||
| Latanoprost 0.005% | 0.02% BAK | ||||
| Human corneal EC | Travoprost 0.004% | 0.001% PQ | LIVE/DEAD viability/cytotoxicity |
latonoprost (BAK) ≥bimatoprost (BAK) |
Whitson and Petroll (2012) |
| Latanoprost 0.005% | 0.02% BAK | >tafluprost (PF) ≒travoprost (PQ) |
|||
| Bimatoprost 0.01% | 0.02% BAK | ||||
| Tafluprost 0.0015% | Free |
Abbreviations: EC, epithelial cell; BAK, benzalkonium chloride; PQ, polyquaternium-1; SB, sodium benzoate; PF, preservative free.
Cell Viability Score (CVS) as a Good Indicator of Ophthalmic Solutions for Toxicity in Cultured Ocular Surface Cell Lines
Cytotoxicity of ophthalmic solutions is a contentious issue because once an ophthalmic solution is applied to the ocular surface, its concentration and drug penetration can change very rapidly. To reflect the actual situation, we have tried to improve cytotoxicity assays for ocular cells by conducting comprehensive investigations covering a variety of concentrations and treatment times, and based on our studies, we proposed the use of a cell viability score (CVS) as a simple parameter to express the cytotoxic potential of ophthalmic solutions.21–24
The methods for cell culture, the cytotoxicity assays, and data evaluation are as follows: The following commercially available cell lines were used: SIRC (rabbit corneal epithelium), BCE (bovine corneal epithelial cells), RC-1 (rabbit corneal epithelium) and Chang conjunctiva (human conjunctival cells). After cells reached confluence, the culture medium was replaced with undiluted, twofold diluted and tenfold diluted test solutions, and cell monolayers were incubated in the presence of these solutions for 10, 30, or 60 minutes. After 10, 30, or 60 minutes of incubation, the ophthalmic solutions were replaced with fresh culture medium and the cells incubated for a further 48 h. Cell viability was measured using the MTT (3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, yellow tetrazole) and neutral red assays, and then calculated as a percentage of control cell viability in medium only.
CVS is used to compare the toxicity of test solutions in the following way. The CVS50 is calculated as the number of measurements indicating ≥50% viability compared with control. The CVS40/80 is calculated as: (the number of measurements indicating >80% viability)–(the number of mea surements indicating <40% viability). The total number of measurements becomes 72 (three concentrations, three exposure times, four cell lines, and two assays). Results are expressed as a percentage of all measurements (%CVS). As such, we can evaluate the effects of a range of drug concentrations and exposure times in four commercially available cell lines because, in the clinical situation, the eyes are exposed to various drug-treatment situations. For example, the drug may be concentrated on the eye due to evaporation or decreased drainage, the drug may adsorb on the eye for a prolonged period of time, or the vulnerability to a particular drug may differ among cell types (eg, conjunctival versus corneal epithelial cells).
In our study,23 the %CVS40/80 values were obtained to estimate the cytotoxicity of PG analog-containing eyedrops as shown in Table 2. The %CVS40/80 of Tapros (Santen, Osaka, Japan) was 99, indicating that the product showed almost no cytotoxic effect, and suggesting that of the five PG analog eyedrops tested, Tapros would be least cytotoxic to ocular surface cells. In addition, the %CVS40/80 of Tapros was higher than that of 0.001% BAK alone, which was the concentration equal to that contained in Tapros. This means that tafluprost may reduce the cytotoxic effect of BAK. Indeed, it was reported that latanoprost and travoprost have protective effects against BAK toxicity on conjunctiva-derived epithelial cells in vitro, probably related to their antioxidative properties.25 Thus, as is the case with latanoprost and travoprost, it is highly possible that tafluprost has protective effects against BAK toxicity on ocular surface cells. Further supporting the idea of the cytoprotective effect of tafluprost, it was reported that tafluprost has a protective effect on cultured retinal ganglion cells (RGCs) and rat RGCs in retinas with optic nerve crush.26 In in vitro study, tafluprost promoted survival and inhibited apoptotic events in serum-deprived and glutamate-exposed RCG-5 cells in a dose-dependent fashion up to 3 μM, suggesting that tafluprost would have a direct anti-apoptotic effect. Regarding the in vivo study in which topically applied tafluprost reduced the number of apoptotic cells and increased the survival of RGCs in rat retinas with optic nerve crush, because lowering normal IOPs might have some effects to protect RGCs, they concluded that one cannot distinguish between a direct neuroprotective effect and the IOP-lowering effect of tafluprost in the in vivo experiments. Yamagishi et al. reported that PG analogs including tafluprost acid exerted an IOP-independent neuroprotective effect, which may be not related to FP receptor stimulation.27
Table 2.
The %CVS40/80 values for prostaglandin analog eyedrops and benzalkonium chloride.
| PRODUCT NAME | PG ANALOGUE | %CVS40/80 | BENZALKONIUN CHLORIDE | |
|---|---|---|---|---|
| CONCENTRATION (%)* | %CVS40/80 | |||
| Xalatan | latanoprost 0.005% | −42 | 0.02 | −46 |
| Travatan | travoprost 0.004% | −54 | 0.015 | −33 |
| TravatanZ** | travoprost 0.004% | 83 | 0 | 100 |
| Lumigan | bimatoprostgan 0.002% | 26 | 0.005 | 39 |
| Tapros | tafluprost 0.0015% | 99 | 0.001 | 85 |
Notes:
Concentrations contained in the corresponding eyedrop products.
Preserved with sofZia. Partially reproduced from Ayaki et al.23 with permission.
In vitro and in vivo toxicity studies on tafluprost other than the direct effect on ocular surface cells
Hos et al. evaluated the vascular effects of tafluprost on the healthy and inflamed cornea, because the potential side effects of PG analogs on the normally avascular cornea, the main application route for eye drops, have so far not been fully defined.28 They conducted in vitro studies, in which blood and lymphatic endothelial cells were treated with tafluprost, and short-term in vivo studies, in which mice with corneal inflammation induced by suture placement received tafluprost eye drops for 1 week. They also assessed proliferation of blood and lymphatic endothelial cells treated with tafluprost in long term in vivo studies in which naive corneas of BALB/c mice were treated with tafluprost eye drops for 4 weeks. They concluded that tafluprost does not affect blood and lymphatic vessel growth, either under resting or under inflammatory conditions, suggesting a safe vascular profile of tafluprost eye drops at the inflammatory neovascularized cornea.
Liang et al. investigated conjunctiva-associated lymphoid tissue (CALT) reactions to anti-glaucoma PGs with or without BAK-preservative in a rabbit acute toxicity study.29 Their study was based on the evidence that BAK, the most widely used preservative in eye drops, could influence local immune regulation. The studies supporting this showed that: exposure of mouse ears to BAK induced significant B cell activation in the draining lymph nodes, with an increase in the percentage of B220+ cells;30 and BAK in experimental irritant contact dermatitis induced a state of metabolic activation in a high proportion of epidermal CD1+ Langerhans cells, suggesting that BAK could influence antigen-presenting cells.31 The results of the study of Liang et al demonstrated that anti-glaucoma PG analog eye drops stimulated inflammatory cell infiltration in the CALT, which seemed to be primarily related to the concentration of their BAK content. They also addressed that these immunoinflammatory changes in CALT may actively participate in the strong inflammatory and apoptotic reactions observed after applications of these BAK containing eye drops.32 Accordingly, BKA-free tafluprost showed no significant effect on CALT reactions.
Safety Evaluation of Tafluprost in Healthy Volunteers
Sutton et al conducted a phase I placebo-controlled study, in which healthy volunteers received sequentially ascending doses of tafluprost (0.0001%, 0.0005%, 0.0025% and 0.005%) in one eye, and placebo in the other for two days of each treatment with five days between the treatment periods.33 They concluded that tafluprost was well tolerated and effective in lowering IOP. Uusitalo et al similarly conducted a randomized, investigator-masked, single-center, crossover phase I study to evaluate the pharmacokinetics, efficacy and safety profiles of preserved and preservative-free tafluprost 0.0015% eyedrops in healthy volunteers who received each formulation once/day for eight days.34 They also concluded that preservative- free tafluprost appeared to have similar pharmacokinetic properties to the preserved formulation and was generally well tolerated.
Mochizuki et al compared the intraocular pressure (IOP) reduction over 24 h achieved with tafluprost 0.0015% with that achieved with latanoprost 0.005%.35 In their study with 27 healthy volunteers, after a 24-h IOP baseline measurement was taken, one ophthalmic solution was applied to the right eye daily for seven days, and the drug was then withdrawn for two weeks. The other agent was then applied to the left eye in the same manner. Although tafluprost showed a greater IOP reduction in the second half of the 24-h measurement period than latanoprost, tafluprost showed a higher rate of conjunctival hyperemia. That is, the incidence of conjunctival hyperemia with latanoprost was 4/27 (14.8%) and that with tafluprost was 8/27 (29.6%). In their study, since Tapros (with 0.01% BAK) and Xalatan (with 0.02% BAK) were used, incidence of conjunctival hyperemia would not be attributable to BAK. They addressed that latanoprost has been reported to induce conjunctival hyperemia less frequently than other PG agents.36–38
Safety Evaluation of Tafluprost in Patients with Glaucoma and Ocular Hypertension
Adverse reactions occurring around the eyes associated with PG analog treatment are conjunctival hyperemia, eyelash changes, eyelid pigmentation, iris pigmentation, hypertrichosis around the eyes, corneal epithelium disorder, iritis, cystoid macula edema, and deepening of the upper eyelid sulcus (DUES).39–50
Inoue et al investigated the frequency of eyelid pigmentation and eyelash bristles after the use of five types of PG analogs including tafluprost.51 Their study included 250 eyes from 250 patients diagnosed with primary open-angle glaucoma or ocular hypertension who were treated with either latanoprost, travoprost, tafluprost, bimatoprost, or isopropyl unoprostone for more than three months in only one eye. As a result, they demonstrated that the appearance frequency of eyelid pigmentation was similar among the five types of PG analogs studied, and eyelash bristles appeared less frequently with isopropyl unoprostone use.
Inoue’s group also examined the frequency of appearance of DUES in Japanese subjects diagnosed with primary open-angle glaucoma or ocular hypertension.52 They noted that DUES occurred more frequently in the bimatoprost group than in the latanoprost, the tafluprost, and the unoprostone groups. In addition, Maruyama et al investigated the incidence of DUES with topical use of tafluprost in Japanese glaucoma patients.53 In their prospective and open-label study, 36 primary open-angle glaucoma Japanese patients who had no history of surgery were prescribed 0.0015% topical tafluprost once daily to one eye that had the more severe visual field disorder, and observed during outpatient visits before and at 30, 60, and 90 days after starting treatment. They concluded that, similar to other PG analogs, topical use of tafluprost ophthalmic solution is associated with DUES as a local adverse reaction. The development of DUES is suspected to be related to the lipolytic action of PG analogs as demonstrated by a magnetic resonance imaging study and a histological study.54,55 Basic in vitro study also showed that latanoprost, travoprost, tafluprost, and bimatoprost, all of which have high affinity to FP receptors, inhibit differentiation of pre-adipocytes through stimulating FP receptors.56,57 However, tafluprost, which has higher affinity to FP receptors than other PG analogs, showed lower incidence of DUES than did bimatoprost in their study. Furthermore, the most recent in vitro study that examined the effects of latanoprost, travoprost, bimatoprost, and tafluprost on pre-adipocyte differentiation reported that bimatoprost has the greatest anti-adipogenic effect, followed by travoprost and tafluprost with similar effects.58 With all this taken into consideration, Maruyama et al suggested that the incidence of DUES is related to multiple mechanisms.55
Although some adverse reactions as described above were reported, as is the case with other PG analog eye drops, most of the clinical studies demonstrated that not only preservative- free tafluprost but also BAK-preserved tafluprost is well tolerated, and safe in patients with glaucoma and ocular hypertension.59–63
Conclusion
Discomfort due to medications for glaucoma, which is a chronic disease requiring lifelong treatment, may affect patients’ quality of life and may cause poor compliance, leading to poor intraocular pressure control. Preparations with lower BAK concentrations, preservative-free preparations and alternative preservatives have been developed to reduce the side effects of long-term treatment. Tafluprost, launched on the ophthalmic market in 2008, is a new PG analog, 15,15-difluoroprostaglandin F2α, for the treatment of glaucoma and ocular hypertension, and recently not only tafluprost preserved with a low concentration of BAK but also BAK-free tafluprost has become clinically available.
Our studies using CVS, and other recent studies, appear to show that the in vitro cytotoxicity of anti-glaucoma PG eyedrops in ocular surface cells is primarily related to the concentration of BAK contained in the eyedrops. Accordingly, preservative-free tafluprost and low concentration of BAK (0.001%)-preserved tafluprost are less toxic than other BAK-preserved PG analogs clinically available.
Besides the in vitro cytotoxic studies, the safety and IOP-lowering efficacy of tafluprost has been demonstrated in various preclinical and clinical studies.
Footnotes
COMPETING INTERESTS: Author(s) disclose no potential conflicts of interest.
Author Contributions
Wrote the first draft of the manuscript: YN. Conceived and designed the experiments for cell viability score: MA, AI, YN. Analyzed the data of cell viability score studies: MA, AI, YN. Agree with manuscript results and conclusions: YN, MA, AI. Jointly developed the structure and arguments for the paper: YN, MA, AI. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
FUNDING: Author(s) disclose no funding sources.
REFERENCES
- 1.Vogel R, Crick RP, Newson RB, et al. Association between intraocular pressure and loss of visual field in chronic simple glaucoma. Br J Ophthalmol. 1990;74(1):3–6. doi: 10.1136/bjo.74.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teus MA, Castejon MA, Calvo MA, et al. Intraocular pressure as a risk factor for visual field loss in pseudoexfoliative and in primary open-angle glaucoma. Ophthalmology. 1998;105(12):2225–9. doi: 10.1016/S0161-6420(98)91220-9. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Kim DM, Lindsey JD. Propagation of ciliary smooth muscle cells in vitro and effects of prostaglandin F2 alpha on calcium efflux. Invest Ophthalmol Vis Sci. 1992;33(9):2679–86. [PubMed] [Google Scholar]
- 4.Mukhopadhyay P, Geoghegan TE, Patil RV, et al. Detection of EP2, EP4, and FP receptors in human ciliary epithelial and ciliary muscle cells. Biochem Pharmacol. 1997;53(9):1249–55. doi: 10.1016/s0006-2952(97)00011-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Andom T, Bhattacherjee P, et al. Intracellular calcium mobilization following prostaglandin receptor activation in human ciliary muscle cells. Curr Eye Res. 1997;16(8):847–53. doi: 10.1076/ceyr.16.8.847.8986. [DOI] [PubMed] [Google Scholar]
- 6.Stjernschantz JW. From PGF2α-isopropyl ester to latanoprost: a review of the development of xalatan: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2001;42(6):1134–45. [PubMed] [Google Scholar]
- 7.Whitson JT. Travoprost—a new prostaglandin analogue for the treatment of glaucoma. Expert Opin Pharmacother. 2002;3(7):965–77. doi: 10.1517/14656566.3.7.965. [DOI] [PubMed] [Google Scholar]
- 8.Easthope SE, Perry CM. Topical bimatoprost: a review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging. 2002;19(3):231–48. doi: 10.2165/00002512-200219030-00008. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima T, Matsugi T, Goto W, et al. New fluoroprostaglandin F2α derivatives with prostanoid FP-receptor agonistic activity as potent ocular-hypotensive agents. Biol Pharm Bull. 2003;26(12):1691–5. doi: 10.1248/bpb.26.1691. [DOI] [PubMed] [Google Scholar]
- 10.Holmstrom S, Buchholz P, Walt J, et al. Analytic review of bimatoprost, latanoprost and travoprost in primary open angle glaucoma. Curr Med Res Opin. 2005;21(11):1875–83. doi: 10.1185/030079905X65600. [DOI] [PubMed] [Google Scholar]
- 11.Terminology and guidelines for glaucoma. European Glaucoma Society website. IIIrd ed. 2008. [Accessed on October 16, 2013]. Available at: http://www.eugs.org/eng/EGS_guidelines.asp.
- 12.Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78(4):767–76. doi: 10.1016/j.exer.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Ota T, Murata H, Sugimoto E, et al. Prostaglandin analogues and mouse intraocular pressure: effects of tafluprost, latanoprost, travoprost, and unoprostone, considering 24-hour variation. Invest Ophthalmol Vis Sci. 2005;46(6):2006–11. doi: 10.1167/iovs.04-1527. [DOI] [PubMed] [Google Scholar]
- 14.Brasnu E, Brignole-Baudouin F, Riancho L, et al. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008;33(4):303–12. doi: 10.1080/02713680801971857. [DOI] [PubMed] [Google Scholar]
- 15.Kahook MY, Ammar DA. In vitro toxicity of topical ocular prostaglandin analogs and preservatives on corneal epithelial cells. J Ocul Pharmacol Ther. 2010;26(3):259–63. doi: 10.1089/jop.2010.0003. [DOI] [PubMed] [Google Scholar]
- 16.Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27(11):837–45. doi: 10.1007/s12325-010-0070-1. [DOI] [PubMed] [Google Scholar]
- 17.Liang H, Pauly A, Riancho L, et al. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional- reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95(6):869–75. doi: 10.1136/bjo.2010.189449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellinen P, Huhtala A, Tolonen A, et al. The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr Eye Res. 2012;37(2):145–54. doi: 10.3109/02713683.2011.626909. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012;53(9):5154–60. doi: 10.1167/iovs.12-9685. [DOI] [PubMed] [Google Scholar]
- 20.Whitson JT, Petroll WM. Corneal epithelial cell viability following exposure to ophthalmic solutions containing preservatives and/or antihypertensive agents. Adv Ther. 2012;29(10):874–88. doi: 10.1007/s12325-012-0057-1. [DOI] [PubMed] [Google Scholar]
- 21.Ayaki M, Iwasawa A, Niwano Y. In vitro assessment of the cytotoxicity of six topical antibiotics to four cultured ocular surface cell lines. Biocontrol Sci. 2012;17(2):93–9. doi: 10.4265/bio.17.93. [DOI] [PubMed] [Google Scholar]
- 22.Ayaki M, Iwasawa A, Niwano Y. Comparative study of in vitro ocular surface cytotoxicity of a fixed combination of 0.5% timolol/1% dorzolamide eyedrop and its components with 0.005% benzalkonium chloride. Biocontrol Sci. 2012;17(3):115–20. doi: 10.4265/bio.17.115. [DOI] [PubMed] [Google Scholar]
- 23.Ayaki M, Iwasawa A, Niwano Y. Cell viability score as an integrated indicator for cytotoxicity of benzalkonium chloride-containing antiglaucoma eyedrops. Biocontrol Sci. 2012;17(3):121–8. doi: 10.4265/bio.17.121. [DOI] [PubMed] [Google Scholar]
- 24.Ayaki M, Iwasawa A, Niwano Y. Comparative assessment of the cytotoxicity of six anti-inflammatory eyedrops in four cultured ocular surface cell lines, as determined by cell viability scores. Clin Ophthalmol. 2012;6:1879–84. doi: 10.2147/OPTH.S36968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guenoun JM, Baudouin C, Rat P, et al. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva- derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(12):4594–9. doi: 10.1167/iovs.05-0776. [DOI] [PubMed] [Google Scholar]
- 26.Kanamori A, Naka M, Fukuda M, et al. Tafluprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1353–60. doi: 10.1007/s00417-009-1122-6. [DOI] [PubMed] [Google Scholar]
- 27.Yamagishi R, Aihara M, Araie M. Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp Eye Res. 2011;93(3):265–70. doi: 10.1016/j.exer.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Hos D, Koch KR, Bock F, et al. Short- and long-term corneal vascular effects of tafluprost eye drops. Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1919–27. doi: 10.1007/s00417-013-2345-0. [DOI] [PubMed] [Google Scholar]
- 29.Liang H, Baudouin C, Labbe A, et al. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS One. 2012;7(3):e33913. doi: 10.1371/journal.pone.0033913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerberick GF, Cruse LW, Ryan CA, et al. Use of a B cell marker (B220) to discriminate between allergens and irritants in the local lymph node assay. Toxicol Sci. 2002;68(2):420–8. doi: 10.1093/toxsci/68.2.420. [DOI] [PubMed] [Google Scholar]
- 31.Willis CM, Stephens CJ, Wilkinson JD. Differential effects of structurally unrelated chemical irritants on the density and morphology of epidermal CD1+ cells. J Invest Dermatol. 1990;95(6):711–6. doi: 10.1111/1523-1747.ep12514510. [DOI] [PubMed] [Google Scholar]
- 32.Malvitte L, Montange T, Vejux A, et al. Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br J Ophthalmol. 2007;91(1):29–32. doi: 10.1136/bjo.2006.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutton A, Gilvarry A, Ropo A. A comparative, placebo-controlled study of prostanoid fluoroprostaglandin-receptor agonists tafluprost and latanoprost in healthy males. J Ocul Pharmacol Ther. 2007;23(4):359–65. doi: 10.1089/jop.2006.0061. [DOI] [PubMed] [Google Scholar]
- 34.Uusitalo H, Kaarniranta K, Ropo A. Pharmacokinetics, efficacy and safety profiles of preserved and preservative-free tafluprost in healthy volunteers. Acta Ophthalmol Suppl (Oxf ) 2008;242:7–13. doi: 10.1111/j.1755-3768.2008.01380.x. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki H, Itakura H, Yokoyama T, et al. Twenty-four-hour ocular hypotensive effects of 0.0015% tafluprost and 0.005% latanoprost in healthy subjects. Jpn J Ophthalmol. 2010;54(4):286–90. doi: 10.1007/s10384-010-0828-7. [DOI] [PubMed] [Google Scholar]
- 36.Konstas AG, Kozobolis VP, Katsimpris IE, et al. Efficacy and safety of latanoprost versus travoprost in exfoliative glaucoma patients. Ophthalmology. 2007;114(4):653–7. doi: 10.1016/j.ophtha.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 37.Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trials. J Glaucoma. 2008;17(8):667–73. doi: 10.1097/IJG.0b013e3181666557. [DOI] [PubMed] [Google Scholar]
- 38.Honrubia F, Garcia-Sanchez J, Polo V, et al. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trials. Br J Ophthalmol. 2009;93(3):316–21. doi: 10.1136/bjo.2007.135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandolfi S, Simmons ST, Sturm R, et al. Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther. 2001;18(3):110–21. doi: 10.1007/BF02850299. [DOI] [PubMed] [Google Scholar]
- 40.Netland PA, Landry T, Sullivan EK, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(4):472–84. doi: 10.1016/s0002-9394(01)01177-1. [DOI] [PubMed] [Google Scholar]
- 41.Demitsu T, Manabe M, Harima N, et al. Hypertrichosis induced by latanoprost. J Am Acad Dermatol. 2001;44(4):721–3. doi: 10.1067/mjd.2001.111625. [DOI] [PubMed] [Google Scholar]
- 42.Brandt JD, VanDenburgh AM, Chen K, et al. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108(6):1023–31. doi: 10.1016/s0161-6420(01)00584-x. [DOI] [PubMed] [Google Scholar]
- 43.Kampik A, Arias-Puente A, O’Brart DP, et al. Intraocular pressure-lowering effects of latanoprost and brimonidine therapy in patients with open-angle glaucoma or ocular hypertension: a randomized observer-masked multicenter study. J Glaucoma. 2002;11(2):90–6. doi: 10.1097/00061198-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Parrish RK, Palmberg P, Sheu WP. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 45.Noecker RS, Dirks MS, Choplin NT, et al. A six-month randomized clinical trial comparing the intraocular pressure-lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003;135(1):55–63. doi: 10.1016/s0002-9394(02)01827-5. [DOI] [PubMed] [Google Scholar]
- 46.Chiba T, Kashiwagi K, Ishijima K, et al. A prospective study of iridial pigmentation and eyelash changes due to ophthalmic treatment with latanoprost. Jpn J Ophthalmol. 2004;48(2):141–7. doi: 10.1007/s10384-003-0039-6. [DOI] [PubMed] [Google Scholar]
- 47.Manni G, Centofanti M, Parravano M, et al. A 6-month randomized clinical trial of bimatoprost 0.03% versus the association of timolol 0.5% and latanoprost 0.005% in glaucomatous patients. Graefes Arch Clin Exp Ophthalmol. 2004;242(9):767–70. doi: 10.1007/s00417-004-0866-2. [DOI] [PubMed] [Google Scholar]
- 48.Elgin U, Batman A, Berker N, et al. The comparison of eyelash lengthening effect of latanoprost therapy in adults and children. Eur J Ophthalmol. 2006;16(2):247–50. doi: 10.1177/112067210601600209. [DOI] [PubMed] [Google Scholar]
- 49.Sharpe ED, Reynolds AC, Skuta GL, et al. The clinical impact and incidence of periocular pigmentation associated with either latanoprost or bimatoprost therapy. Curr Eye Res. 2007;32(12):1037–43. doi: 10.1080/02713680701750625. [DOI] [PubMed] [Google Scholar]
- 50.Birt CM, Buys YM, Ahmed II, Trope GE. Prostaglandin efficacy and safety study undertaken by race (the PRESSURE study) J Glaucoma. 2010;19(7):460–7. doi: 10.1097/IJG.0b013e3181c4aeac. [DOI] [PubMed] [Google Scholar]
- 51.Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111–6. doi: 10.2147/OPTH.S27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue K, Shiokawa M, Wakakura M, et al. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22(8):626–31. doi: 10.1097/IJG.0b013e31824d8d7c. [DOI] [PubMed] [Google Scholar]
- 53.Maruyama K, Tsuchisaka A, Sakamoto J, Shirato S, Goto H. Incidence of deepening of upper eyelid sulcus after topical use of tafluprost ophthalmic solution in Japanese patients. Clin Ophthalmol. 2013;7:1441–6. doi: 10.2147/OPTH.S47783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayaprakasam A, Ghazi-Nouri S. Periorbital fat atrophy—an unfamiliar side effect of prostaglandin analogues. Orbit. 2010;29(6):357–9. doi: 10.3109/01676830.2010.527028. [DOI] [PubMed] [Google Scholar]
- 55.Maruyama K, Shirato S, Tsuchisaka A. Incidence of Deepening of the Upper Eyelid Sulcus After Topical Use of Travoprost Ophthalmic Solution in Japanese. J Glaucoma. doi: 10.1097/IJG.0b013e31826a7e09. Epub August 23, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun. 1997;233(1):200–2. doi: 10.1006/bbrc.1997.6433. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Clipstone NA. Prostaglandin F2α inhibits adipocyte differentiation via a G alpha q-calcium-calcineurin-dependent signaling pathway. J Cell Biochem. 2007;100(1):161–73. doi: 10.1002/jcb.21044. [DOI] [PubMed] [Google Scholar]
- 58.Choi HY, Lee JE, Lee JW, Park HJ, Jung JH. In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J Ocul Pharmacol Ther. 2012;28(2):146–52. doi: 10.1089/jop.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamacher T, Airaksinen J, Saarela V, et al. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis. Acta Ophthalmol Suppl (Oxf ) 2008;242:14–9. doi: 10.1111/j.1755-3768.2008.01381.x. [DOI] [PubMed] [Google Scholar]
- 60.Traverso CE, Ropo A, Papadia M, et al. A phase II study on the duration and stability of the intraocular pressure-lowering effect and tolerability of Tafluprost compared with latanoprost. J Ocul Pharmacol Ther. 2010;26(1):97–104. doi: 10.1089/jop.2009.0066. [DOI] [PubMed] [Google Scholar]
- 61.Uusitalo H, Pillunat LE, Ropo A. Efficacy and safety of tafluprost 0.0015% versus latanoprost 0.005% eye drops in open-angle glaucoma and ocular hypertension: 24-month results of a randomized, double-masked phase III study. Acta Ophthalmol. 2010;88(1):12–9. doi: 10.1111/j.1755-3768.2010.01862.x. [DOI] [PubMed] [Google Scholar]
- 62.Erb C, Lanzl I, Seidova SF, et al. Preservative-free tafluprost 0.0015% in the treatment of patients with glaucoma and ocular hypertension. Adv Ther. 2011;28(7):575–85. doi: 10.1007/s12325-011-0038-9. [DOI] [PubMed] [Google Scholar]
- 63.Rossi GC, Blini M, Scudeller L, et al. Effect of Preservative-Free Tafluprost on Keratocytes, Sub-Basal Nerves, and Endothelium: A Single-Blind One-Year Confocal Study on Naive or Treated Glaucoma and Hypertensive Patients Versus a Control Group. J Ocul Pharmacol Ther. doi: 10.1089/jop.2013.0069. Epub August 14, 2013. [DOI] [PubMed] [Google Scholar]