Abstract
Previous studies have reported cerebellar abnormalities or static ataxia associated with risk for chronic use of alcohol and drugs. Adverse childhood experience (ACE) is another strong risk factor for later substance abuse. We therefore, sought to ascertain the relationship between morphological phenotypes of the lingula (Lobule I) of the anterior cerebellar vermis (ACV), and exposure to emotional (EM) versus physical (PM) maltreatment,on the degree of ongoing alcohol or drug use. The study design consisted of a cross-sectional in vivo neuroimaging study, utilizing retrospective assessment of maltreatment history and self-reports of alcohol and substance use. Study participants were 153 subjects (54M/99F, 21.9±2.2 years) selected for imaging from a database of 1,402 community participants 18–25 years of age, who completed a detailed online screening instrument, and met rigorous inclusion/exclusion criteria. Subjects were exposed to only physical abuse or harsh corporal punishment (PM group, n=37); parental verbal abuse and/or witnessing domestic violence (EM group, n= 58); or had no history of maltreatment or Axis I disorders (n=58). The main outcomes measures consisted of the grey matter volume of Lobule I as measured by manual tracing, number and type of alcoholic beverages consumed during a drinking session, number of sessions per month, and monthly drug use, along with family history of drug and alcohol abuse. Lingula thickness was not attenuated by alcohol use or maltreatment history. However, increased lingula thickness was associated with greater consumption of drugs and hard liquor, particularly in physically maltreated subjects who consumed 2.5- and 2.7-fold more alcohol, and used drugs 6.1- and 7.8-fold more frequently than controls or EM subjects, respectively. In conclusion, physical maltreatment was observed to interact with cerebellar morphology resulting in a strong association with alcohol and substance use. Lingula thickness may represent a novel, experientially-sensitive, phenotypic risk factor for enhanced alcohol and drug use, that perhaps modulates sensitivity to these agents.
Introduction
The elucidation of developmental risk factors for alcoholism and drug abuse has been a focus of research for decades. Risk appears to involve the complex interplay of genetic susceptibility, a host of experiential factors, parental and peer influences, age of initiation, and comorbid disorders (1, 2). Genetics appears to modify or moderate a number of endophenotypes that interact with environmental events to produce risk for substance abuse (3). A particularly compelling endophenotype is a low-level of response to alcohol (3, 4). Individuals, who require greater amounts of alcohol to feel high or nauseous, and to show impairments in postural stability or psychomotor performance, are at enhanced risk for developing alcohol use disorders (4, 5). Neurobiological factors responsible for differences in alcohol response have not been clearly identified, but cerebellar differences may be a good candidate (6). For example, individual differences in cerebellar structure (6, 7) or static ataxia (8, 9) have been identified as possible risk factors for the development of alcohol dependence and substance abuse. Further, key signs of intoxication (slurred speech, lack of coordination, unsteady gait, impairment of attention and memory) may be related, at least in part, to alcohol effects on cerebellar functions.
Genetic susceptibility interacts with experience to determine outcome, and several studies have identified exposure to childhood adversity as a major experiential risk factor. Perhaps the most compelling evidence stems from the Adverse Childhood Experience (ACE) study, in which exposure to eight categories of adversity was assessed in 17,337 adults enrolled in the Kaiser-Permanete Health Plan. Types of adversity included: recurrent physical abuse (PA), recurrent emotional abuse (EA), sexual abuse (SA), witnessing domestic violence (WDV), and multiple forms of household dysfunction. A striking dose-dependent relationship emerged between number of ACEs and risk for alcoholism or drug abuse. The population attributable risk associated with early adversity was 50% for drug abuse, 65% for alcoholism, and 78% for intravenous drug abuse. (10–12)
The ACE study assumed, for convenience, that all forms of adversity were equally consequential, and that the total number of ACEs was the most relevant determinant. While this approach has merit, a more detailed understanding of the pathway between early adversity and substance abuse dictates that we examine these risk factors independently to assess their relative importance. For instance, we recently reported that exposure to SA and EA were associated with higher symptom ratings of depression, anxiety and dissociation than exposure to PA (13).
In this study we were able to specifically compare the association between exposures to emotional maltreatment (EM) versus physical maltreatment (PM) in groups of young adults. In accordance with our previous findings (13) we observed higher ratings of depression and anxiety in young adults exposed to EM than PM. However, exposure to PM was associated with a marked increase in drug or alcohol abuse compared to controls, whereas exposure to EM was not.
Interestingly, a primary region of vulnerability to the pathogenic effects of chronic alcohol use is the vermis, which has a complex tree-like structure that differs dramatically among individuals in foliation and lobulation (14). Pathology studies by Thomas in 1905 and Victor in 1959 (15) showed that the anterior cerebellar vermis (ACV) was selectively atrophied in chronic alcoholics, and this has been confirmed using modern imaging techniques (16, 17). Similarly, fetal alcohol exposure results in a hypoplastic ACV (18–21).
Hence, during the course of our investigation into the effects of abuse on brain structure and alcohol/drug use, we carefully examined ACV morphology. This led to the observation that high-level consumption of hard liquor in young adults who have not previously demonstrated alcohol dependence was associated with a distinct cerebellar morphology. Marked thickening of lobule I of the ACV (a.k.a., lingula) was noted in some subjects with the highest levels of alcohol use, and with a strong preference for hard liquor. The finding was unexpected since chronic excessive use of alcohol (or fetal exposure) leads to atrophic changes in this region (16, 17, 19–21). A thick lingula results from the in utero (14) fusion of lobule I and II. We therefore propose for the first time that individual differences in ACV morphology may serve to increase risk for alcoholism and drug abuse in young adults. We are unaware of any previous studies that have examined the potential influence of human anterior cerebellar vermis phenotypes as describe by Larsell (1972; p.39, Fig. 54) on early alcohol or drug use behaviors. We have used Larsell’s description of individual variations of the anterior cerebellar lobe of man as a guide in the measurement and description of our current MRI findings (see Figure 1).
Figure 1.
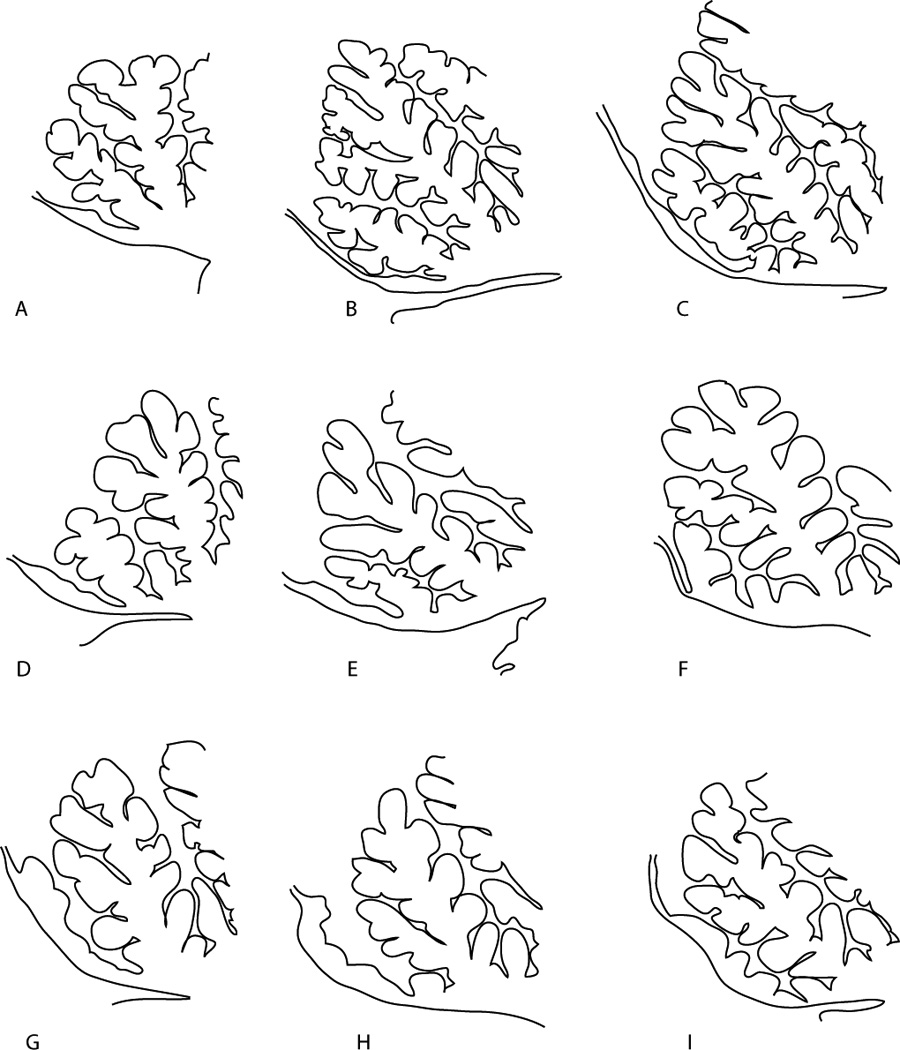
Hand tracing of Larsell’s (14) Figure 54 of medial sagittal sections of adult anterior cerebellum which according to Larsell represented “gradations in the central lobule and lingual that have significance when compared with the vermian segments between the preculminate fissure and the anterior meduallary velum of the subhuman cerebella and with the development of this part of the vermis in man. p. 40–41.” Parts A-E of this figure illustrate the unfused thin variations of lobule I with a distinctive lobule II presentation. Parts F-I of the figure illustrate the fusing of lobule I+II common in our sample. Hatching in the figure illustrate typical sampling of the lingual in these hypothetical cases.
This novel hypothesis is plausible given the function of this structure. Lingula lesions in primates disrupt vestibulocerebellar and spinocerebellar integration, resulting in marked disequilibrium (22). Similarly, ingestion of alcohol and certain drugs disrupts vestibulocerebellar integration (23). Hence, differences in vestibulocerebellar sensitivity to alcohol or abusable drugs may be due, at least in part, to differences in lingula morphometry. Specifically, we hypothesized that individuals with thick phenotypes may have a blunted vestibulocerebellar response to alcohol and may learn that higher proof beverages deliver alcohol at a sufficiently rapid rate to produce vestibular feelings of inebriation. Therefore, we shifted focus from the chronic effects of alcohol or drugs on the cerebellum, to the influence of individual differences in ACV phenotypes on alcohol and drug use, and to the potential interaction between vermal phenotype and exposure to early adversity.
Methods
Participants
Subjects were right-handed, healthy, unmedicated young adults, recruited from the community by advertisements with the title “Memories of Childhood”. Subjects were recruited for entry into one of three studies, which looked at the effects of EM (parental verbal abuse or witnessing domestic violence), harsh corporal punishment, or childhood trauma (physical or sexual abuse) on trajectories of brain development. The aim was to assess the effects of exposure to specific forms of maltreatment, and to recruit these subjects from the community rather than from clinical sources. Selection of subjects was a two-step process. During the first phase a large number of subjects interested in participating in the second (neuroimaging) phase, provided detailed information on their degree of exposure to a host of abusive or traumatic experiences, along with medical, psychiatric, developmental and family history. Applicants were aware that the purpose of the second phase was to study of the effects of early experience on brain development, but unaware of our specific emphasis on EM, CP or physical/sexual abuse, so no candidate could fake or embellish a history to gain eligibility. Potentially interested respondents (who provided written informed consent) were provided with a password to an internet-based enrollment system that requested detailed information (2,342 fields) on childhood history, development, and symptomatology. Screened respondents (n=1,402) were then selected for enrollment in phase two based on either a complete absence of exposure to any type of abuse (control group), or a history of PM or EM. Subjects with a history head trauma, fetal exposure to alcohol or drugs, perinatal or neonatal complications, neurological disorders, or medical conditions that could adversely affect growth and development were excluded. Subjects could be included if they used alcohol or drugs up to several times per month, but were excluded for substance abuse or dependence. The goal of the screening was to identify subjects in the community who were exposed to only one type of maltreatment, who could then undergo neuroimaging. This means of screening enabled us to identify potentially eligible subjects in the community. All who met these inclusion and exclusion criteria were invited to the laboratory for comprehensive screening and possible neuroimaging. The McLean Hospital IRB approved all procedures. The purpose and meaning of this study was explained to subjects, who gave their written informed consent for each step. Controls were required to have no history of any DSM-IV Axis I disorders. EM or PM subjects were enrolled regardless of psychiatric diagnoses, to provide a balanced assessment of the effects of exposure. Selecting maltreated subjects with specific diagnoses (e.g., PTSD, depression) may overestimate the impact of exposure. Similarly, selecting maltreated subjects without any psychiatric history may underestimate the impact. Enrolled subjects were tested for recent drug or alcohol use. Subjects ranged in age from 18 to 25 years (mean= 21.87, SD=2.15 years); 99 were women, and 54 were men (Table I). Controls consisted of 19 males and 39 females with no history of exposure to any form of early maltreatment or trauma. Most of the study subjects were white 70.6%, 7.2% were black, 7.9% were Hispanic, 10.5% were Asian, and 3.9% were from other ethnic groups.
Table I.
Demographics and subject characteristics by maltreatment history
| Maltreatment Groupings |
P - Values |
|||||||
|---|---|---|---|---|---|---|---|---|
| No | Physical (P) | Emotional (E) | F2,150 or Χ2 | 3 Groups | No vs P | No vs E | P vs E | |
| Subjects | 58 | 37 | 58 | |||||
| Female/Male | 39F/19M | 18F/19M | 42F/16M | Χ2=5.849, df=2 | 0.054 | 0.088 | 0.686 | 0.029 |
| Age | 21.9±2.0 | 21.8±2.1 | 22.0±2.3 | 0.099 | 0.906 | 0.976 | 0.999 | 0.966 |
| Weighta | 152.1±33.6 | 160.8±34.1 | 155.6±28.3 | 1.009 | 0.367 | 0.403 | 0.887 | 0.783 |
| Education (yrs)ab | 14.5±1.6 | 14.5±1.7 | 14.1±1.6 | 2.878 | 0.059 | 0.999 | 0.082 | 0.202 |
| FSIQabcd | 124.8±0.0 | 119.8±1.2 | 117.6±1.5 | 2.26 | 0.109 | 0.468 | 0.114 | 0.939 |
| Family History - Alcohol | 0.08±0.21 | 0.26±0.48 | 0.36±0.75 | 4.15 | 0.018 | 0.305 | 0.015 | 0.728 |
| Family History - Drugs | 0.03±0.16 | 0.24±0.51 | 0.27±0.77 | 3.062 | 0.05 | 0.196 | 0.066 | 0.995 |
| Financial Sufficiency | 3.57±0.23 | 3.00±0.62 | 2.84±0.27 | 15.81 | <0.001 | 0.001 | <.001 | 0.608 |
| Parental Education (yrs) | 16.1±2.32 | 14.7±2.62 | 15.5±2.73 | 3.29 | 0.04 | 0.034 | 0.462 | 0.424 |
| SQ - Anxietya | 04.6±3.4 | 05.2±3.3 | 07.9±4.8 | 10.463 | <0.001 | 0.885 | <0.001 | 0.007 |
| SQ - Depressiona | 03.7±3.7 | 04.4±4.3 | 06.9±4.9 | 7.988 | 0.001 | 0.848 | 0.001 | 0.029 |
| SQ - Somatizationa | 03.6±3.6 | 04.4±3.3 | 06.6±4.3 | 8.836 | <0.001 | 0.684 | <0.001 | <0.001 |
| SQ - Hostilitya | 04.1±3.1 | 04.8±3.7 | 06.1±4.3 | 4.065 | 0.019 | 0.769 | 0.016 | 0.307 |
| LSCL-33a | 10.7±8.7 | 16.5±12.2 | 21.8±14.6 | 12.233 | <0.001 | 0.074 | <0.001 | 0.123 |
| DESa | 05.6±4.5 | 08.3±8.6 | 10.8±10.4 | 5.907 | 0.003 | 0.335 | 0.002 | 0.408 |
| Lingula Sizea (voxels) | 19.7±0.0 | 20.7±1.1 | 19.1±1.4 | 0.103 | 0.902 | 0.992 | 0.994 | 0.958 |
Adjusted for gender
Adjusted for age
Adjusted for education
Adjusted for parental education
Physical Maltreatment Group
This group consisted of 19 males and 18 females exposed to harsh corporal punishment (HCP, n = 29) or PA by primary caretakers (n = 8). HCP involves the chronic administration of parental physical force to correct behavior that caused pain without physical injury. The basic requirement was at least 3 years exposure with monthly or greater frequency during early childhood (birth to 13 years old), with at least one incident per year involving an implement, such as a belt, hairbrush or paddle. HCP subjects reported that these events were accompanied by feelings of fear or apprehension; anticipatory anxiety or avoidance of the punisher. However, they also needed to indicate that HCP occurred specifically for discipline and that primary care givers were not hitting them out of anger, rage or loss of control. Traumatic PA was defined as episodes of physical violence by a primary care giver in which the subject believed that they were going to be seriously injured or killed. These episodes fulfilled the A(1) and A(2) criteria for a traumatic experience in DSM-IV(24), and involved physical assaults that received, or should have received, medical attention or left permanent scars. Episodes must have occurred during at least two years of the subject’s life prior to age 18. PM subjects had no history of exposure to sexual abuse or EM.
Emotional maltreatment group
This group consisted of 17 males and 41 females exposed to parental verbal abuse (PVA) and/or WDV, which are the two primary forms of EM. These two forms of exposure are typically combined on rating instruments, such as the Childhood Trauma Questionnaire (CTQ) (29) to provide a measure of exposure to emotional abuse (EA). PVA was defined by elevated parental scores on the verbal abuse scale (13) (VAS) (see below). WDV was defined as the visual observation of at least one event where one parent deliberately inflicted physical injury to another parent. EM subjects also had CTQ EA subscale scores greater than 16 (25). EM subjects had no history of significant PA or SA, exposure to significant corporal punishment, or any additional traumatic childhood events.
Assessment
SCID-I, SCID-II, TAI
Subjects were evaluated using the SCID-I and SCID-II Clinical Versions (26) for history of Axis I and II DSM-IV disorders. This included comprehensive evaluation for alcohol and substance use disorders. Exposure to trauma was assessed in detail using the semi-structured 100-item Trauma Antecedents Interview(27); (28), CTQ (29) and the Straus Conflict Tactics scales (CTS) (30). Subjects meeting criteria for exposure to maltreatment needed to be consistent in their reports of trauma exposure between self-report and interviews.
Exposure to verbal aggression
The VAS (13) consists of 15 items that cover the key components of verbal abuse—scolding, yelling, swearing, blaming, insulting, threatening, demeaning, ridiculing, criticizing, belittling, etc. In a separate group of 48 college students, the questionnaire showed high internal consistency as applied to both maternal and paternal behaviors (Cronbach alphas, 0.98 and 0.94, respectively). The VAS provides a continuous measure of exposure. A cut off score (average PVAS > 40 or maximal [mother or father] PVAS > 50) was used to identify subjects exposed to a substantial degree of verbal aggression (31). This cutoff designates the top 10–15% of scores, and is associated with increased psychiatric symptoms ratings (13), and alterations in white matter tract integrity of arcuate fasciculus, cingulum bundle and fornix (31).
Alcohol and Drug Use
Alcohol and recreational drug use histories were obtained though the online survey. Subjects detailed consumption of beer, wine, mixed drinks and shots of hard liquors during typical drinking occasions at college and home as well as the number of episodes of use per month. Use of beer, wine, mixed drinks and shots were calculated by multiplying the number of drinks consumed per daily drinking session times the number of sessions per month of use. This approach is similar to methods used in epidemiological surveys such as NSDUH (32), except that we quantified use of each type of alcoholic beverage separately, rather than number of drinks regardless of type. Self-reported monthly use of street drugs at college and home (e.g., marijuana, sedatives, stimulants, opioids, cocaine, PCP, hallucinogens, steroids, ecstasy & inhalants) were totaled and expressed as the number of days per month in which drugs were used. History of substance abuse or dependence was assessed by interview using SCID (26).
Financial Sufficiency
Low income and poverty may be important developmental risk factors for drug or alcohol abuse. Subjects were often uncertain about parental income, but were well aware of the degree of perceived financial sufficiency, or stress, experienced while growing up. This was rated on a Likert scale ranging from 1 (much less than enough money for our needs) to 5 (much more than enough money for our needs).
Family History
Subjects indicated on the online assessment whether each of their first degree and some second degree relatives (uncles, aunts, nephews, nieces) had no, possible or definite history of mental health problems, problems with alcohol, problems with drugs, depression, mania or bipolar disorder, schizophrenia or psychosis, ADHD or ADD, anxiety or fears, criminal behavior or incarceration. Further, they were asked to specify the types of treatments they may have received (talk therapy, medication, hospitalization). In addition they also provided information during each stage of life (0–2, 2–4,…,16–18 years) whether a parent was unavailable due to mental illness, use of drugs, use of alcohol, or incarceration.
During the Traumatic Antecedents Interview a detailed assessment was made of parental interactions with the subjects, which included inappropriate parental behaviors or absences due to use of drugs, alcohol or mental illness. This provided additional data on parental psychiatric history.
MRI Acquisition
MRI scans were performed at the McLean Hospital Brain Imaging Center using a Siemens 3 Tesla Trio scanner and an 8-element phased-array head coil. A three-plane scout series was acquired to verify subject position. Scans for clinical review included volumetric T1 weighted sagittal MPRAGE, and double echo TSE and FLAIR. Lingula measurements were made from sagittal T2 Relaxometry (T2-RT) image sets composed of segmented spin echo EPI scans with 7 progressively stepped TE values: TE(7)/TR = 17ms, to 113ms/5s; matrix = 128×128 on (220mm)2 FOV; 26 × 5mm slices with no gap; segmented < BW 1775 Hz/px = 227kHz, 0.71 esp, 5/8 partphase >. T2-weighted matched Turbo Spin Echo (TSE) images were also used to aid ROI definition (TE/TR = 90ms/4.5s; matrix 384×384 on (220mm)2 FOV; 26 × 5mm slices with no gap; GRAPPA, 2 averages, with a reduced refocus pulse of 150deg. < BW 99 Hz/px = 38kHz, turbo factor =9; 17.1 esp >.
ROI Analysis
Manual ROI tracing of lobule I of the CV was performed using in house algorithms developed for the Functional Analysis Tool, an IDL-based (Research Systems Inc.) software tool for displaying overlapping image sets and curve fitting T2 relaxation time data. Using the Functional Analysis Tool a region was carefully visualized on a midline sagittal TSE image, and traced onto a matched midsaggital voxel-wise T2 map for segmentation. The TSE image allowed optimum visualization of the fourth ventricle, superior medullary vellum and anterior cerebellar lobules. The precentral fissure and culmen (lobules IV & V) were identified. As the ACV shows great variation among individuals, the rostral central lobule (lobule III) and lingula (lobules I or I + II) were classified with reference to Larsell’s descriptions of typical human variations (Figure 1; Larsell, Fig 54, A-I) (14). The principal distinction between human lingula variants is the presence or absence of lobule II (Larsell, p.40; see Figure 2A) (14). Therefore, this variation was identified, recorded and a cursor placed at the thinnest rostral-most region of the superior medullary vellum, near the junction with the brainstem. The lingula was then outlined with the cursor, enclosing the region within a ROI while avoiding excursions into lobule II (if present) or the adjacent central lobule (lobule III; see Figure 2C for representative ROIs). The T2 ROI contains 1.72mm by 1.72 mm by 5mm rectangular cuboid-shaped voxels, and one sagittal slice effectively occupies the space filled by the medullary vellum. The ROI measurement thus represents the number of cuboids that fill the gray matter of the lingual.
Figure 2.
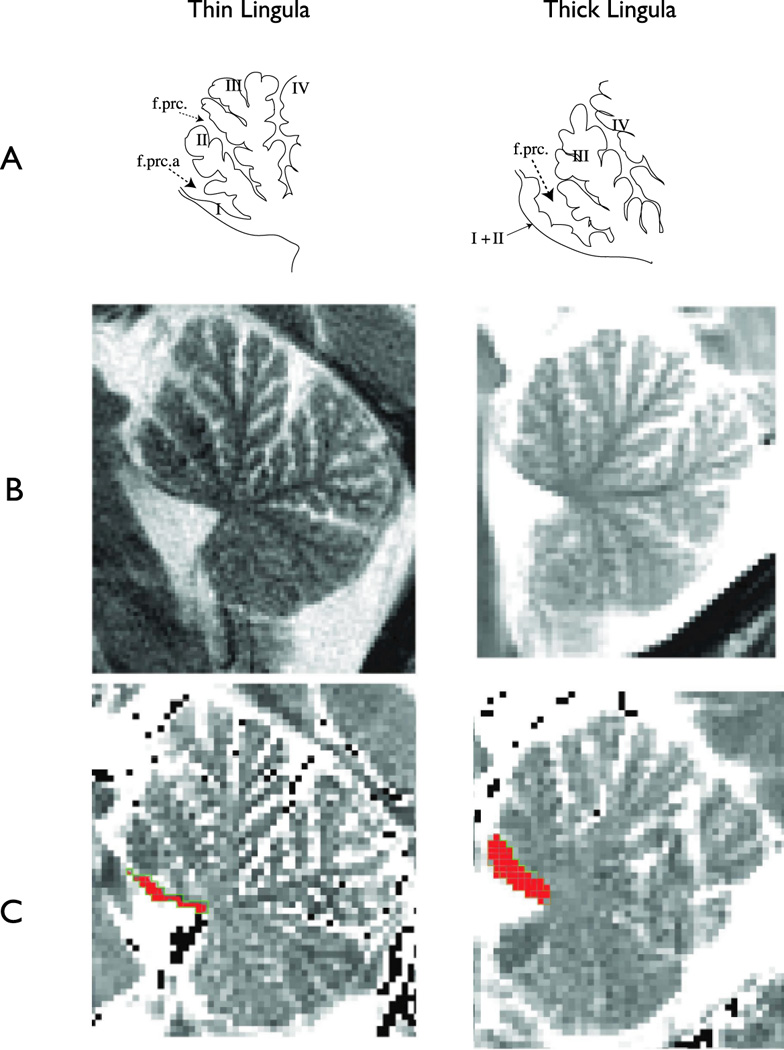
Common phenotypes of the anterior lobes of the cerebellar vermis. Adult anterior cerebellar vermis (A) hand traced from (14) p. 41, Figure 54A & H) depicting the normal thin phenotype of lobule I and typical lobule II (left) and the normal thick phenotype (right) lacking a discrete lobule II. It appears that the folia of the missing lobule II have migrated to lobule I; thus Larsell’s use of the term “lobule I+II”. The presence (left) or absence (right) of the precentral fissure a (f.prc.a) which was described by Larsell (14) (p. 18) as the defining characteristic of a thin lobule I with normal lobule II. (B) Sagittal T2-weighted matched turbo spin echo images of individuals displaying thick lobules. (C) T2-relaxation time maps collected in the same plane as A, illustrating the ROI placement on lobule I.
Voxel-wise T2 maps were calculated using linear least-squares regression assuming mono-exponential relaxation decay (33). To eliminate non-cerebellar tissue from the calculation of lingula midsaggital area, only voxels ranging from 30 to 90 msec were counted within cerebellar ROIs. T2 values outside of this range were indicative of large vessels or cerebral spinal fluid, and excluded from analysis. For convenience we refer to this measure of midsaggital area as lingula thickness (LT). Two skilled raters (C.M.A.) & (K. R.) blind to subject information preformed the LT measurements. The inter-rater reliability coefficient for these raters was 0.77.
Statistical Methods
There were three identifiable types of vermal morphometry: thin, moderate and thick. K-means cluster analysis was used to impartially assign LT voxel measures to one of these three groupings. Main and interactive effects of exposure to early maltreatment and LT grouping were assessed using linear mixed effect models, which provides more accurate measures of significance when cell sizes are uneven than ANOVA, and can better estimate interactive effects if cell sizes are relatively sparse. Gender, financial sufficiency, and family histories of drug abuse and alcohol abuse by first-degree relatives were included as random effect covariates. Calculations were performed using SPSS (version 16, Chicago Ill) with a Sidak correction for multiple comparisons.
Results
Maltreatment groups and controls did not differ significantly by age, gender, weight, years of education or full scale IQ (Table I). However, the EM group had significantly higher ratings of depression, anxiety, and somatization than either the PM or control groups. There were no differences between PM and controls on these ratings. Subjects in PM and EM groups reported a significantly lower level of perceived financial sufficiency during childhood than non-maltreated controls (Table I).
LT measures, in voxels clustered into 3 groups of unequal size (Table II). There were no differences between these LT clusters in age, education, gender ratio, financial sufficiency, parental education, or ratings of depression and anxiety. LT was not found to differ by maltreatment groups (F2,146 = 0.26, P > 0.8). There was a trend for LT to be about 13.1% smaller in females (F1,147 = 3.513, P > 0.06). However, there were no differences between genders in the distribution of subjects into LT clusters I, II or III (χ2 = 2.51, df=2, P > 0.2). Age, within this narrow range, exerted no significant covariate effect on LT (F1,146= 0.508, P > 0.4).
Table II.
Subject characteristics based on lingula thickness groupings
| Lingula Groupings |
P - Values |
|||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | F2,150 or Χ2 | 3 Groups | I vs II | I vs III | II vs III | |
| Subjects | 88 | 47 | 18 | |||||
| Female/Male | 61F/27M | 28F/19M | 10F/8M | Χ2=2.022, df=2 | 0.364 | 0.261 | 0.28 | 0.785 |
| Lingula Sizea | 13.9±3.9 | 23.9±3.4 | 37.3±7.1 | 258.785 | <0.001 | <0.001 | <0.001 | <0.001 |
| Age | 21.8±2.1 | 22.1±2.1 | 22.1±2.5 | 0.43 | 0.651 | 0.793 | 0.906 | 0.999 |
| Weighta | 155.2±33.2 | 154.7±30.3 | 159.3±31.9 | 0.178 | 0.837 | 0.999 | 0.929 | 0.919 |
| Educationab | 14.4±1.7 | 14.3±1.5 | 14.2±1.6 | 0.269 | 0.764 | 0.99 | 0.849 | 0.944 |
| Family History - Alcohol | 0.18±0.40 | 0.23±0.44 | 0.47±1.12 | 2.227 | 0.111 | 0.912 | 0.106 | 0.308 |
| Family History - Drugs | 0.14±0.43 | 0.11±0.31 | 0.53±1.16 | 4.463 | 0.013 | 0.986 | 0.017 | 0.016 |
| SQ - Anxietya | 05.7±3.9 | 06.8±5.0 | 05.5±4.2 | 1.014 | 0.365 | 0.478 | 0.994 | 0.628 |
| SQ - Depressiona | 04.9±4.4 | 05.4±5.0 | 05.1±3.9 | 0.177 | 0.838 | 0.911 | 0.997 | 0.994 |
| SQ - Somatizationa | 04.9±4.2 | 05.2±4.0 | 04.6±3.4 | 0.174 | 0.841 | 0.961 | 0.991 | 0.931 |
| SQ - Hostilitya | 04.6±3.4 | 06.2±4.6 | 03.9±2.7 | 3.624 | 0.029 | 0.065 | 0.847 | 0.08 |
| LSCL-33a | 15.5±13.5 | 17.6±12.9 | 16.5±10.0 | 0.4 | 0.671 | 0.754 | 0.99 | 0.983 |
| DESa | 07.7±7.1 | 08.9±10.6 | 09.5±8.3 | 0.53 | 0.59 | 0.822 | 0.783 | 0.99 |
Adjusted for gender
Adjusted for age
Linear mixed effect modeling indicated that there were robust main and interactive effects of exposure to early adversity and LT on drug and alcohol use, even after controlling for family history of drug and alcohol abuse, differences in gender and perceived financial sufficiency (Table II). For instance, use of drugs (predominantly marijuana) was affected by history of adversity (F2,137.053 = 20.565, p < 0.0001), LT (F2,137.849 = 15.438, p<0.001) and their interaction (F4,139.742 = 10.683, p<0.0001).
Alcohol use, particularly use of hard liquor, was markedly increased by exposure to PM, and most fervent in subjects with the thickest lingula (Table III) {Table IV}. Consumption of hard liquor was affected by exposure to maltreatment (F2,142.376 = 15.440, p < 0.001), LT (F2,139.626 = 8.100, p < 0.0001) and their interaction (F4,143.483 = 7.179, p < 0.0001). In contrast, early maltreatment and LT failed to exert significant main effects on consumption of wine+beer (maltreatment: F2,141.595 = 0.677, p > 0.5; LT: F2,143.544 = 0.309, p > 0.7).
Table III.
Main effects of maltreatment history on alcohol and drug use
| Maltreatment Groupings |
P - Values |
|||||||
|---|---|---|---|---|---|---|---|---|
| No | Physical (P) | Emotional (E) | F2,150 | 3 Groups | No vs P | No vs E | P vs E | |
| Subjects | 58 | 37 | 58 | |||||
| Alcohol Use (drinks/month)a | 19.34±29.54 | 51.98±52.54 | 18.74±42.85 | 5.19 | 0.007 | 0.012 | 0.999 | 0.012 |
| Wine/Beer (drinks/month)a | 12.82±18.46 | 15.84±15.76 | 10.72±28.90 | 0.32 | 0.727 | 0.95 | 0.971 | 0.811 |
| Hard Liquor (drinks/month)a | 06.52±13.88 | 36.14±37.90 | 08.02±15.57 | 14.07 | <0.001 | <0.001 | 0.987 | <0.001 |
| Drug Use (days/month)a | 0.41±0.84 | 3.47±4.11 | 0.34±0.78 | 18.87 | <0.001 | <0.001 | 0.998 | <0.001 |
Adjusted for gender, age and family history of drug and alcohol abuse
Table IV.
Main effects of lingula thickness grouping on alcohol and drug use
| Lingula Groupings |
P - Values |
|||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | F2,150 | 3 Groups | I vs II | I vs III | II vs III | |
| Subjects | 88 | 47 | 18 | |||||
| Alcohol Use (drinks/month)a | 20.12±37.83 | 20.59±35.31 | 49.35±65.00 | 3.254 | 0.042 | 0.999 | 0.04 | 0.063 |
| Wine/Beer (drinks/month)a | 12.05±25.23 | 11.46±17.83 | 15.87±18.54 | 0.207 | 0.813 | 0.999 | 0.917 | 0.895 |
| Hard Liquor (drinks/month)a | 08.07±15.23 | 09.13±20.45 | 33.48±47.92 | 8.481 | <0.001 | <0.001 | 0.991 | <0.001 |
| Drug Use (days/month)a | 0.46±00.88 | 0.26±05.42 | 3.49±02.18 | 14.690 | <0.001 | 0.933 | <0.001 | <0.001 |
Adjusted for gender, age and family history of drug and alcohol abuse
As illustrated in table III & IV, drug use was markedly increased in subjects exposed to PM, and in subjects with the thickest lingula. It is interesting that exposure to EM did not increase rates of drug use, even though EM was associated with greater symptom ratings of depression (F1,89=7.69, p=0.007) and anxiety (F1,89 = 5.19, p=0.025) than exposure to PM.
The PM group consisted of subjects who experienced either HCP or PA by parents. There were no differences between HCP and PA subjects in degree of drug (F1,29.982 = 0.099, p > 0.7) or alcohol use (F1,30.85 = 0.008, p > 0.9). Similarly, the EM group consisted of subjects with PVA, WDV, or both). There were no differences in drug (F2,48.o = 0.164, P > 0.8) or alcohol use (F2,48.853 = 0.294, p > 0.7) or symptom ratings between these subgroups.
Interestingly, LT was associated with a family history of drug and alcohol abuse in first-degree relatives, particularly siblings. We computed the number of first-degree relatives (FDR) with drug abuse and with alcohol abuse by scoring each relative with 0.5 points for a possible history of drug or alcohol abuse and 1.0 point for a definite history, and summed these together. Subjects with thin, intermediate, and thick lingula had FDR scores of 0.13, 0.09, and 0.69 respectively (F2,143 = 8.09, p < 0.0001).
Although the interactions of maltreatment group and LT grouping on alcohol and drug use were highly significant, these interactions were based on small cell sizes for the thickest LT group. We therefore utilized linear regression to probe the association between LT in voxels with degree of alcohol or drug use, within each maltreatment grouping. For the PM group there were significant correlations between LT voxels and days per month of drug use (r = 0.393, P < 0.02) and drinks per month (r = 0.323, P = 0.05). However, in the EM and control groups there were no significant correlations between LT thickness and drug use (EM: r = 0.065, P > 0.7; controls: r =0.179, P > 0.2), or alcohol use (EM: r = 0.140, P > 0.2; controls: r = 0.129, P > 0.3).
Discussion
A great deal of research has revealed that adverse childhood experiences such as CP, EM, PA or SA may interact with hereditary factors to influence later alcohol and drug use (34–40). While most human twin and adoption studies agree that alcohol intake is a highly heritable trait (41–45), heritability estimates can differ widely among studies (46), perhaps due to discernible differences in particular brain structures that have not until now been taken into account.
Our findings are surprising for a number of reasons. First, as previously reported, childhood exposure to EM was associated with higher ratings of anxiety, depression and somatization (Table I) than PM. However, the PM group had a much higher degree of drug and alcohol use than subjects exposed to EM. Hence, our hypothesis that drug use is often mediated by symptoms of depression or anxiety may be naïve, and early exposure to physical pain may be an important risk factor.
The prominent association between lingula thickness and degree of drug and alcohol use was both serendipitous and surprising. While it is well known that the ACV is vulnerable to pathological effects of chronic alcohol exposure, the cerebellum has not traditionally been considered a brain region associated with risk for substance abuse. However, much is known that makes this plausible (47). First, the vermis, through its fastigial projections to substantia nigra and ventral tegmental area exerts strong effects on the turnover of dopamine in the caudate and nucleus accumbens (48–52). Second, vermal blood flow and metabolism are affected by a host of abusable substances (i.e., nicotine (53), cocaine (54), methylenedioxymethamphetamine (55), barbiturates (56), opiates (57, 58), marijuana (59, 60), and alcohol (61–63)). Methylphenidate, in particular, exerts robust effects on blood flow in this region (64–66). Third, CV and/or cerebellar hemispheric activation has been observed in response to presentation of cues for cocaine (67–70), heroin (71), or alcohol (72), during recall or imagery of cocaine-use experiences (68, 73) and during stimulant expectancy (65). Fourth, the putative anti-addictive agent ibogaine exerts profound and potentially selective effects on the vermis (74, 75). Fifth, ADHD is a significant risk factor for development of substance abuse (76–78), and the most consistent anatomical finding in ADHD is reduced posterior inferior vermal size (79, 80). Finally, the lingula lies in close proximity to the choroids plexus in the fourth ventricle. Alcohol appears to rapidly affect the choroids plexus and disrupt the blood-CSF-brain barrier, resulting in enhanced exposure to neuroactive substances (81). Hence, the vermis may be a component of a neural circuit modulating risk for substance abuse.
Our speculation is that abusable substances alter vermal activity and produce disturbances in vestibulocerebellar introception that can be perceived as feelings of dizziness or ataxia, which may be interpreted as being ’spaced out’, ’drugged’, ’stoned’, or ‘inebriated’. We know that environmental context, such as drug paraphernalia and rituals, are critical factors in abuse (82). Similarly, drug-induced interoceptive perceptual distortions may also provide critical context.
Schuckit and colleagues have published extensively on risk for alcoholism based on individual differences in sensitivity, quantified as reports of negative feelings (e.g., nausea) positive feelings (e.g., ’high’), and degree of body sway in response to an alcohol challenge. They found that low-level response to alcohol challenge was associated with a four-fold greater likelihood of future alcoholism (4), and, also was related to family history of alcoholism (83). Our finding of a strong association between LT and drug and alcohol use is consistent with the findings of Hill and colleagues (6, 7) that individual differences in cerebellar structure may be a possible risk factors for the development of alcohol dependence and substance abuse.
It is also noteworthy that exposure to PM and a thick lingula appeared to be associated with increased consumption of hard liquor but not wine+beer (Table II). Our hypothesis is that individuals with thicker lingulas may consume hard liquor preferentially, as they may be less susceptible to vestibulocerebellar effects, and may experience more of a sense of 'being high' or inebriated with higher proof beverages that they can consume rapidly (eg., ’shots’). Reduced susceptibility to vestibulocerebellar effects of drugs and alcohol may be due to physical differences related to compartment size and diffusion of neuroactive substances from CSF. Alternatively, having an increased number of granule cells may result in a greater reserve capacity and correspondingly greater tolerance to the effects of alcohol. Genetic factors that altered sensitivity in other ways may incidentally lead to thickening or fusion of lobules I and II providing an interesting but non-functional marker. Lack of association with degree of use of wine+beer may relate to the demographics of the study participants who were predominantly college students. These individuals are frequently in social situations where beer and wine are consumed and are endeavoring to fit in. Hence, degree of use of these beverages may be significantly dictated by social factors. There may have been less pressure to imbibe hard liquor, and consequently their degree of consumption of hard liquor may have been more sensitive to differences in early experience and lingula morphology.
Exploration of individual cerebellar phenotypes has been hampered by the limits of current MRI imaging and analysis techniques(84, 85). Single mid-sagittal images of the vermis provide better visualization of characteristic landmarks and surface features of lobules than do axial images. Precision and standardization of subject alignment is important to ensure comparability of single mid-sagittal slices. Second, parcellation of this complex lobular structure is also an issue, as it is critical to separate gray matter from CSF. Axial T1-weighted images, collected in most studies, are not optimal for precise anatomical visualization of vermis lobules I, II and III. Hence, we used matched sagittal T2-weighted TSE images, and T2-relaxation time maps to overcome these limitations, and to provide both a qualitative and quantitative perspective on posterior fossa anatomy. However, there is still a need for greater resolution and contrast (31).
Another limitation is that the information on drug and alcohol use was obtained by self-report on a survey instrument. Although we adopted methods used in epidemiological studies (32), more precise quantification would have been possible through use of a calendar method with memory aids, such as the Alcohol Timeline Followback (86). However, there is no reason to suspect that lingula size or PM would bias response to a self-report survey.
Overall, we observed an association between LT and degree of alcohol and drug use. The major cause of increased thickness is fusion of lobules I and II, which occurs in utero. Further, chronic alcohol abuse leads to atrophy, particularly of the ACV. We cannot however exclude the possibility that increased use leads to a transient state of swelling or hypertrophy (but not fusion) before atrophy. It would be most desirable to assess in a longitudinal study whether increased LT early in childhood predicts degree of alcohol use in adolescents or adulthood. In the absence of such data our interpretation of these findings must be considered preliminary.
Several studies have previously shown associations between exposure to PM and propensity to abuse drugs and alcohol (87, 88). Most individuals experiencing abuse have been exposed to multiple forms of abuse, which can produce an additive or synergistic increase in substance use or abuse (11). This study is relatively unique in its selective recruitment of individuals who experienced only one type of maltreatment. It remains to be seen if exposure to PM and EM in combination would be associated with a greater degree of use than PM alone. The fact that the EM group had higher ratings of depression and anxiety than the PM group, and the observation that individuals experiencing PA and HCP had comparably high rates of substance use, suggest that early exposure to pain may be a risk factor separate and distinct from symptoms of anxiety and depression. Overall, these novel findings may provide new insights and perspective that may enhance our understanding of experiential and neurobiological determinants and role of the cerebellum in substance abuse.
Acknowledgements
The authors thank the following individuals: (data collection) Cynthia E. McGreenery & Ann Polcari, Ph.D., R.N; (editing) Mary R. Kolodny, MA, & Steven B. Lowen, Ph.D; (imaging) Michael L. Rohan, Ph.D., & Kathleen Thangaraj, BS RTR MR.
Funding/Support:
CMA received partial support from DA-016746 during the course of this work. DA-016934, DA-017846 and MH-66222, to MHT, providing funding for the recruitment, assessment and scanning of participants in the study.
References
- 1.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. The American journal of psychiatry. 2000 May;157(5):745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 2.Swadi H. Individual risk factors for adolescent substance use. Drug Alcohol Depend. [Review] 1999 Jul;55(3):209–224. doi: 10.1016/s0376-8716(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 3.Schuckit MA. An overview of genetic influences in alcoholism. Journal of substance abuse treatment. 2009 Jan;36(1):S5–S14. [PubMed] [Google Scholar]
- 4.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 5.Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Archives of General Psychiatry. 1985;42(4):375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- 6.Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, et al. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological psychiatry. 2007 Jan 1;61(1):41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction biology. 2007 Mar;12(1):122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill SY, Armstrong J, Steinhauer SR, Baughman T, Zubin J. Static ataxia as a psychobiological marker for alcoholism. Alcoholism, clinical and experimental research. 1987 Aug;11(4):345–348. doi: 10.1111/j.1530-0277.1987.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 9.Lipscomb TR, Carpenter JA, Nathan PE. Static ataxia: a predictor of alcoholism? The British journal of addiction to alcohol and other drugs. 1979 Sep;74(3):289–294. doi: 10.1111/j.1360-0443.1979.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 10.Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, et al. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv. 2002 Aug;53(8):1001–1009. doi: 10.1176/appi.ps.53.8.1001. [DOI] [PubMed] [Google Scholar]
- 11.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003 Mar;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 12.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006 Apr;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. The American journal of psychiatry. 2006 Jun;163(6):993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 14.Larsell O, Jansen J. In: The comparitive anatomy and histology of the cerebellum: the human cerebellum, cerebellar connections, and cerebellar cortex. 1st ed. Jansen J, editor. Minneapolis: The University of Minnesota Press; 1972. [Google Scholar]
- 15.Victor M, Adams RD, Mancall EL. A Restricted Form of Cerebellar Cortical Degeneration Occurring in Alcoholic Patients. Arch Neurol. [Review] 1959;1(6):579–688. [Google Scholar]
- 16.Cavanagh JB, Holton JL, Nolan CC. Selective damage to the cerebellar vermis in chronic alcoholism: a contribution from neurotoxicology to an old problem of selective vulnerability. Neuropathology & Applied Neurobiology. 1997;23(5):355–363. [PubMed] [Google Scholar]
- 17.Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, et al. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry. 2000;57(9):894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- 18.Autti-Ramo I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Developmental Medicine & Child Neurology. 2002;44(2):98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- 19.Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001 Mar;43(3):148–154. [PubMed] [Google Scholar]
- 20.O'Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, et al. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005 Aug 22;16(12):1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- 21.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal Development of the Cerebellar Vermis in Children Prenatally Exposed to Alcohol - Size Reduction in Lobules I-V. Alcoholism, Clinical & Experimental Research. 1996;20(1):31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 22.Crosby EC, Taren JA, Davis R. The anterior lobe and the lingula of the cerebellum in monkeys and man. Bibliotheca Psychiatrica. 1970;143:22–39. doi: 10.1159/000385814. [DOI] [PubMed] [Google Scholar]
- 23.Chiang HH, Young YH. Impact of alcohol on vestibular function in relation to the legal limit of 0.25 mg/l breath alcohol concentration. Audiology & Neuro-Otology. 2007;12(3):183–188. doi: 10.1159/000099022. [DOI] [PubMed] [Google Scholar]
- 24.APA APA. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition. Washington, DC: 1994. (DSM-IV) [Google Scholar]
- 25.Bernstein DP, Fink L. Childhood Trauma Questionnaire Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders - clinician version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 27.Herman JL, Perry JC, van der Kolk BA. Traumatic Antecedents Interview. Boston: The Trauma Center; 1989. [Google Scholar]
- 28.Roy CA, Perry JC. Instruments for the assessment of childhood trauma in adults. J Nerv Ment Dis. 2004 May;192(5):343–351. doi: 10.1097/01.nmd.0000126701.23121.fa. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect [see comments] The American journal of psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 30.Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse Negl. 1998;22(4):249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- 31.Cho Z-H, Kim Y-B, Han J-Y, Min H-K, Lim K-N, Choi S-H, et al. New brain atlas - mapping the human brain in vivo with 7.0T MRI and comparison wiht postmortem histology: Will these images change modern medicine? Int J Imaging Syst Technol. 2008;18:2–8. [Google Scholar]
- 32.Gfroerer J, Eyerman J, Chromy J, editors. DHHS Publication No. SMS 03–3768. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2002. Redesigning an Ongoing National Household Survey: Methodological Issues. [Google Scholar]
- 33.Anderson CM, Kaufman MJ, Lowen SB, Rohan M, Renshaw PF, Teicher MH. Brain T2 relaxation times correlate with regional cerebral blood volume. Magma. 2005 Mar;18(1):3–6. doi: 10.1007/s10334-004-0076-2. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, et al. Genetic and environmental predictors of early alcohol use. Biological psychiatry. 2007 Jun 1;61(11):1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Langeland W, Draijer N, van den Brink W. Psychiatric comorbidity in treatment-seeking alcoholics: The role of childhood trauma and perceived parental dysfunction. Alcoholism (NY) 2004 Mar;28(3):441–447. doi: 10.1097/01.alc.0000117831.17383.72. [DOI] [PubMed] [Google Scholar]
- 36.Lynch SK, Turkheimer E, D'Onofrio BM, Mendle J, Emery RE, Slutske WS, et al. A genetically informed study of the association between harsh punishment and offspring behavioral problems. J Fam Psychol. 2006 Jun;20(2):190–198. doi: 10.1037/0893-3200.20.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makhija N, Sher L. Childhood abuse, adult alcohol use disorders and suicidal behaviour. QJM-An Int J Med. 2007 May;100(5):305–309. doi: 10.1093/qjmed/hcm024. [DOI] [PubMed] [Google Scholar]
- 38.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: Results from a large population-based sample of men and women. Child Abuse Negl. 2007 May;31(5):517–530. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartor CE, Lynskey MT, Bucholz KK, McCutcheon VV, Nelson EC, Waldron M, et al. Childhood sexual abuse and the course of alcohol dependence development: Findings from a female twin sample. Drug Alcohol Depend. 2007 Jul 10;89(2–3):139–144. doi: 10.1016/j.drugalcdep.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon HH, Iacono WG, Malone SM, McGue M. Using the brain P300 response to identify novel phenotypes reflecting genetic vulnerability for adolescent substance misuse. Addict Behav. 2006 Jun;31(6):1067–1087. doi: 10.1016/j.addbeh.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Decastro JM. A Twin Study Of Genetic And Environmental-Influences On The Intake Of Fluids And Beverages. Physiol Behav. 1993 Oct;54(4):677–687. doi: 10.1016/0031-9384(93)90076-r. [DOI] [PubMed] [Google Scholar]
- 42.Heath AC, Martin NG. Types Of Alcoholics. New York: New York Acad Sciences; 1994. Genetic Influences On Alcohol-Consumption Patterns And Problem Drinking - Results From The Australian Nh-And-Mrc Twin Panel Follow-Up Survey; pp. 72–85. [DOI] [PubMed] [Google Scholar]
- 43.Heath AC, Meyer J, Jardine R, Martin NG. The Inheritance Of Alcohol-Consumption Patterns In A General-Population Twin Sample .2. Determinants Of Consumption Frequency And Quantity Consumed. J Stud Alcohol. 1991 Sep;52(5):425–433. doi: 10.15288/jsa.1991.52.425. [DOI] [PubMed] [Google Scholar]
- 44.Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic Influences On Use And Abuse Of Alcohol - A Study Of 5638 Adult Finnish Twin Brothers. Alcoholism (NY) 1987 Aug;11(4):349–356. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 45.Reed T, Slemenda CW, Viken RJ, Christian JC, Carmelli D, Fabsitz RR. Correlations Of Alcohol-Consumption With Related Covariates And Heritability Estimates In Older Adult Males Over A 14-Year To 18-Year Period - The Nhlbi Twin Study. Alcoholism (NY) 1994 Jun;18(3):702–710. doi: 10.1111/j.1530-0277.1994.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz JG, Long JC, Linnoila M, Goldman D, Suomi SJ, Higley JD. Genetic and other contributions to alcohol intake in rhesus Macaques (Macaca mulatta) Alcoholism (NY) 2006 Mar;30(3):389–398. doi: 10.1111/j.1530-0277.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 47.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27(1–2):231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 48.Albert TJ, Dempesy CW, Sorenson CA. Anterior cerebellar vermal stimulation: effect on behavior and basal forebrain neurochemistry in rat. Biological psychiatry. 1985;20(12):1267–1276. doi: 10.1016/0006-3223(85)90111-8. [DOI] [PubMed] [Google Scholar]
- 49.Nieoullon A, Cheramy A, Glowinski J. Release of dopamine in both caudate nuclei and both substantia nigrae in response to unilateral stimulation of cerebellar nuclei in the cat. Brain Research. 1978;148(1):143–152. doi: 10.1016/0006-8993(78)90384-0. [DOI] [PubMed] [Google Scholar]
- 50.Snider RS, Maiti A, Snider SR. Cerebellar pathways to ventral midbrain and nigra. Experimental Neurology. 1976;53(3):714–728. doi: 10.1016/0014-4886(76)90150-3. [DOI] [PubMed] [Google Scholar]
- 51.Snider SR, Snider RS. Structural and functional relationships betwen cerebellum and catecholamine systems: an overview. Experimental Brain Research. 1982;(Suppl. 6) [Google Scholar]
- 52.Tellerman K, Astrow A, Fahn S, Snider SR, Snider RS, Glassgold JM. Cerebellar control of catecholaminergic activities implications for drug therapy of movement disorders. International Journal of Neurology. 1979;13(1–4):135–155. [PubMed] [Google Scholar]
- 53.Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, et al. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000 Dec 1;38(3):313–321. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.London ED, Wilkerson G, Goldberg SR, Risner ME. Effects of L-cocaine on local cerebral glucose utilization in the rat. Neurosci Lett. 1986;68(1):73–78. doi: 10.1016/0304-3940(86)90232-6. [DOI] [PubMed] [Google Scholar]
- 55.Wilkerson G, London ED. Effects of methylenedioxymethamphetamine on local cerebral glucose utilization in the rat. Neuropharmacology. 1989;28(10):1129–1138. doi: 10.1016/0028-3908(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 56.Marietta CA, Wixon HN, Weight FF, Eckardt MJ. Cerebral glucose utilization in rat brain during phenobarbital withdrawal. Brain Res. 1989;496(1–2):173–179. doi: 10.1016/0006-8993(89)91063-9. [DOI] [PubMed] [Google Scholar]
- 57.Walsh SL, Gilson SF, Jasinski DR, Stapleton JM, Phillips RL, Dannals RF, et al. Buprenorphine reduces cerebral glucose metabolism in polydrug abusers. Neuropsychopharmacology. 1994 May;10(3):157–170. doi: 10.1038/npp.1994.18. [DOI] [PubMed] [Google Scholar]
- 58.Firestone LL, Gyulai F, Mintun M, Adler LJ, Urso K, Winter PM. Human brain activity response to fentanyl imaged by positron emission tomography. Anesthesia and analgesia. 1996 Jun;82(6):1247–1251. doi: 10.1097/00000539-199606000-00025. [DOI] [PubMed] [Google Scholar]
- 59.O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002 Jun;26(6):802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 60.Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006 May;129:1096–1112. doi: 10.1093/brain/awl064. [Article] [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, et al. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Research. 1988;24(2):201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 62.Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. 1990 Apr;35(1):39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 63.Ingvar M, Ghatan PH, Wirsen-Meurling A, Risberg J, Von Heijne G, Stone-Elander S, et al. Alcohol activates the cerebral reward system in man. J Stud Alcohol. 1998 May;59(3):258–269. doi: 10.15288/jsa.1998.59.258. [DOI] [PubMed] [Google Scholar]
- 64.Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. The American journal of psychiatry. 2002;159(8):1322–1328. doi: 10.1176/appi.ajp.159.8.1322. [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003 Dec 10;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003 May;28(5):967–973. doi: 10.1038/sj.npp.1300110. [DOI] [PubMed] [Google Scholar]
- 67.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001 Apr;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 69.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002 Mar;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 70.Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31(6):1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- 71.Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. The European journal of neuroscience. 1999 Mar;11(3):1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 72.Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. The American journal of psychiatry. 2001 Jul;158(7):1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 73.Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life sciences. 1999;64(9):775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 74.O'Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55(2):303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- 75.Molinari HH, Maisonneuve IM, Glick SD. Ibogaine neurotoxicity: a re-evaluation. Brain Res. 1996;737(1–2):255–262. doi: 10.1016/0006-8993(96)00739-1. [DOI] [PubMed] [Google Scholar]
- 76.Schubiner H, Tzelepis A, Milberger S, Lockhart N, Kruger M, Kelley BJ, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61(4):244–251. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- 77.Wilens TE, Biederman J, Mick E. Does ADHD affect the course of substance abuse? Findings from a sample of adults with and without ADHD. Am J Addict. 1998;7(2):156–163. [PubMed] [Google Scholar]
- 78.Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis. 1997;185(8):475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, et al. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50(4):1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- 80.Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, et al. Quantitative brain magnetic resonance imaging in girls with attention- deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58(3):289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 81.Nixon PF. Glutamate export at the choroid plexus in health, thiamin deficiency, and ethanol intoxication: review and hypothesis. Alcoholism: Clinical & Experimental Research. 2008;32(8):1339–1349. doi: 10.1111/j.1530-0277.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 82.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008 Feb 1;39(3):1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuckit MA, Smith TL. Level of response (LR) to alcohol as a phenotype related to the alcoholism risk. Alcoholism (NY). [Meeting Abstract] 2006 Sep;30(9) 123A-A. [Google Scholar]
- 84.Toga AW, Holmes C. High-resolution cerebellar anatomy. In: Manto MU, Pandolfo M, editors. The Cerebellum and its Disorders. Cambridge: Cambridge University Press; 2002. pp. 30–37. [Google Scholar]
- 85.Courchesne E, Plante E. Measurement and analysis issues in neurodevelopmental magnetic resonance imaging. In: Thatcher RW, Lyon GR, Rumsey J, Krasnegor N, editors. Developmental Neuroimaging: Mapping the Development of Brain and Behavior. San Diego: Academic Press; 1996. [Google Scholar]
- 86.Sobell LC, Sobell MB. Timeline folowback: A technique for assessing self-reported alcohol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and biological methods. Totowa, NJ: Humana; 1992. pp. 41–72. [Google Scholar]
- 87.Straus MA, Kantor GK. Corporal punishment of adolescents by parents: a risk factor in the epidemiology of depression, suicide, alcohol abuse, child abuse, and wife beating. Adolescence. 1994;29(115):543–561. [PubMed] [Google Scholar]
- 88.Simpson TL, Miller WR. Concomitance between childhood sexual and physical abuse and substance use problems. A review. Clin Psychol Rev. 2002 Feb;22(1):27–77. doi: 10.1016/s0272-7358(00)00088-x. [DOI] [PubMed] [Google Scholar]


