Abstract
Between 2005 and 2008, we conducted separate phase II clinical testing of 3 distinct anti-VEGF therapies for patients with relapsed/refractory CLL. Collectively, 46 patients were accrued to trials of single-agent anti-VEGF antibody (bevacizumab, n=13) or 1 of 2 receptor tyrosine kinase inhibitors (AZD2171, n=15; sunitinib malate, n=18). All patients have completed treatment. Patients received a median of 2 cycles of bevacizumab, AZD2171, or sunitinib malate. All 3 trials were closed early due to lack of efficacy. No complete or partial remissions were observed. Individually and collectively, these studies indicate single-agent anti-VEGF therapy has minimal clinical activity for patients with relapsed/refractory CLL.
Keywords: chronic lymphocytic leukemia, angiogenesis, vascular endothelial growth factor (VEGF), therapy, bevacizumab, receptor tyrosine kinase inhibitor
INTRODUCTION
Despite significant improvement in response rates and progression-free survival with chemoimmunotherapy approaches, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) is uncurable with conventional treatment and novel non-cross resistant agents are needed. Angiogenesis and signaling via angiogenic cytokines have increasingly been recognized as an important process in the growth of both solid tumors[1] and hematologic malignancies[2] including CLL[3]. Early work in CLL demonstrated the CLL B-cell synthesizes and secretes pro-angiogenic molecules.[4] In addition, bone marrow microvessel density, a marker of angiogenesis, correlates with CLL disease stage[5,6] and identifies patients with a shorter progression free survival.[7] Other reports also suggest serum and urine levels of pro-angiogenic factors are increased in CLL.[8]
Based on this work, several preliminary investigations have explored the relationship between vascular endothelial growth factor (VEGF) and survival in CLL. VEGF is a physiologic stimulator of angiogenesis and is also believed to be a significant mediator of tumor angiogenesis.[8] CLL B-cells express VEGF receptors (R1 and R2)[9–11], and these receptors are constitutively phosphorylated.[12] Culture of CLL B-cells with exogenous VEGF is associated with increased levels of the anti-apoptotic proteins MCL1 and XIAP as well as a reduction in both spontaneous and drug induced apoptosis.[12,13] Furthermore, inhibition of VEGF receptor signaling with receptor tyrosine kinase (RTK) inhibitors in vitro decreases levels of the anti-apoptotic proteins MCL-1 and XIAP[12] and blocks both the cytoprotective effect of the CD40-CD40 ligand interaction as well as CD40-CD40 ligand induced increases in survivin and NF-kB.[14] VEGF has also been implicated in CLL B-cell migration [15,16] and can modulate expression of B-cell receptor signaling through effects on protein kinase C (beta) II.[17] In addition clinical studies found that patients with early stage CLL who had higher serum VEGF levels had significantly shorter progression free survival[8] and that pre-treatment plasma VEGF levels were associated with response to chemoimmunotherapy treatment in CLL patients.[18]
In aggregate, these results suggest that signaling via the VEGF pathway may be an important process in the pathogenesis of CLL and could provide an important therapeutic target for patients with this disease. This postulate also builds on the clinical trial data in solid tumors where anti-VEGF therapy has already been found to improve clinical outcome in patients with colon, lung, and renal cell carcinoma.[19–21] To test the efficacy of anti-VEGF therapy in CLL patients, we initiated and completed separate phase II clinical testing of 3 different anti-VEGF therapies for patients with relapsed/refractory CLL: AZD2171 (a potent, oral, pan VEGF receptor inhibitor), bevacizumab (a recombinant humanized monoclonal antibody to VEGF) and sunitinib malate (a multi-targeted, small molecule inhibitor of RTKs involved in tumor proliferation and angiogenesis including VEGFR-1, VEGFR-2, VEGFR-3, and platelet-derived growth factor receptor [PDGFR]).
MATERIALS and METHODS
We performed separate phase II trials evaluating the safety and efficacy of three distinct anti-VEGF or RTK inhibitor therapies each targeting a different aspect of VEGF receptor signaling (Figure. 1). Each trial was registered with the National Cancer Institute at clinicaltrials.gov (NCT00290810; NCT00398112; NCT00321724). All patients were required to have a confirmed diagnosis of CLL or SLL by standard criteria.[22,23] All three trials accrued relapsed or refractory CLL patients with an indication for treatment as defined by marrow failure, measurable and progressive lymphadenopathy, measurable and progressive lymphocytosis, and/or clinically significant constitutional symptoms due to CLL. All patients had received prior treatment (the AZD2171 trial required prior purine nucleoside analogue treatment; the trials of sunitinib malate and bevacizumab trials required prior purine nucleoside analogues and/or alkylating agent treatment). Mantle cell lymphoma was excluded in all patients by fluorescence in situ hybridization (FISH) assessment for the presence of the t(11;14) or immunophenotypic analysis (CD23 expression and intensity of surface immunoglobulin expression).
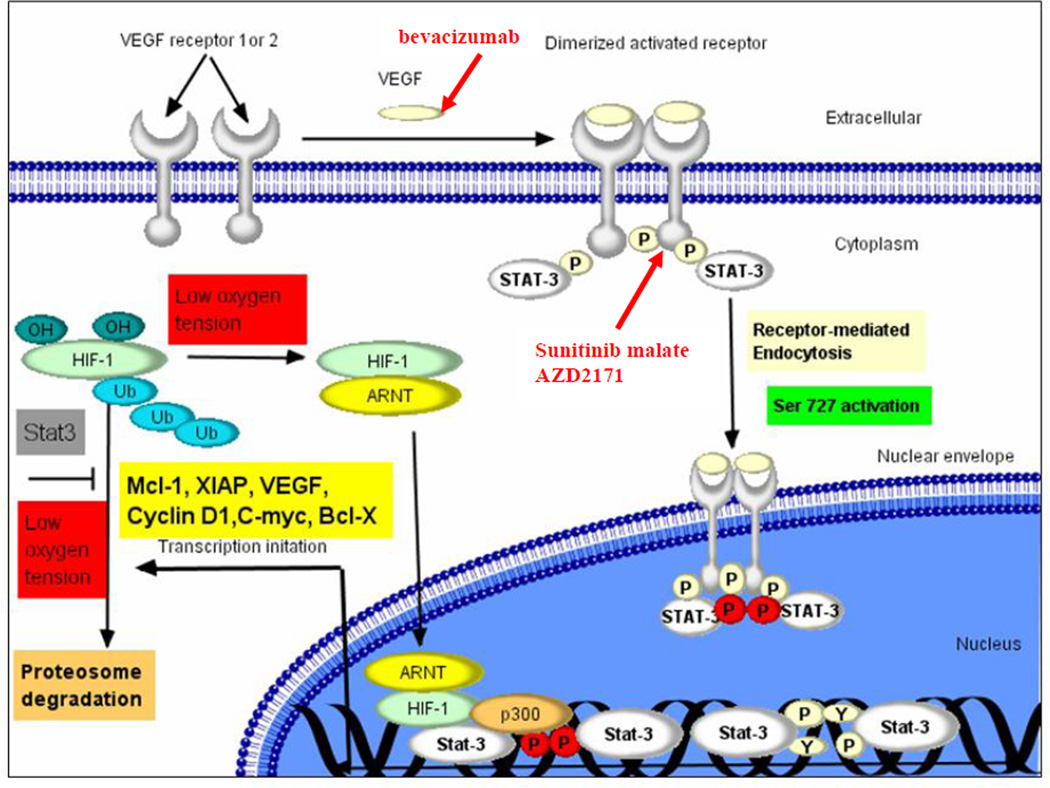
Figure 1. VEGF Pathway in CLL.
Figure shows scheme of VEGF receptor signaling pathway in CLL as well as aspects of pathway targeted by bevacizumab, AZD2171, and sunitinib malate.
The eligibility criteria of all three trials required participants to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 along with adequate renal and hepatic function. Bone marrow biopsy and baseline echocardiogram were required at study entry in all 3 trials. Concurrent chemotherapy, immunotherapy, radiotherapy, or steroid treatment was not allowed and patients who had received recent chemotherapy (< 4–6 weeks), antibody (4–8 weeks), or other experimental therapy (< 4 weeks) were not eligible. Patients with uncontrolled hypertension, significant proteinuria, severe thrombocytopenia, recent myocardial infarction or stroke were excluded from all 3 studies. Patients with QTc prolongation (> 500 msec), other arrhythmia, or on potentially pro-arrhythmic drugs were excluded from the trials of AZD2171 and sunitinib malate. Patients with recent gastrointestinal fistula, gastrointestinal perforation, a serious non-healing wound, ulcer or bone fracture were explicitly excluded from the trials of sunitinib malate and bevacizumab. Patients with known bleeding diathesis or with pathologic conditions carrying a high risk for bleeding (e.g. varices) were excluded from the bevacizumab trial, while those with pulmonary embolus in the last 12 months were excluded from the sunitinib malate trial. Those who had received prior anti-VEGF therapy were explicitly excluded from the sunitinib malate trial.
All three protocols were reviewed and approved by the Mayo Clinic Institutional Review Board and registered with the National Institute of Health (clinicaltrials.gov). All patients provided written informed consent prior to study enrollment in accordance with the Declaration of Helsinki. Toxicity was graded using NCI Common Terminology Criteria for Adverse Events version 3.0, except for hemoglobin and platelets which were graded according to the Grading Scale for Hematological Toxicity in CLL Studies.[22] Response was evaluated using the NCI-WG criteria.[22]
PROTOCOL TREATMENT FOR TRIAL 1: AZD2171
Protocol 1 was a multi-center phase II trial of AZD2171 conducted in the North Central Cancer Treatment Group (NCCTG). Patients received AZD2171 45 mg orally once daily on days 1–28 as part of 28 day cycles. Patients were assessed by physical examination, CBC, and chemistries prior to each cycle. Blood pressure (BP) was assessed prior to each cycle as well as assessed by patients twice daily using a home BP device with results recorded in a BP diary. Urinalysis evaluating for proteinuria was assessed prior to each cycle. Patients with significant proteinuria underwent 24-hour urine protein analysis. ECG was obtained at baseline and prior to each cycle to evaluate QTc prolongation. The dose of AZD2171 was reduced to 30 mg daily (dose level minus 1), 20 mg daily (dose level minus 2), or 10 mg daily (dose level minus 3) as indicated by adverse events. In general, for grade 3 adverse events AZD2171 was reduced by 1 dose level while for grade 4 adverse events AZD2171 was held and restarted at next lower dose level when symptoms had resolved to ≤ grade 2. Blood samples were obtained for research purposes at study entry, after every other cycle, and at time of progression. Patients continued on treatment until they experienced disease progression or prohibitive toxicity.
PROTOCOL TREATMENT FOR TRIAL 2: BEVACIZUMAB
Protocol 2 was a multi-center phase II trial of bevacizumab conducted through the Mayo Clinic and Ohio State University Phase II Consortia. Patients received bevacizumab 10 mg/kg intravenously, every 14 days (+/− 3 days) as part of 28 day cycles. Patients were assessed by physical examination, CBC, and chemistries prior to each cycle. BP and urine protein:creatinine ratio were assessed prior to each dose. Patients who developed a urine protein:creatinine >3 underwent 24-hour urine protein analysis. Bevacizumab was held in patients with grade 3 proteinuria. Bevacizumab was discontinued in patients with grade 4 proteinuria or those with bowel perforation, wound dehiscence, or arterial thromboembolic events (any grade). Blood samples were obtained for correlative research purposes at study entry and prior to each cycle. Patients continued on treatment provided they did not experience disease progression or prohibitive toxicity.
PROTOCOL TREATMENT FOR TRIAL 3: SUNITINIB MALATE
Protocol 3 was a multi-center phase II trial of sunitinib malate conducted in the NCCTG. Patients received sunitinib malate 37.5 mg orally once daily on days 1–28 as part of 28 day cycles. Patients were assessed by physical examination, CBC, and chemistries prior to each cycle. BP was assessed weekly during cycle 1 and every 2 weeks during cycles 2–12. ECG was obtained at baseline and prior to cycle 2 to evaluate QTc prolongation. The dose of sunitinib malate was reduced to 25 mg daily (dose level minus 1) or 12.5 mg daily (dose level minus 2) as indicated by adverse events. In general, sunitinib malate was held for ≥ grade 3 adverse events and restarted at the next lower dose level when symptoms had resolved to ≤ grade 1. Blood samples were obtained for correlative research purposes at study entry, after 8 weeks of treatment and at the time study treatments were discontinued. Patients continued on treatment for up to 12 cycles unless they experienced disease progression or prohibitive toxicity.
PLASMA LEVELS OF ANGIOGENIC CYTOKINES
To test the effect of anti-VEGF therapies on plasma levels of VEGF, we measured VEGF prior to treatment and after 8 weeks of therapy (for patients still on active treatment). VEGF (isoform 165) was measured using Quantikine kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions as previously described.[18]
STATISTICAL ANALYSIS
Response to treatment based on intent to treat analysis was the primary outcome for all three trials. Confirmed clinical responses (NCI-WG complete or partial remission[22,24]) on 2 consecutive evaluations at least 4 weeks apart were summarized by simple descriptive statistics. The same one-stage design with an interim analysis was used for each trial independently. A total of 32 evaluable patients were required to test the null hypothesis that the true response rate is at most 5% vs. the alternative hypothesis that it is at least 20%. Assuming that the number of responses is binomially distributed, each study had 90% power, with a 7% Type I error rate. In all 3 trials, an interim analysis was planned/conducted after 16 evaluable patients were enrolled, where at least one response was required to continue accrual. A patient was considered evaluable for response if they were eligible and received treatment. Progression-free survival (PFS) was defined as the time from registration to progression or death due to any cause. The distribution of PFS was estimated using the Kaplan-Meier method.
RESULTS
PATIENT DEMOGRAPHICS
Fifteen patients were accrued to the AZD2171 trial between May 2006 and June 2007, 13 patients to the bevacizumab trial between December 2005 and March 2009, and 18 patients to the trial of sunitinib malate between August 2007 and December 2008. One patient was deemed ineligible for the AZD2171 trial after receiving 1 cycle or treatment because he/she had not received prior purine nucleoside analogue treatment. One patient on the bevacizumab trial never received protocol treatment due to a high protein/creatinine ratio and high 24-hour urine protein excretion. Accordingly 14, 12, and 18 eligible patients were included in the outcome analysis for the AZD2171, bevacizumab, and sunitinib malate trials, respectively. Patient characteristics for the 3 trials are shown in Table 1.
Table 1.
Patient Characteristics
| AZD2171 N=14 |
Bevacizumab N=12 |
Sunitinib malate N=18 |
||
|---|---|---|---|---|
| DEMOGRAPHIC CHARACTERISTICS | ||||
| AGE (Median; range) | 64.5 (44.0–77.0) | 73.0 (60.0–80.0) | 67.5 (51.0–89.0) | |
| MALE | 10 (71.4%) | 5 (41.7%) | 9 (50%) | |
| ALC (Median; range) | 46.5 (8.3–225.1) | 125 (9.3–760.9) | 35.1 (0.4–134.7) | |
| STAGE | ||||
| Low (Rai 0) | 0 | 0 | 0 | |
| Intermediate (Rai I–II) | 6 (42.9%) | 2 (16.6%) | 8 (44.4%) | |
| High (Rai III–IV) | 8 (57.1%) | 10 (83.3%) | 10 (55.6%) | |
| ZAP-70 | ||||
| Positive | 7 (50%) | 5 (41.7%) | 10 (55.6%) | |
| Negative | 7 (50%) | 7 (58.3%) | 8 (44.4%) | |
| CD38 | ||||
| Positive | 10 (71.4%) | 3 (25%) | 9 (50%) | |
| Negative | 4 (28.6%) | 9 (75%) | 9 (50%) | |
| FISH | ||||
| Del (17p13) | 0 (0%) | 4 (33.3%) | 6 (33.3%) | |
| Del (11q23) | 6 (46.2%) | 2 (16.7%) | 1 (5.6%) | |
| Trisomy 12 | 1 (7.7%) | 2 (16.7%) | 5 (27.8%) | |
| Del 13q14 | 3 (23.1%) | 2 (16.7%) | 2 (11.1%) | |
| Other | 1 (7.7%) | 1 (8.3%) | 0 (0%) | |
| Normal | 2 (15.4%) | 1 (8.3%) | 4 (22.2%) | |
| Missing | 1 | 0 | 0 | |
| IGHV | ||||
| Unmutated | 3 (75%) | 3 (50%) | 1 (100%) | |
| Mutated | 1 (25%) | 3 (50%) | 0 | |
| Missing | 10 | 6 | 17 | |
| PRIOR TREATMENT: | ||||
| # prior therapies (Median; range) | 2.0 (1.0–10.0) | 3.0 (1.0–10.0) | 2.5 (1.0–7.0) | |
| Prior purine analogue treatment | 14 (100%) | 10 (83%) | 14 (78%) | |
| Prior alkylating agent treatment | 13 (93%) | 10 (83%) | 17 (94%) | |
| Prior rituximab | 13 (93%) | 12 (100%) | 15 (83%) | |
| Prior alemtuzumab | 2 (14%) | 1 (8%) | 3 (17%) | |
| Time (days) from last prior treatment (Median; range) | 365.5 (39–1085) | 249.5 (47–1410) | 386.5 (62–1816) | |
The results of prognostic testing with ZAP-70, IGHV mutation status, CD38, and cytogenetic analysis by FISH generally demonstrated biologically aggressive disease typical of patients with relapsed/refractory CLL. Thus, with respect to high risk cytogenetic abnormalities, 39–50% of patients on the 3 trials had deletion 17p13 and/or deletion 11q23. Patients in all three trials had received a median of ≥2 prior therapies (range 1–10) and nearly all patients in each of the three trials had received prior purine nucleoside analogue based therapy and/or alkylating agent-based therapy.
TOXICITY AND TOLERABILITY
At the time of this report, all patients in all 3 trials have completed active treatment. Overall, 10 (71%) patients in the AZD2171 trial, 4 (33%) in the bevacizumab trial, and 16 (89%) in the sunitinib malate trial experienced a grade 3 or higher adverse event attributed to study medication. The frequency of specific side effects attributed to study therapy differed for the 3 agents tested and are shown in Table 2. In the AZD2171 trial, the most frequent grade ≥ 3 adverse events were thrombocytopenia (5/14 patients), fatigue (5/14 patients), diarrhea (3/14 patients), muscle weakness (3/14 patients) and hypertension (3/14 patients). In the bevacizumab trial, the most frequent grade ≥3 adverse events were proteinuria (2/12 patients) and fatigue (2/12 patients). In the sunitinib malate trial, the most frequent grade ≥ 3 adverse events were thrombocytopenia (10/18 patients), fatigue (6/18 patients), neutropenia (5/18 patients), and anorexia (4/18 patients).
Table 2.
Grade 3–4 Adverse Events Attributed to Study Treatment
| CTCAE Classification | AZD2171 N=14 |
Bevacizumab N=12 |
Sunitinib malate N=18 |
|---|---|---|---|
| Platelet Count Decreased | 5 | 0 | 10 |
| Fatigue | 5 | 2 | 6 |
| Neutrophil Count Decreased | 2 | 0 | 5 |
| Anorexia | 1 | 0 | 4 |
| Diarrhea-No Colostomy | 3 | 0 | 2 |
| Headache | 2 | 1 | 1 |
| Hypertension | 3 | 0 | 0 |
| Muscle Weakness | 3 | 0 | 0 |
| Hyperkalemia | 1 | 0 | 1 |
| Nausea | 2 | 0 | 0 |
| Proteinuria | 0 | 2 | 0 |
| Clostridial Infection | 0 | 0 | 1 |
| Dehydration | 0 | 0 | 1 |
| Dyspnea | 0 | 1 | 0 |
| Edema Limbs | 0 | 0 | 1 |
| Hyponatremia | 1 | 0 | 0 |
| Hypothyroidism | 1 | 0 | 0 |
| Neuro | 1 | 0 | 0 |
| Petechiae | 1 | 0 | 0 |
| Rash | 0 | 0 | 1 |
| Rectum Infection | 0 | 0 | 1 |
| Skin Reaction-Hand/Foot | 0 | 0 | 1 |
| Urinary Tract Infection | 1 | 0 | 0 |
| Vision | 0 | 1 | 0 |
| Voice Change | 1 | 0 | 0 |
Patients received a median of 2 cycles of AZD2171, 2 cycles of bevacizumab, and 2 cycles of sunitinib malate. In the AZD2171 trial, 5 patients discontinued treatment due to adverse events (grade 4 fatigue; grade 3 infection and fatigue; grade 3 peripheral neuropathy of the hands; grade 3 hypertension; grade 3 reversible posterior leukoencephalopathy syndrome), 7 due to disease progression, 1 due to patient refusal, and 1 at their physician’s discretion. In the bevacizumab trial, 3 patients discontinued treatment due to an adverse event (grade 2 hypertension; potential retinal detachment and subsequently grade 3 fatigue; worsening performance status and grade 3 fatigue), 7 due to disease progression, 1 due to patient refusal, and 1 at their physician’s discretion. In the sunitinib malate trial, 4 patients discontinued treatment due to an adverse event (low platelets; grade 3 rash; neutropenia; grade 3 platelets), 9 progressed, 1 received alternative treatment (rituxan and fludarabine), 3 due to patient refusal, and 1 died on study.
RESPONSE TO THERAPY
All three trials were closed early due to lack of efficacy. No complete or partial remissions were observed (Table 3). Although no complete or partial responses were obtained, 5/14 patients on AZD2171, 10/12 patients on bevacizumab, 10/18 patients on sunitinib had stabilization of disease for a median duration of 2.7, 2.9, and 4.4 months respectively. The ALC values declined by at least 10% during treatment for 5/14 patients on AZD2171, 3/12 patients on bevacizumab, and 6/18 patients on sunitinib malate.
Table 3.
Number of Cycles Received and Response to Treatment
| AZD2171 N=14 |
Bevacizumab N=12 |
Sunitinib malate N=18 |
||
|---|---|---|---|---|
| # Cycles administered (median) | 2 | 2 | 2 | |
| BEST RESPONSE | ||||
| Complete remission | 0 | 0 | 0 | |
| Partial remission | 0 | 0 | 0 | |
| Stable disease | 5 | 10 | 10 | |
| Progression | 6 | 2 | 6 | |
| PROGRESSION FREE SURVIVAL (median) | 1.8 months (95% CI: 1.0–3.4) | 2.9 months (95% CI: 1.8–3.7) | 2.7 months (95% CI: 1.8–4.6) | |
CORRELATIVE STUDIES
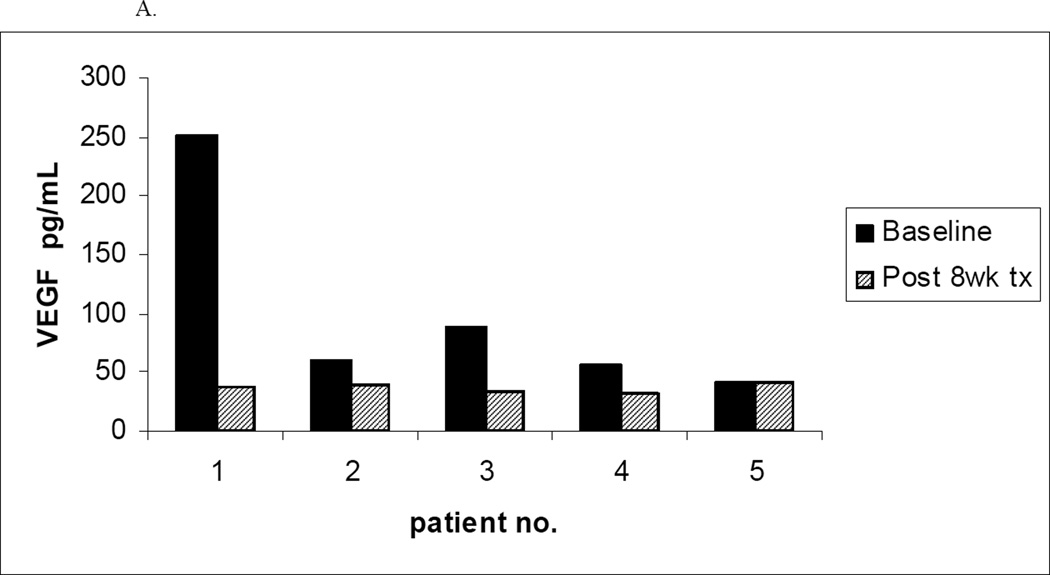
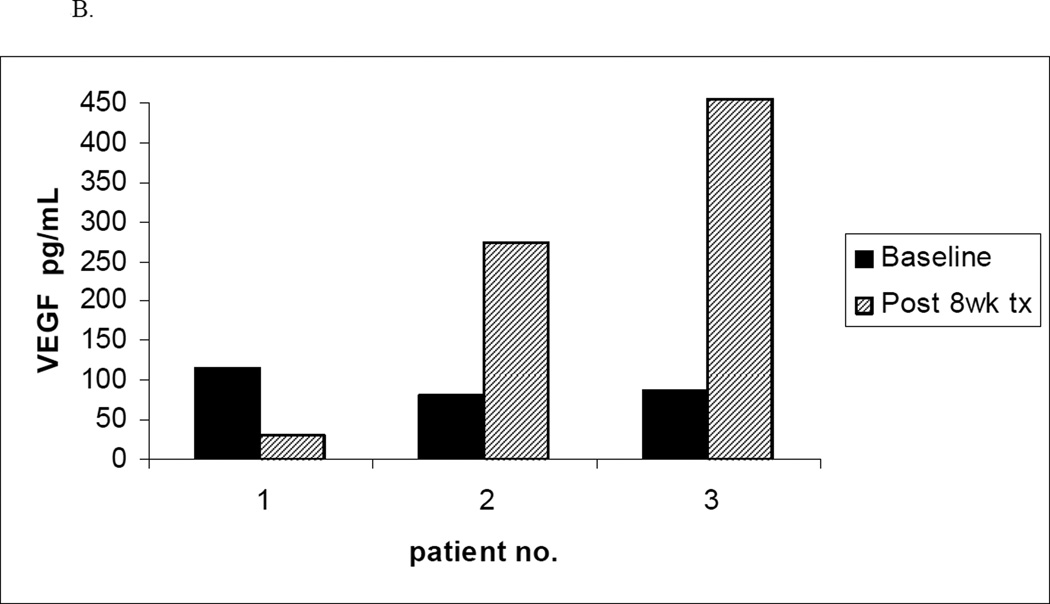
To evaluate the effect of treatment on plasma levels of VEGF at baseline and 8 week follow-up, plasma samples were collected from patients on the trials of bevacizumab and sunitinib malate. Unfortunately, since few patients completed at least 8 weeks of active treatment, baseline and 8 week follow-up serum specimens while still on active treatment were only available for 5 patients on the bevacizumab trial and 3 patients on the trial of sunitinib malate. A decline in plasma VEGF levels after 8 weeks of treatment was observed in 4 of the 5 patients on the bevacizumab trial as compared to 1 of the 3 patients on the sunitinib malate trial (Figure 2). Leukemia cell specimens intended to allow assessment of VEGF receptor phosphorylation status were also collected and cryopreserved at baseline and after 8 weeks of therapy in all 3 trials. However, on thawing of cells to make lysates, the high level of spontaneous cell death precluded meaningful analysis.
Figure 2. Plasma VEGF Levels Before and After 8 Weeks of Treatment.
A. Plasma levels of VEGF before and after 8 weeks of bevacizumab therapy (n=5)
B. Plasma levels of VEGF before and after 8 weeks of sunitinib therapy (n=3)
DISCUSSION
We report here the first 3 trials evaluating anti-VEGF therapy for patients with CLL. The VEGF pathway was inhibited using either anti-VEGF anti-bodies or by blocking VEGF receptor tyrosine kinase. Despite the strong biologic basis for testing anti-VEGF therapy in patients with CLL, minimal clinical activity (stabilization of disease; declines in ALC) was observed and all 3 trials were closed early due to lack of efficacy. Individually and collectively, the results of the 3 studies show that single agent anti-VEGF therapy has minimal clinical efficacy for patients with relapsed/refractory CLL.
These findings should be interpreted with a number of important caveats. First, all 3 trials enrolled heavily pretreated CLL patients with a high proportion of patients having del 17p13 and del 11q23, a very difficult group of patients to treat. Second, although single agent anti-VEGF therapy has clear clinical activity in some human malignancies, [21,25–28] in others the benefits are primarily observed when anti-VEGF therapy is combined with chemotherapy.[19,20] In this regard, the anti-VEGF therapies tested were generally well tolerated and combination with other CLL treatment approaches appears feasible. Since pre-treatment VEGF levels have been found to predict response to chemoimmunotherapy treatment in CLL patients,[18] and to upregulate anti-apoptotic proteins known to relate to chemotherapy resistance[12,13] testing of anti-VEGF therapy in combination with chemotherapy appears warranted. Third, the lack of efficacy in these 3 trials could reflect either i) failure to inhibit the intended biologic target (VEGF signaling) or ii) effective targeting but a lack of biologic effect. Although the doses of bevacizumab and AZD2171 tested were consistent with the established doses for treatment of solid tumors, we were unable to definitively determine whether the doses used effectively inhibited the VEGF pathway in the limited correlative studies performed because too few patients were still on active therapy at the time the follow-up samples were collected. The dose of sunitinib malate (37.5 mg daily, continuous) was slightly different from the standard dose in solid tumors (50 mg daily for 4 weeks followed by 2 weeks off); however, this was the dose recommended by the NCI for testing in hematologic malignancy at the time the trial was initiated and the total dose over a 6 week interval (daily, continuous: 1575 mg; 4 weeks on/2 weeks off: 1400 mg) is similar in both schedules. Fourth, aberrant angiogenesis in human malignancy is now recognized as a multi-step process that is regulated at least by a complex balance of multiple pro and anti-angiogenic molecules[29]. It is possible that targeting VEGF signaling alone may be inadequate to kill CLL cells.
Despite the lack of clinical activity observed in these trials, scientific insights continue to indicate that VEGF and other related angiogenic events play a role in CLL biology.[3] These include recent studies indicating that marrow vascular density is significantly higher in CLL patients with high risk FISH and CD38 positivity,[30] a pro-angiogenic profile favors disease progression,[31] circulating endothelial cells correlate with more advanced disease stage,[32] pro-angiogenic molecules such as angiopoietin-2 and matrix metalloproteinase 9 are associated with progressive CLL,[33,34] and that use of combination chemoimmunotherapy may work in part via anti-angiogenic effects.[35] Newer VEGF receptor RTK inhibitors have also recently demonstrated activity against CLL B-cells in vitro as well as in a xenograft model and appear to increase the efficacy of purine nucleoside analogues against CLL on in vitro testing.[36] These observations suggest VEGF inhibition remains a potential therapeutic target in CLL and suggest combining anti-VEGF therapy with more traditional therapeutic agents may be a useful strategy for patients with this disease. Indeed we and others have already initiated clinical trials exploring the benefits of this approach as part of efforts to improve outcomes for patients with CLL.
Acknowledgment
Role of the funding source:
Support through grants from the National Institutes of Health (NIH), National Cancer Institute (NCI) CA113408, CA116237, N01-CM62205; CA25224; CA114740, CA15083, N01-CM62207 the Commonwealth Foundation for Cancer Research, and Polyphenon E International are gratefully acknowledged. Study sponsors had no input in the study design, collection/analysis/interpretation of data, writing of the manuscript; or decision to submit the manuscript for publication.
Footnotes
DECLARATION OF INTERESTS
Conflict of Interest:
The authors have no conflicts of interest to disclose. Mayo Clinic filed a provisional patent on EGCG in combination with chemotherapy for treatment of CLL based on the work presented here.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Vacca A, Ribatti D, Ruco L, Giacchetta F, Nico B, Quondamatteo F, Ria R, Iurlaro M, Dammacco F. Angiogenesis extent and macrophage density increase simultaneously with pathological progression in B-cell non-Hodgkin's lymphomas. Br J Cancer. 1999;79:965–970. doi: 10.1038/sj.bjc.6690154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Kay NE. The clinical and biologic importance of neovascularization and angiogenic signaling pathways in chronic lymphocytic leukemia. Semin Oncol. 2006;33:174–185. doi: 10.1053/j.seminoncol.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Kay NE, Bone ND, Tschumper RC, Howell KH, Geyer SM, Dewald GW, Hanson CA, Jelinek DF. B-CLL cells are capable of synthesis and secretion of both pro- and anti-angiogenic molecules. Leukemia. 2002;16:911–919. doi: 10.1038/sj.leu.2402467. [DOI] [PubMed] [Google Scholar]
- 5.Kini AR, Kay NE, Peterson LC. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia. 2000;14:1414–1418. doi: 10.1038/sj.leu.2401825. [DOI] [PubMed] [Google Scholar]
- 6.Szmigielska-Kaplon A, Lech-Maranda E, Jesionek-Kupnicka D, Gora-Tybor J, Blonski JZ, Kasznicki M, Kordek R, Robak T. Prognostic value of the bone marrow microvessel density in progressive B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 51:1351–1353. doi: 10.3109/10428194.2010.486092. [DOI] [PubMed] [Google Scholar]
- 7.Molica S, Vacca A, Ribatti D, Cuneo A, Cavazzini F, Levato D, Vitelli G, Tucci L, Roccaro AM, Dammacco F. Prognostic value of enhanced bone marrow angiogenesis in early B-cell chronic lymphocytic leukemia. Blood. 2002;100:3344–3351. doi: 10.1182/blood-2002-01-0084. [DOI] [PubMed] [Google Scholar]
- 8.Molica S, Vitelli G, Levato D, Gandolfo GM, Liso V. Increased serum levels of vascular endothelial growth factor predict risk of progression in early B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;107:605–610. doi: 10.1046/j.1365-2141.1999.01752.x. [DOI] [PubMed] [Google Scholar]
- 9.Aguayo A, Manshouri T, O'Brien S, Keating M, Beran M, Koller C, Kantarjian H, Rogers A, Albitar M. Clinical relevance of Flt1 and Tie1 angiogenesis receptors expression in B-cell chronic lymphocytic leukemia (CLL) Leuk Res. 2001;25:279–285. doi: 10.1016/s0145-2126(00)00139-9. [DOI] [PubMed] [Google Scholar]
- 10.Bairey O, Boycov O, Kaganovsky E, Zimra Y, Shaklai M, Rabizadeh E. All three receptors for vascular endothelial growth factor (VEGF) are expressed on B-chronic lymphocytic leukemia (CLL) cells. Leuk Res. 2004;28:243–248. doi: 10.1016/s0145-2126(03)00256-x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrajoli A, Manshouri T, Estrov Z, Keating MJ, O'Brien S, Lerner S, Beran M, Kantarjian HM, Freireich EJ, Albitar M. High levels of vascular endothelial growth factor receptor-2 correlate with shortened survival in chronic lymphocytic leukemia. Clin Cancer Res. 2001;7:795–799. [PubMed] [Google Scholar]
- 12.Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19:513–523. doi: 10.1038/sj.leu.2403667. [DOI] [PubMed] [Google Scholar]
- 13.Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788–794. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 14.Farahani M, Treweeke AT, Toh CH, Till KJ, Harris RJ, Cawley JC, Zuzel M, Chen H. Autocrine VEGF mediates the antiapoptotic effect of CD154 on CLL cells. Leukemia. 2005;19:524–530. doi: 10.1038/sj.leu.2403631. [DOI] [PubMed] [Google Scholar]
- 15.Till KJ, Spiller DG, Harris RJ, Chen H, Zuzel M, Cawley JC. CLL, but not normal, B cells are dependent on autocrine VEGF and alpha4beta1 integrin for chemokine-induced motility on and through endothelium. Blood. 2005;105:4813–4819. doi: 10.1182/blood-2004-10-4054. [DOI] [PubMed] [Google Scholar]
- 16.Ugarte-Berzal E, Redondo-Munoz J, Eroles P, Del Cerro MH, Garcia-Marco JA, Terol MJ, Garcia-Pardo A. VEGF/VEGFR2 interaction down-regulates matrix metalloproteinase-9 via STAT1 activation and inhibits B chronic lymphocytic leukemia cell migration. Blood. 2010;115:846–849. doi: 10.1182/blood-2009-08-239426. [DOI] [PubMed] [Google Scholar]
- 17.Abrams ST, Brown BR, Zuzel M, Slupsky JR. Vascular endothelial growth factor stimulates protein kinase CbetaII expression in chronic lymphocytic leukemia cells. Blood. 2010;115:4447–4454. doi: 10.1182/blood-2009-06-229872. [DOI] [PubMed] [Google Scholar]
- 18.Shanafelt TD, Byrd JC, La PB, Zent CS, Call T, Secreto C, Grever MR, Lin TS, Kay NE. Pretreatment angiogenic cytokines predict response to chemoimmunotherapy in patients with chronic lymphocytic leukaemia. Br J Haematol. 2009;146:660–664. doi: 10.1111/j.1365-2141.2009.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 20.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 23.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 24.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, et al. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 29.Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Leukemia. 2007;21:44–52. doi: 10.1038/sj.leu.2404402. [DOI] [PubMed] [Google Scholar]
- 30.Antic D, Jovanovic MP, Fekete MD, Cokic V. Assessment of bone marrow microvessel density in chronic lymphocytic leukemia. Appl Immunohistochem Mol Morphol. 2010;18:353–356. doi: 10.1097/PAI.0b013e3181d18ae2. [DOI] [PubMed] [Google Scholar]
- 31.Frater JL, Kay NE, Goolsby CL, Crawford SE, Dewald GW, Peterson LC. Dysregulated angiogenesis in B-chronic lymphocytic leukemia: morphologic, immunohistochemical, and flow cytometric evidence. Diagn Pathol. 2008;3:16. doi: 10.1186/1746-1596-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gora-Tybor J, Jamroziak K, Szmigielska-Kaplon A, Krawczynska A, Lech-Maranda E, Wierzbowska A, Jesionek-Kupnicka D, Blonski JZ, Robak T. Evaluation of circulating endothelial cells as noninvasive marker of angiogenesis in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:62–67. doi: 10.1080/10428190802549883. [DOI] [PubMed] [Google Scholar]
- 33.Martinelli S, Maffei R, Castelli I, Santachiara R, Zucchini P, Fontana M, Bonacorsi G, Leonardi G, Marasca R, Torelli G. Increased expression of angiopoietin-2 characterizes early B-cell chronic lymphocytic leukemia with poor prognosis. Leuk Res. 2008;32:593–597. doi: 10.1016/j.leukres.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Quiney C, Billard C, Mirshahi P, Fourneron JD, Kolb JP. Hyperforin inhibits MMP-9 secretion by B-CLL cells and microtubule formation by endothelial cells. Leukemia. 2006;20:583–589. doi: 10.1038/sj.leu.2404134. [DOI] [PubMed] [Google Scholar]
- 35.Smolej L, Andrys C, Krejsek J, Belada DZ, Zak P, Siroky O, Maly J. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are elevated in peripheral blood plasma of patients with chronic lymphocytic leukemia and decrease after intensive fludarabine-based treatment. Vnitr Lek. 2007;53:1171–1176. [PubMed] [Google Scholar]
- 36.Paesler J, Gehrke I, Gandhirajan RK, Filipovich A, Hertweck M, Erdfelder F, Uhrmacher S, Poll-Wolbeck SJ, Hallek M, Kreuzer KA. The vascular endothelial growth factor receptor tyrosine kinase inhibitors vatalanib and pazopanib potently induce apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Clin Cancer Res. 16:3390–3398. doi: 10.1158/1078-0432.CCR-10-0232. [DOI] [PubMed] [Google Scholar]