Abstract
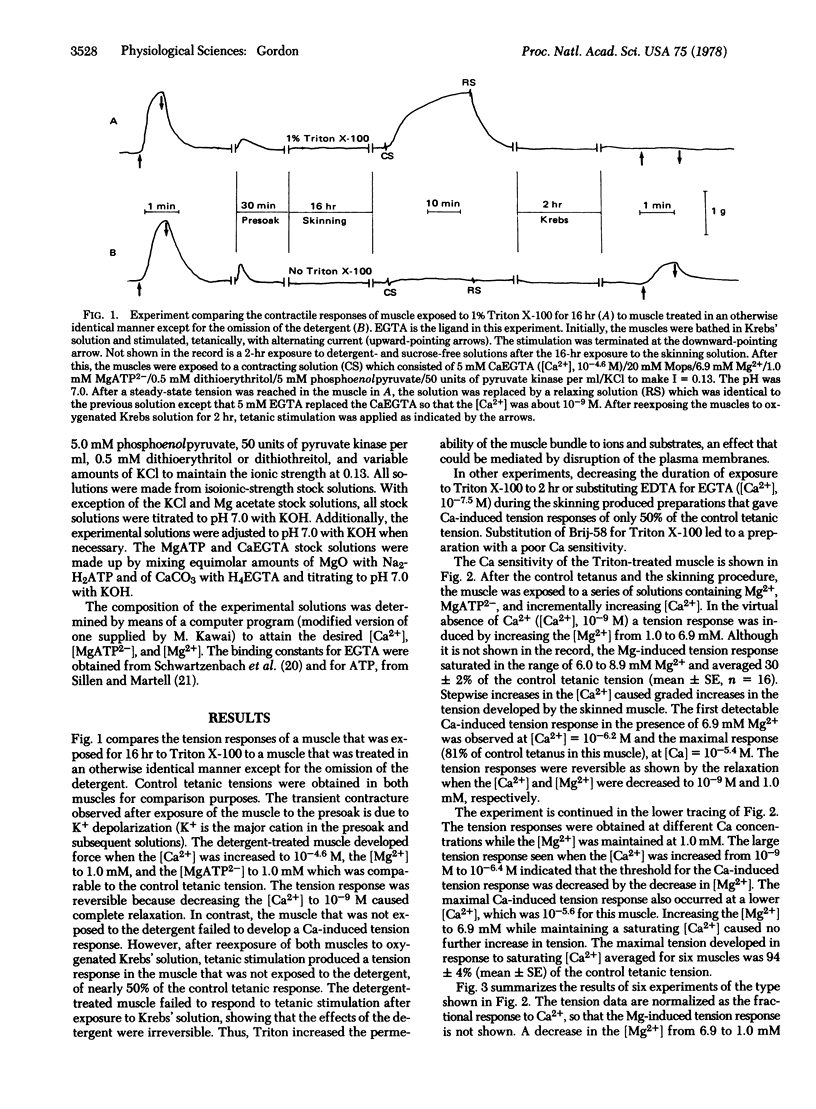
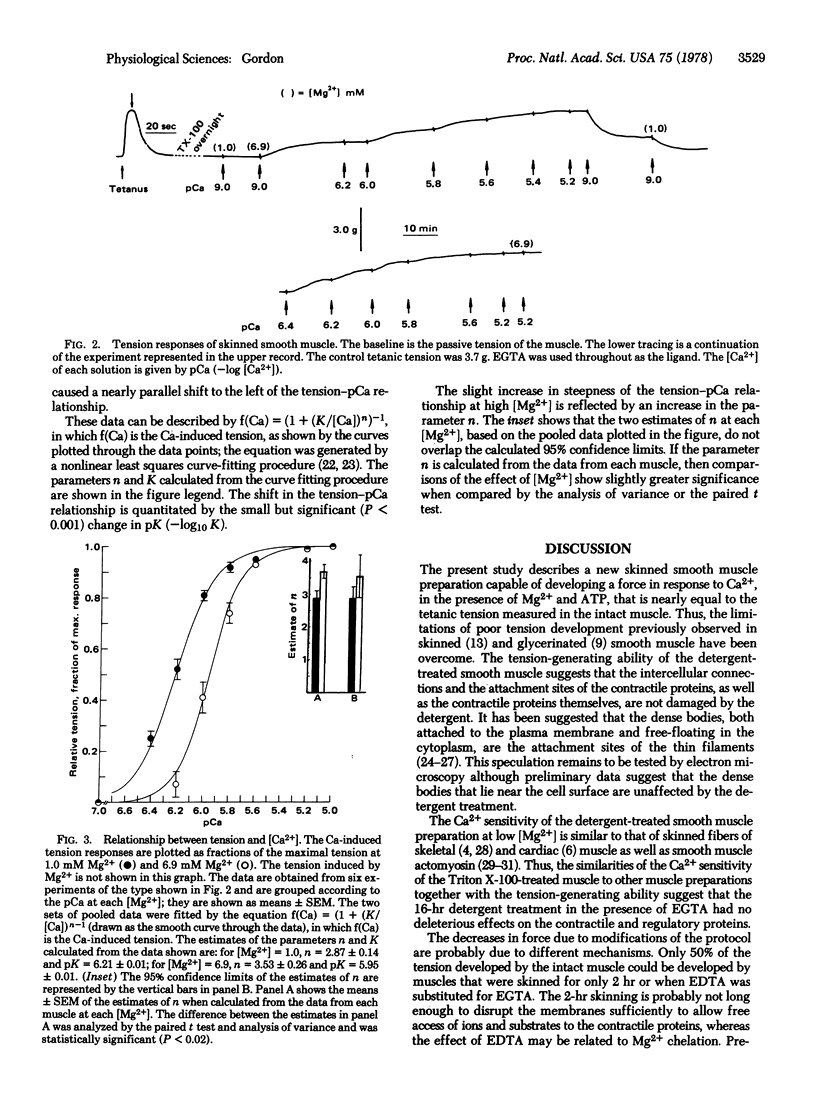
After exposure of segments of rabbit taenia coli to the nonionic detergent Triton X-100, tension could be induced by increasing the [Ca2+] in the micromolar range. In the presence of a saturating [Ca2+], this preparation developed nearly 100% of the control tetanus tension recorded from the intact muscle prior to the detergent treatment. In addition, tension could be induced by increasing the [Mg2+], in the virtual absence of Ca2+. Mg2+ seems to inhibit the Ca2+-induced tension in a predominantly competitive manner.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOZLER E. Evidence of an ATP-actomyosin complex in relaxed muscle and its response to calcium ions. Am J Physiol. 1952 Mar;168(3):760–765. doi: 10.1152/ajplegacy.1952.168.3.760. [DOI] [PubMed] [Google Scholar]
- BOZLER E. Mechanism of relaxation in extracted muscle fibers. Am J Physiol. 1951 Oct;167(1):276–283. doi: 10.1152/ajplegacy.1951.167.1.276. [DOI] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J., Davies R. E. Rigor and resistance to stretch in vertebrate smooth muscle. Am J Physiol. 1976 Nov;231(5 Pt 1):1509–1514. doi: 10.1152/ajplegacy.1976.231.5.1509. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driska S., Hartshorne D. J. The contractile proteins of smooth muscle. Properties and components of a Ca2+-sensitive actomyosin from chicken gizzard. Arch Biochem Biophys. 1975 Mar;167(1):203–212. doi: 10.1016/0003-9861(75)90457-9. [DOI] [PubMed] [Google Scholar]
- FILO R. S., BOHR D. F., RUEGG J. C. GLYCERINATED SKELETAL AND SMOOTH MUSCLE: CALCIUM AND MAGNESIUM DEPENDENCE. Science. 1965 Mar 26;147(3665):1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. R., Siegman M. J. Mechanical properties of smooth muscle. I. Length-tension and force-velocity relations. Am J Physiol. 1971 Nov;221(5):1243–1249. doi: 10.1152/ajplegacy.1971.221.5.1243. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megerman J., Murphy R. A. Myosin from arterial smooth muscle: isolation following actin depolymerization. Biochim Biophys Acta. 1975 Dec 15;412(2):241–255. doi: 10.1016/0005-2795(75)90038-0. [DOI] [PubMed] [Google Scholar]
- Morgan P. H., Mercer L. P., Flodin N. W. General model for nutritional responses of higher organisms. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4327–4331. doi: 10.1073/pnas.72.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrwa U., Achtig I., Ruegg J. C. Influences of calcium concentration and pH on the tension development and ATPase activity of the arterial actomyosin contractile system. Blood Vessels. 1974;11(5-6):277–286. doi: 10.1159/000158021. [DOI] [PubMed] [Google Scholar]
- Ne'eman Z., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. II. Solubilization and enzymic activities of Acholeplasma laidlawii membrane proteins. Biochim Biophys Acta. 1971 Oct 12;249(1):169–176. doi: 10.1016/0005-2736(71)90093-9. [DOI] [PubMed] [Google Scholar]
- Orentlicher M., Reuben J. P., Grundfest H., Brandt P. W. Calcium binding and tension development in detergent-treated muscle fibers. J Gen Physiol. 1974 Feb;63(2):168–186. doi: 10.1085/jgp.63.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROSSER C. L., BURNSTOCK G., KAHN J. Conduction in smooth muscle: comparative structural properties. Am J Physiol. 1960 Sep;199:545–552. doi: 10.1152/ajplegacy.1960.199.3.545. [DOI] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. Free-energy relations and contraction of actomyosin. Biol Bull. 1949 Apr;96(2):140–161. [PubMed] [Google Scholar]
- Shoenberg C. F. An electron microscope study of the influence of divalent ions on myosin filament formation in chicken gizzard extracts and homogenates. Tissue Cell. 1969;1(1):83–96. doi: 10.1016/s0040-8166(69)80007-8. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., North S. R. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol. 1971 Dec;51(3):722–741. doi: 10.1083/jcb.51.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Devine C. E., Rice R. V. Aggregation of thick filaments into ribbons in mammalian smooth muscle. Nat New Biol. 1971 Jun 23;231(25):243–246. doi: 10.1038/newbio231243a0. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Studies of cardiac muscle with a high permeability to calcium produced by treatment with ethylenediaminetetraacetic acid. J Gen Physiol. 1971 Jul;58(1):71–93. doi: 10.1085/jgp.58.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu H., Uchida I., Miyazaki E. Participation of native tropomyosin in the ATP-contraction of an intestinal glycerinated muscle bundle. Jpn J Physiol. 1971 Oct;21(5):465–473. doi: 10.2170/jjphysiol.21.465. [DOI] [PubMed] [Google Scholar]



