Abstract
Neuropeptides and catecholamines act as neurotransmitters within circuits of the central and peripheral nervous systems that mediate both systemic and psychological stress responses, as well as long-term adaptation and maladaptation to stress recognizable clinically as survival with resilience, or survival with cost, as manifested in anxiety, depression, PTSD, and other human behavioral disorders. The interactions between catecholamines and neuropeptides within some of these circuits are summarized in this chapter and described in detail in the three chapters following.
1. INTRODUCTION
It has long been appreciated that central nervous system (CNS) noradrenergic systems set the tone for organismic response to stress, in particular at the level of the locus coeruleus (LC) and its projections to limbic cortex, extended amygdala, and hypothalamus. Noradrenergic neurons also mediate the autonomic effector limb of the stress response, via increased heart rate and peripheral vascular resistance, and visceral organ activation. Epinephrine release from the adrenal medulla constitutes the very hallmark of acute stress responses. Between the perception of threat and the autonomic response to it, a complex intervening circuitry sets the sensitivity and gain of the stress response. Neuropeptides are employed as transmitters in this circuitry. There is a growing understanding of opiate peptide effects on arousal and hedonic tone. Pituitary adenylate cyclase-activating polypeptide (PACAP) has been discovered to be a critical neurotransmitter mediating activation of the hypothalamic–pituitary adrenal (HPA) and hormonal sympathetic adrenal (HSA) axes by stress. Corticotropin-releasing hormone (CRH) not only initiates HPA activation via release of adrenocorticotropic hormone (ACTH) from the pituitary but also is released from hypothalamic and extrahypothalamic neurons to feed back on noradrenergic systems driving central to peripheral stress “executive” programs. Striking new findings in the last decade or so have accelerated progress in understanding how, where, and when neuropeptide–catecholamine interactions occur in brain and periphery. A new picture of stress circuitry has emerged, in which catecholamine and neuropeptide systems are intimately intercalated, both centrally and peripherally, during response to both systemic and psychogenic stress. This neurochemical and anatomical integration allows responses to acute stressors to be translated into long-term changes. These can be both adaptive, and maladaptive, for modern individuals experiencing a range of stressors perceived as threats to homeostasis by limbic and hypothalamic circuits whose final output is activation of the HPA and HSA axes, or the sympathetic nervous system (SNS).
2. CATECHOLAMINE AND NEUROPEPTIDE INTERACTIONS IN CIRCUITS AND SUBCIRCUITS MEDIATING STRESS RESPONSES
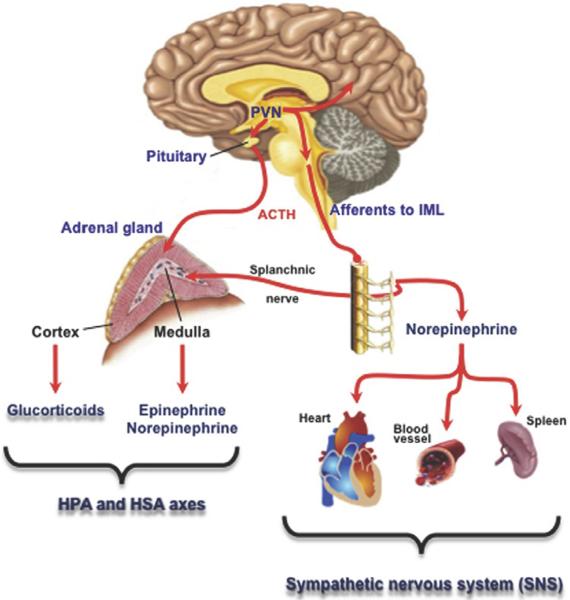
Several contributions to this volume describe recent advances in our understanding of the final output systems shown in Fig. 18.1, that is, how adrenal cortex, adrenal medulla, and postganglionic sympathetic neurons effect acute stress responses and adaptively transduce chronic stress responses. The CNS circuits mediating acute and chronic stress responses, however, are not above the fray after causing the activation of the axes depicted in Fig. 18.1 (and see Stroth et al., 2011). Rather, the brain is itself affected by peripherally generated glucocorticoids, and catecholamine-dependent metabolic changes occurring in acute and chronic stress. A clear indication of this is adaptive and maladaptive behaviors associated with chronic psychological stress that include depression, overeating, sleep disturbance, and immune dysregulation and, in perhaps, the most clinically dramatic fashion, posttraumatic stress disorder (PTSD).
Figure 18.1.
Stress effector systems. Adapted from Stroth, Holighaus, Ait-Ali, & Eiden, 2011.
The contributions to this volume on neuropeptide–catecholamine interactions in stress, following on this overview, sum up to an overarching picture of catecholamine–neuropeptide systems that are “sandwiched” between the arousal response conveyed from the sensorium to the brain in large part via the noradrenergic system of the LC, and the final effector system shown in Fig. 18.1, a hybrid catecholamine/corticosteroid hormone output. In Chapter 21, Tomris Mustafa summarizes the role of PACAP as a neuropeptide important in modulating the stress response at several levels. First, PACAP is released from the splanchnic nerve during both acute and chronic stress, whether systemic/physical (hypoglycemia, cold, sepsis) or psychogenic/psychological (restrain/immobilization, social defeat) to allow catecholamine release. Second, PACAP controls activation of the HPA axis at a central level, but this level of control is operative for psychogenic stress only, and not for the systemic stress response.
This control appears to be exerted primarily at the level of activation of CRH neurons in the paraventricular hypothalamus. In Chapter 20, Watts and Kahn elegantly describe the fully complementary regulation of CRH during systemic—but not psychogenic—stress by noradrenergic inputs (presumably arising mainly from A1/A2 noradrenergic brain stem cell groups— see Itoi et al. in Chapter 8 of this volume). This regulation is likely mediated by precise ERK-dependent control of both CRH synthesis and CRH secretion into the portal circulation—the actual final effector for pituitary ACTH release and subsequent hormonal secretion of corticosterone/cortisol.
The LC, in addition to mediating the gain of the initial stress response, is “carbon-copied” on activation of CRH neurons by feedback from projections from the amygdala, as well as potentially from the PVN, back to the LC, as outlined by Van Bockstaele and Valentino in Chapter 19 of this volume. Multiple other opiopeptidergic inputs to LC from limbic stations (dynorphin), and via corelease with other transmitters from the PGi, the pathway through which LC is first activated by sensory cues/stimuli (enkephalin) may be the substrates through which opiate peptide agonists and antagonists, and CRH antagonists, can exert marked effects on stress-dependent anhedonia, depression, and cognitive dysfunction.
3. CONCLUSION
Catecholamine neurotransmitters and neuropeptides both interact with GPCRs and are released from large dense-cored vesicles (and in some cases from tubulovesicular structures, e.g., dopamine release in substantia nigra) in response to high-frequency or burst neuronal firing of the type associated with the conduction of stress signaling in the CNS and peripheral nervous system. Ultimately, the stress response requires a sensory input, or a representation of a threat to the conscious brain, to trigger convergence on the hypothalamus leading to AHS/SNS and HPA activation. Activation of CNS circuits to complete this loop requires a “stressed brain” and one that is furthermore additionally acted upon by the stress hormones released peripherally (Fig. 18.2). In some cases, processing of threat responses by the brain may transition it from a homeostatically to an allostatically responding organ, with pathophysiological consequences (PTSD, depression, anxiety) as suggested by McEwen (2008). Integration of the new neurochemical, neurophysiological, and neuroanatomical facts put forward about catecholamine–neuropeptide interactions in the contributions following should help provide translationally relevant answers to two major questions about the neurochemistry and neuropharmacology of stress responses. The first is how are catecholaminergic–peptidergic circuit interactions patterned in brainstem, hypothalamus, and extended amygdala to integrate HPA axis activation not only in response to an acute stressor but also in stressor response that is conditioned by past experience? Glucocorticoids play an important role in this plasticity, both acutely and long term, and at virtually all levels: hippocampus, extended amygdala, hypothalamus, and pituitary (Radley et al., 2011). A second, corollary question is to what extent do inputs from the periphery besides glucocorticoids, such as peripheral catecholamine release from sympathetic nerves and the adrenal medulla, also promote plasticity in brain stress response circuitry? The chapters following represent major steps forward in framing these questions, based on a new and expanded understanding of the neurochemistry and neuroanatomy of catecholamine–neuropeptide interactions during the stress response.
Figure 18.2.
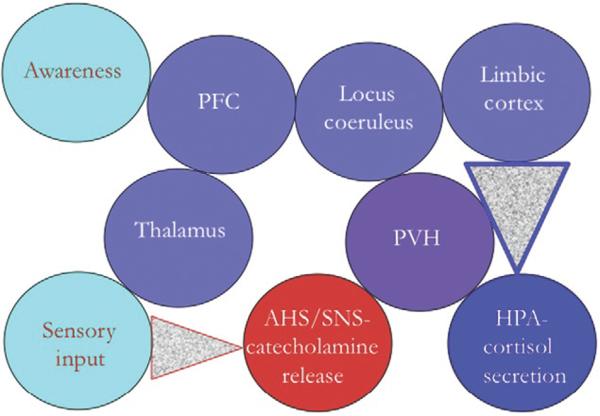
Attentional, limbic and neuroendocrine effector systems: interactions in psychophysiological homeostasis.
ABBREVIATIONS
- CNS
central nervous system
- CRH
corticotropin-releasing hormone
- HPA axis
hypothalamic–pituitary adrenal axis
- HSA axis
hormonal sympathetic adrenal axis
- LC
locus coeruleus
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PFC
prefrontal cortex
- PGi
nucleus paragigantocellularis lateralis
- PVH
paraventricular hypothalamus
- SNS
sympathetic nervous system
Footnotes
CONFLICT OF INTEREST The author has no conflicts of interest to declare.
Publisher's Disclaimer: This chapter was originally published in the book Advances in Pharmacology, Vol. 68, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution's administrator.
REFERENCES
- Itoi K, Ohara S, Kobayashi K. Selective ablation of dopamine beta-hydroxylase neurons in the brain by immunotoxin-mediated neuronal targeting. Advances in Pharmacology. doi: 10.1016/B978-0-12-411512-5.00008-7. this volume. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. Stress risk factors and stress-related pathology: Neuroplasticity, epigenetics and endophenotypes. Stress. 2011;14:481–497. doi: 10.3109/10253890.2011.604751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: A master regulator of neuroendocrine stress circuits and the cellular stress response. Annals of the New York Academy of Sciences. 2011;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]