Abstract
Background
Prophylaxis of hemophilia B, at present, requires multiple infusions of human factor IX (FIX) concentrates per week. A FIX molecule with a prolonged half-life has the potential to greatly improve convenience of, and adherence to, prophylaxis.
Objectives
The aim of our studies was to investigate the pharmacokinetic and pharmacodynamic profile of a recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP).
Methods
Cynomolgus monkeys and hemophilia B dogs received single intravenous doses of rIX-FP (50–500 IU kg−1). rIX-FP plasma levels were determined by an activity-based assay (dogs only) and anti-FIX enzyme-linked immunosorbent assay methods. Additionally, activated partial thromboplastin time (aPTT) was determined in hemophilia B dogs. Data were compared with a direct study comparator (recombinant FIX [rFIX]) or previously published data.
Results
Terminal half-life of rIX-FP was prolonged in both species compared with FIX reference data. In hemophilia B dogs, human FIX antigen levels remained above 0.05 IU mL−1 more than three times longer following rIX-FP (7.3 days) compared with rFIX (2.3 days), whereas respective calculations based on activity levels confirmed observed superior profile. Prolonged pharmacodynamics of rIX-FP was demonstrated with aPTT <60 seconds sustained around four times longer with rIX-FP (5.9 days) than rFIX (1.5 days).
Conclusions
These studies indicate that the recombinant albumin fusion technology successfully improves the pharmacokinetic profile of FIX. Clinical studies will test whether the improved kinetics result in a significant half-life extension in patients with hemophilia B.
Keywords: factor IX, fusion protein, hemophilia B, rIX-FP, hemophilia B dog, cynomolgus monkey
Introduction
Hemophilia B is a blood-clotting disorder affecting approximately 1 in 25,000 human male births [1]. It is caused by an X-linked recessive mutation resulting in a deficiency in coagulation factor IX (FIX), a critical component of the coagulation cascade. Bleeding is characteristic of this condition, with symptom severity dependent on the baseline FIX activity. Management of hemophilia B, at present, requires plasma-derived (pd) or recombinant FIX (rFIX) to be administered by intravenous (IV) infusion for both prophylaxis and treatment.
As a result of the short half-life of current FIX treatments (17–34 hours [2–5]), patients with severe hemophilia B, managed with prophylactic regimens, require an IV infusion 2–3 times a week [6]. This frequency of prophylaxis may affect patient compliance and quality of life. Consequently, there is an unmet need for FIX preparations requiring decreased frequency of administration.
Novel FIX preparations have been developed that attempt to reduce the required infusion frequency [7–10]. One method involves genetic fusion with albumin, a protein with a long physiological half-life, to improve the pharmacokinetic (PK) profile of FIX. This technique has been successful in extending the half-lives of several proteins and peptides [11–16]. Recombinant albumin fusion constructs have been developed in which human FIX complementary DNA (cDNA) was joined to human albumin cDNA. Both proteins are connected by a cleavable linker sequence derived from the natural activation peptide of human FIX [10]. Thrombin generation assays demonstrated that the recombinant fusion protein linking coagulation FIX with albumin (rIX-FP) generated thrombin levels similar to those of wild-type rFIX, and preclinical PK studies in rodents and rabbits investigating rIX-FP (derived from Chinese hamster ovary (CHO) cells) revealed increased in vivo recovery (ratio of rIX-FP/rFIX, rats=1.71; rabbits=1.57), extended terminal half-life (t½) (ratio of rIX-FP/rFIX, rats=4.70; rabbits=3.96) and increased area under the curve (AUC) versus rFIX (ratio of rIX-FP/rFIX, rats=4.64; rabbits=7.18) [10].
The aim of our studies was to further explore the preclinical PK and pharmacodynamic (PD) characteristics and tolerability of rIX-FP in normal cynomolgus monkeys and FIX-deficient hemophilia B dogs.
Methods
The rIX-FP used in the studies was expressed in CHO cells with a cleavable linker sequence between recombinant human FIX and recombinant human albumin as described by Metzner et al [10]. The lyophilized rIX-FP was prepared by reconstitution with water for injection at a fixed concentration of 200 IU mL−1. Visual assessment of the reconstituted rIX-FP was performed to ensure that all contents were in solution. Animal studies were approved by the animal care committees of the respective institutions.
Single-dose pharmacokinetics of rIX-FP in cynomolgus monkeys
The pharmacokinetics of rIX-FP were assessed in four cynomolgus monkeys (Belgrave Services, Long Thanh District, Vietnam) following a single IV dose (bolus injection) into a saphenous vein. Two monkeys (one male, one female) received rIX-FP at a dose of 50 IU kg−1; another two monkeys (one male, one female) received rIX-FP at a dose of 100 IU kg−1. Throughout the study, twice-daily clinical observations assessed the animals for health status and treatment reactions. Blood samples were drawn from each monkey prior to treatment and throughout the 19-day study (5 minutes, 15 minutes, and 1, 3, 8, 23, 47, 72, 96, 120, 216, 312 and 456 hours post-dose). Citrate plasma was prepared and stored frozen until PK and immunogenicity analyses. Validated enzyme-linked immunosorbent assay (ELISA) techniques determined plasma concentrations of rIX-FP and anti-drug antibodies (for immunogenicity investigations). Human FIX plasma concentrations (displayed in IU mL−1) were evaluated using a FIX-ELISA Kit (Kordia, Leiden, Netherlands). ELISA techniques to evaluate the presence of antibodies against human FIX and human albumin, respectively, consisted of human FIX (CSL Behring, Marburg, Germany) or human albumin (20%, CSL Behring) as capture reagents; a horseradish peroxidase (HRP)-conjugated antibody against monkey immunoglobulins (Acris, Herford, Germany) was used as detection antibody. After 216 hours, it was considered that background levels of FIX had been reached; thus average concentrations at pre-dose as well as 216, 312 and 456 hours post-dose were calculated for each animal to account for endogenous FIX levels. This value was then subtracted from the measured concentration at each time point. PK parameter estimates were derived by non-compartmental methods.
Single-dose toxicokinetics of rIX-FP in cynomolgus monkeys
Cynomolgus monkeys were used to assess the systemic tolerability and toxicokinetic (TK) profile of rIX-FP following a single IV dose (75, 150 or 500 IU kg−1) into a cephalic vein. Three female and three male monkeys received each dose; a control group of three males and three females received a single dose of isotonic saline (0.9%). Throughout the study, animals were visually assessed twice daily for health status and adverse reactions to treatment. Blood samples were drawn from each animal before treatment, and 0.25, 1, 5, 24, 72 and 120 hours post-dose (and 240 hours post-dose from one male and female animal from each group) followed by citrate plasma preparation and storage at around −70°C until TK evaluation of rIX-FP plasma levels. Additional blood samples were taken from each animal before treatment and 5 days after dosing for analysis of hematology and blood chemistry; overnight urine samples were collected (before treatment and 5 days after dosing) and assessed for appearance, volume, composition and sediment.
Two males and two females from each group were euthanized (with an overdose of sodium pentobarbitone solution [200 mg mL−1]) 5 days after treatment; the remaining animals were euthanized 10 days after dosing. Detailed necropsy procedures were completed on the day the animals were euthanized to allow a full macroscopic evaluation of the tissues.
Plasma concentrations of human FIX (displayed in IU mL−1) were measured with a validated ELISA method using a FIX-ELISA Kit (Kordia). Thereafter, normalized plasma FIX concentrations (endogenous FIX concentration subtracted from the measured plasma concentration) were used for TK analyses using non-compartmental methods. All TK parameter estimates refer to the 5-day sampling unless otherwise stated.
Single-dose pharmacokinetics/pharmacodynamics of rIX-FP in hemophilia B dogs
Hemophilia B dogs (UNC Chapel Hill FOBRL, NC, USA) were used to assess the PK/PD profile and immunogenicity of a single IV bolus dose (100 IU kg−1) of rIX-FP versus a single IV dose of a marketed rFIX product (BeneFIX, Pfizer, Philadelphia, PA, USA). The 100 IU kg−1 dose was selected as it represents the upper end of the intended clinical dose range. Three dogs received rIX-FP injected into the cephalic vein and two received rFIX. Clinical assessments and body weights were recorded periodically throughout the treatment and observation periods. Blood samples were drawn up to 35 days after dosing for PK/PD (activated partial thromboplastin time [aPTT]) parameter assessments; additional samples were used for hematology, clinical chemistry and immunogenicity investigations.
Human FIX antigen plasma concentrations were evaluated using a FIX-ELISA Kit (Kordia). ELISA techniques to evaluate the presence of antibodies against human FIX and human albumin, respectively, consisted of human FIX (CSL Behring) or human albumin (20%, CSL Behring) as capture reagents; a HRP-conjugated antibody against dog IgG (Acris) was used as detection antibody. Human FIX antigen plasma concentration data (displayed in IU mL−1) were analyzed using a two-compartmental model. PK calculations were adjusted to a comparable dose of 100 IU kg−1 by multiplying the measured human FIX plasma concentrations by a ratio of nominal dose/actual dose.
Additionally, human FIX activity plasma levels were determined using an aPTT-based assay on a Behring Coagulation Timer (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany). FIX-deficient and standard human plasma (both for preparation of standard curve) and aPTT reagent (silicone dioxide particles and plant phospholipids) were obtained from Siemens Healthcare Diagnostics Products GmbH. Mixtures of dog study plasma samples and FIX-deficient human plasma were tested in the aPTT assay and interpreted using the standard curve. Thereafter, human FIX activity data were analyzed using a two-compartmental model, including a background signal. PK calculations were adjusted to a comparable dose of 100 IU kg−1 as described above.
To evaluate the presence of neutralizing antibodies, a Bethesda inhibitor assay was performed [17]. Thereby, a dog’s plasma with a residual factor IX activity of 50% of the normal control is defined as one “Bethesda unit” of inhibitor per mL.
The PD parameter aPTT was determined in the ST4 coagulation analyzer (Diagnostica Stago, Asnieres, France). For aPTT determination, mixtures comprised equal portions of partial thromboplastin (aPTT reagent), 0.025 M CaCl2 and citrated test plasma. Hematology analyses (including platelet, white blood cell and hematocrit levels) were assessed up to 35 days after dosing using a cell counter (Heska ABC Analyzer, Grayslake, IL, USA) calibrated for dog cells. Biochemical serum parameters (including glucose, urea and bilirubin) were assayed automatically using a ‘Superchem’ panel (Antech Diagnostics, Cary, NC, USA) up to 24 hours after administration.
Results
Pharmacokinetics of rIX-FP in cynomolgus monkeys
Single-dose PK study
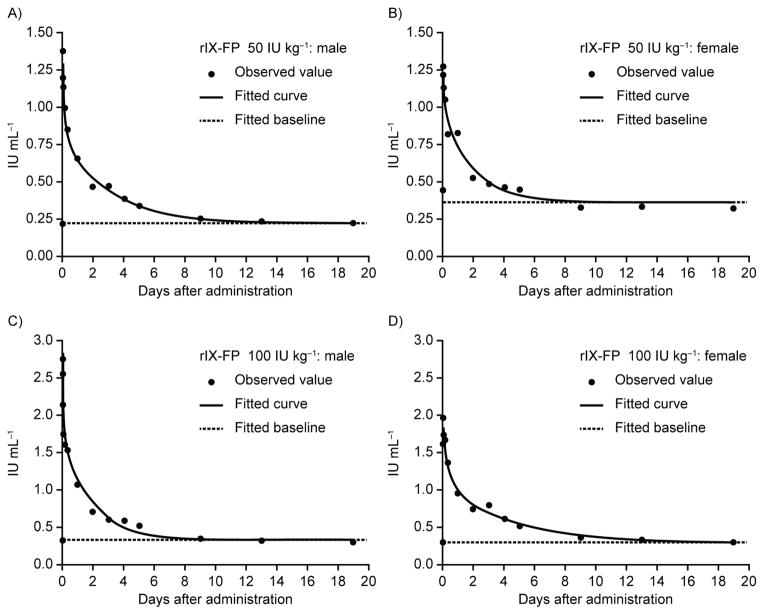
Following IV administration to cynomolgus monkeys, the single-dose pharmacokinetics of rIX-FP were linear over the dose range 50–100 IU kg−1, and independent of sex (Fig. 1); individual terminal t½ range was 39.8–44.4 hours (mean: 42.2 hours) (Table 1). Systemic clearance of rIX-FP (1.26 mL h−1 kg−1) was low relative to monkey liver and kidney plasma flow (around 1740 mL h−1 kg−1 and 1100 mL h−1 kg−1, respectively) [18]. Antibodies against human FIX or human albumin were not detected.
Fig. 1. Observed and fitted human FIX antigen plasma levels in male and female cynomolgus monkeys treated once with rIX-FP (intravenously).
(A) and (B) 50 IU kg−1; (C) and (D) 100 IU kg−1 (A/C = male, B/D = female). Curves were fitted according to a two-compartment model plus an endogenous background level (baseline).
Table 1.
Pharmacokinetic parameters in cynomolgus monkeys
| rIX-FP dose (IU kg−1) | Sex | Cmax IU mL−1 | AUC h·IU mL−1 | Clearance mL h−1 kg−1 | t½ h |
|---|---|---|---|---|---|
| 50 | Male | 1.14 | 43.0 | 1.11 | 39.8 |
| Female | 0.91 | 33.5 | 1.38 | 44.2 | |
| 75 | Male | 1.02 | 41.9† | 1.69† | 44.2 |
| Female | 1.15 | 48.5† | 1.35† | 42.8 | |
| 100 | Male | 2.23 | 72.7 | 1.29 | 44.4 |
| Female | 1.64 | 74.1 | 1.26 | 40.3 | |
| 150 | Male | 2.18 | 97.8 | 1.41 | 39.8 |
| Female | 2.60 | 101* | 1.29* | 55.9† | |
| 500 | Male | 7.29 | 302 | 1.45 | 48.4 |
| Female | 9.45 | 403 | 1.08 | 42.1 |
Data estimated by non-compartmental methods. N=1 for male and female values obtained from the pharmacokinetic study (50 and 100 IU kg−1); n=3 for male and female values (mean) obtained from the toxicokinetic study (5-day values) unless otherwise stated (75, 150 and 500 IU kg−1).
n=1;
n=2 (some animal values were excluded from the summary statistics since the coefficient of determination was < 0.9 or the extrapolated fraction of AUC was > 20%).
AUC, area under the curve; Cmax, maximum concentration; t½, terminal half-life.
Single-dose TK study
rIX-FP plasma concentrations increased with increasing dose (Table 1). The increases in maximum concentration (Cmax) and AUC were proportional to rIX-FP dose after single IV administration for doses of 75–500 IU kg−1 and mean terminal t½ range was 39.8–55.9 hours (Table 1; in selected animals in which blood sampling was extended to 240 hours, terminal t½ up to 83.4 hours was calculated). Data from this study revealed that the effect of rIX-FP on terminal t½ or total plasma clearance was independent of dose. Following the single rIX-FP dose, no apparent immune response against rIX-FP was observed.
Pharmacokinetics of rIX-FP in hemophilia B dogs
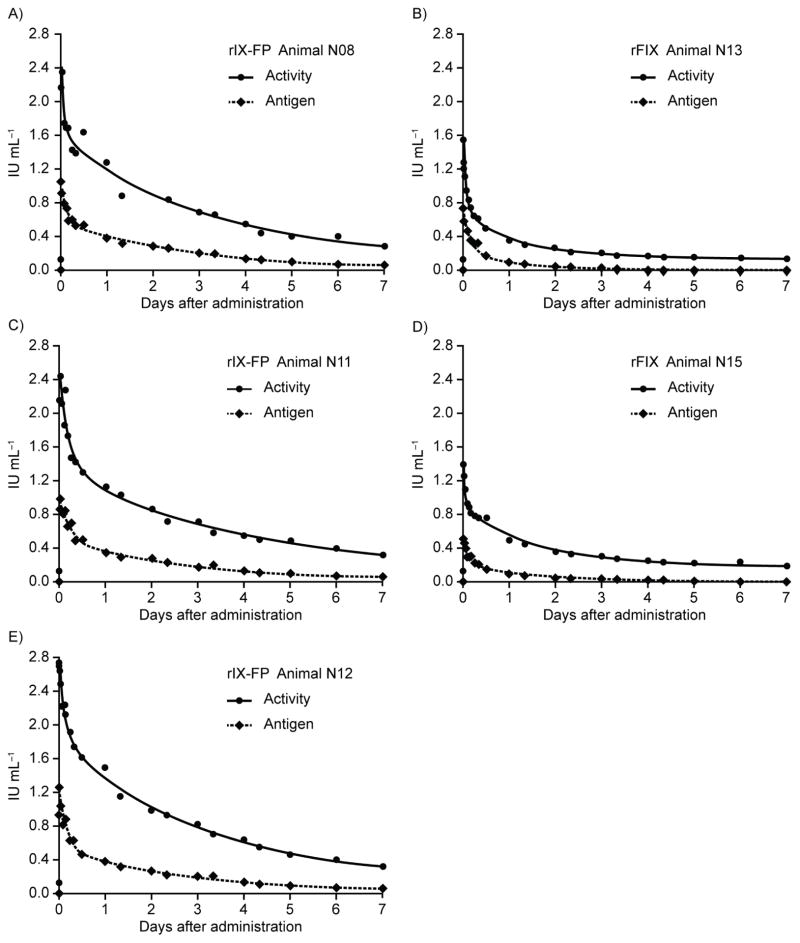
Observed human FIX antigen plasma Cmax was higher with rIX-FP versus rFIX (Fig. 2; Table 2). PK analysis revealed that the estimated clearance of rIX-FP (2.41 mL h−1 kg−1) was more than four times lower than for rFIX (10.35 mL h−1 kg−1) (Table 2). Accordingly, the AUC was more than four times higher with rIX-FP versus rFIX (41.5 vs. 9.7 h·IU mL−1, respectively). Time until levels of human FIX reached values below 0.05 IU mL−1 (the suggested protective level in patients with hemophilia B) was more than three times longer with rIX-FP versus rFIX (7.3 days vs. 2.3 days, respectively) (Fig. 3A; Table 2). Moreover, rIX-FP had a longer terminal t½ and a higher recovery rate than rFIX (51.9 hours vs. 33.6 hours, respectively, for terminal t½ and 43% vs. 23%, respectively, for recovery; Table 2).
Fig. 2. Observed and fitted human FIX activity and antigen plasma levels of hemophilia B dogs treated once with rIX-FP or recombinant factor IX (rFIX) (100 IU kg−1; intravenously).
(A), (C) and (E) rIX-FP; (B) and (D) rFIX. Curves were fitted according to a two-compartment model (including a background signal for activity data).
Table 2.
Pharmacokinetic parameters in hemophilia B dogs (human FIX antigen)
| Group | Animal | Cmax IU mL−1 | AUC h·IU mL−1 | Clearance mL h−1 kg−1 | t½α h | t½β h | Days above 0.05 IU mL−1 | Vss mL kg−1 | Recovery (%) |
|---|---|---|---|---|---|---|---|---|---|
| rIX-FP | N08 | 1.05 | 41.7 | 2.40 | 2.0 | 49.6 | 7.2 | 166 | 42 |
| N11 | 0.97 | 39.2 | 2.55 | 4.6 | 52.4 | 7.1 | 178 | 39 | |
| N12 | 1.19 | 43.7 | 2.29 | 3.3 | 53.8 | 7.6 | 166 | 47 | |
| rFIX | N13 | 0.68 | 9.8 | 10.20 | 3.7 | 32.2 | 2.1 | 352 | 27 |
| N15 | 0.49 | 9.5 | 10.50 | 3.1 | 35.1 | 2.4 | 458 | 19 | |
| rIX-FP | G.Mean | 1.07 | 41.5 | 2.41 | 3.1 | 51.9 | 7.3 | 170 | 43 |
| CV (%) | 10 | 6 | 6 | 41 | 4 | 4 | 4 | 10 | |
| rFIX | G.Mean | 0.57 | 9.7 | 10.35 | 3.4 | 33.6 | 2.3 | 402 | 23 |
| CV (%) | 23 | 2 | 2 | 14 | 6 | 10 | 19 | 23 |
Parameters estimated from a two-compartment model using only the plasma concentration data (human FIX antigen levels, displayed in IU mL−1) up to hour 168. Recovery was calculated for a plasma volume of 40 mL kg−1.
AUC, area under the curve; Cmax, maximum concentration; CV, coefficient of variation; G.Mean, geometric mean; rFIX, recombinant factor IX; t½α initial half-life; t½β terminal half-life; Vss, volume of distribution at steady state.
Fig. 3. Pharmacokinetic and pharmacodynamic profile of single-dose rIX-FP versus recombinant factor IX (rFIX) (100 IU kg−1; intravenously) in hemophilia B dogs.
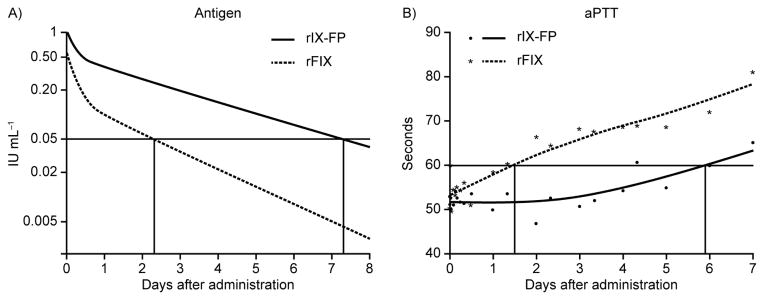
(A) Human FIX antigen plasma levels of an average hemophilia B dog, whose central compartment volume, initial and terminal elimination half-lives, and clearance were the geometric mean of the individual parameter values. (B) Average activated partial thromboplastin time (aPTT) values of hemophilia B dogs after baseline and smoothing spline curve.
The PK evaluation of human FIX activity levels confirmed the improved PK profile of rIX-FP versus rFIX (Fig. 2; Table 3). While estimated clearance of rIX-FP (0.84 mL h−1 kg−1) was more than five times lower than for rFIX (4.5 mL h−1 kg−1), terminal t½ was prolonged around 2.3-fold (i.e., 55.2 hours vs. 23.5 hours, respectively) (Table 3).
Table 3.
Pharmacokinetic parameters in hemophilia B dogs (human FIX activity)
| Group | Animal | Cmax IU mL−1 | AUC h·IU mL−1 | Clearance mL h−1 kg−1 | t½α h | t½β h | Days above 0.05 IU mL−1 | Vss mL kg−1 | Recovery (%) |
|---|---|---|---|---|---|---|---|---|---|
| rIX-FP | N08 | 2.27 | 109.9 | 0.91 | 1.4 | 51.1 | 10.4 | 66 | 91 |
| N11 | 2.25 | 116.2 | 0.86 | 3.5 | 62.6 | 12.1 | 75 | 90 | |
| N12 | 2.60 | 131.8 | 0.76 | 2.8 | 52.6 | 11.1 | 56 | 104 | |
| rFIX | N13 | 1.29 | 17.5 | 5.71 | 1.5 | 20.2 | 2.9 | 152 | 52 |
| N15 | 1.21 | 28.2 | 3.55 | 0.8 | 27.4 | 4.3 | 137 | 49 | |
| rIX-FP | G.Mean | 2.37 | 118.9 | 0.84 | 2.4 | 55.2 | 11.2 | 65 | 95 |
| CV (%) | 8 | 9 | 9 | 42 | 11 | 7 | 14 | 8 | |
| rFIX | G.Mean | 1.25 | 22.2 | 4.50 | 1.1 | 23.5 | 3.5 | 144 | 50 |
| CV (%) | 4 | 33 | 33 | 44 | 21 | 28 | 7 | 4 |
Parameters estimated from a two-compartment model (including a background signal) using only the plasma concentration data (human FIX activity levels) until hour 168. Parameter values refer to net plasma levels (i.e., after subtracting the fitted background signal). Recovery was calculated for a plasma volume of 40 mL kg−1.
AUC, area under the curve; Cmax, maximum concentration; CV, coefficient of variation; G.Mean, geometric mean; rFIX, recombinant factor IX; t½α initial half-life; t½β terminal half-life; Vss, volume of distribution at steady state.
Antibodies against human FIX (and against human albumin around the same time [rIX-FP]) were identified in rIX-FP-treated and rFIX-treated animals approximately 10 and 14 days after drug administration, respectively. Guided by the results from an initial inhibitor screening assay (an aPTT “mixing” assay; data not shown), the Bethesda inhibitor assay was performed on selected blood samples that indicated the presence of an inhibitor. Resulting Bethesda units ranged from 0.99 to 7.04 on day 15 (rIX-FP) and 2.97 to 14.8 on day 22 (rFIX). Since the immune response seen in both groups most likely affected plasma levels prior to the above-mentioned time points, PK analysis was only completed up to 168 hours after drug administration.
Duration of correction of prolonged aPTT by rIX-FP and rFIX in hemophilia B dogs
In hemophilia B dogs, aPTT values decreased following study drug administration in both groups. A return to pre-treatment aPTT values (mean: 97 seconds) was observed following clearance of the study drugs from the circulation. However, aPTT values remained at the lower level (below 60 seconds) approximately four times longer with rIX-FP versus rFIX (5.9 days vs. 1.5 days, respectively), demonstrating a sustained PD effect (Fig. 3B).
Tolerability in cynomolgus monkeys and hemophilia B dogs
A single IV administration of rIX-FP was well tolerated in cynomolgus monkeys at the doses studied (50–500 IU kg−1) with no toxicologically relevant findings. In hemophilia B dogs, two animals (one treated with rIX-FP and one with rFIX) experienced a clinical bleed (i.e., a hematoma). As neither animal ultimately responded to medical treatment (application of normal canine plasma), both were euthanized 21 days after dosing following concerns over animal welfare. Since the appearance of inhibitory anti-drug antibodies could be confirmed in these animals, cross-reactivity of these neutralizing antibodies against the canine FIX accounted for this non-responsiveness of the treatment with normal canine plasma. There were no further drop outs in this study. In the remaining animals, no drug-related clinical signs were recorded following treatment with rIX-FP or rFIX. Furthermore, white blood cell, hemoglobin, hematocrit and platelet levels were not affected by rIX-FP treatment (Table 4), and no drug-related changes in clinical chemistry parameters were found.
Table 4.
Overview of hematology in hemophilia B dogs 24 hours after study dose
| Animal | Platelets (×103 mm3–1) | White blood cells (×103 mm3–1) | Hematocrit (%) | Hemoglobin (g dL−1) | |
|---|---|---|---|---|---|
| Normal range | 200–500 | 6.0–17.0 | 37–55 | 12–18 | |
| rIX-FP | N08 | 434 | 9.8 | 42.1 | 13.5 |
| N11 | * | * | 41.1 | 12.8 | |
| N12 | 347 | 9.9 | 39.4 | 13.0 | |
| rFIX | N13 | 340 | 9.2 | 42.1 | 13.3 |
| N15 | 327 | 11.6 | 45.1 | 14.4 | |
| rIX-FP | G.Mean | 272.3 | 13.5 | 40.9 | 13.1 |
| SD | 209.2 | 6.3 | 1.4 | 0.4 | |
| rFIX | G.Mean | 333.5 | 10.4 | 43.6 | 13.9 |
| SD | 9.2 | 1.7 | 2.1 | 0.8 |
Clumping due to analysis delay.
G.Mean, geometric mean; rFIX, recombinant factor IX.
Discussion
These studies were performed to further explore the preclinical PK/PD characteristics and tolerability of rIX-FP in a) hemophilia B dogs, which are deficient in endogenous FIX and, b) cynomolgus monkeys with normal endogenous FIX levels. In both cynomolgus monkeys and hemophilia B dogs, rIX-FP had a favorable PK profile. A mean terminal t½ of 39.8–55.9 hours was calculated in cynomolgus monkeys for the dose range evaluated (50–500 IU kg−1); whereas in hemophilia B dogs, a mean terminal t½ of 51.9 hours (FIX antigen levels) and 55.2 hours (FIX activity levels) was calculated after a single IV dose of 100 IU kg−1. Comparing different dose levels, pharmacokinetics of rIX-FP were linear in cynomolgus monkeys up to 500 IU kg−1, and independent of sex. In hemophilia B dogs, a higher recovery rate and a lower clearance rate were seen with rIX-FP versus rFIX, therefore prolonging the time at which FIX levels were above 0.05 IU mL−1 (the suggested protective level for patients with hemophilia B). Furthermore, aPTT values remained at a lower level for approximately four times longer with rIX-FP versus rFIX, demonstrating a sustained PD effect.
A previous study examined the PK/PD characteristics of 200 IU kg−1 rFIX following a single IV bolus in cynomolgus monkeys [19]. Evaluation of these data revealed a Cmax of 1.98 IU mL−1, which is lower than the values reported here for 150 IU kg−1 rIX-FP (Cmax 2.18–2.6 IU mL−1). Furthermore, McCarthy et al. reported a terminal t½ of 12.7 ± 1.8 hours for rFIX [19], versus 39.8–55.9 hours for rIX-FP in our study. These results indicate that linking recombinant human FIX to recombinant human albumin prolongs the half-life of this fusion protein at least three-fold.
Canine models of hemophilia are widely used to test the PK/PD characteristics of anti-hemophilic replacement products [20]. Brinkhous et al. compared the PK profiles of rFIX and pdFIX in hemophilia B dogs [21]. Following IV infusions of either product (50 IU kg−1), the PK parameters of the treatments were similar over the first 24 hours using human FIX antigen levels for calculations (terminal t½: 22.5 and 22.2 hours; clearance: 8.3 and 7.1 mL h−1 kg−1 for rFIX and pdFIX, respectively). Comparable results regarding terminal t½ (19 [rFIX] and 17.9 [pdFIX] hours) were achieved using FIX activity levels for analyses. While terminal t½ values of our internal study comparator rFIX are approximately comparable with published data (23.5 [activity data] and 33.6 [antigen data] hours), analysis of the rIX-FP data demonstrate that the recombinant albumin fusion protein has a longer terminal t½ (55.2 [activity] and 51.9 [antigen] hours) and decreased clearance rate (0.84 [activity] and 2.41 [antigen] mL h−1 kg−1) versus rFIX and pdFIX in hemophilia B dogs. Observed differences in PK values based on activity or antigen FIX levels in our study might be due to a higher sensitivity of the activity assay versus the antigen assay. However, although absolute PK values were different using antigen or activity FIX levels for calculations, the respective relative differences between rIX-FP and rFIX are comparable. Differences between PK values based on activity or antigen data have also been observed in another study using rFIX in hemophilia B dogs [19].
Furthermore, data from our study in hemophilia B dogs demonstrated a lower recovery rate for rFIX than for rIX-FP. Based on FIX antigen levels, mean recovery was 23% for rFIX and 43% for rIX-FP (Table 2), while mean recovery was 50% for rFIX and 95% for rIX-FP based on FIX activity data (Table 3). These activity data of rFIX are comparable to published data showing also reduced human FIX recovery values (44% 15 minutes post-infusion) following administration of rFIX to hemophilia B dogs (100 IU kg−1; IV) [21]. Interestingly, a lower recovery rate was also reported for rFIX versus pdFIX in hemophilia B patients [3,22]. Possible mechanisms to explain the differences in recovery rate include intra- and extravascular binding, endothelial cell binding, and post-translational modification of specific amino acids [9,23–25].
No immune response was seen in cynomolgus monkeys under the study conditions with an observation period of up to 19 days. However, it has to be mentioned that these animals were immunologically tolerant against cynomolgus FIX (and cynomolgus albumin) and only received a single IV application of rIX-FP. In hemophilia B dogs, anti-human FIX antibodies (and anti-human albumin antibodies [rIX-FP]) were identified around 10–14 days following a single dose of rIX-FP or rFIX. Apparently, these anti-drug antibodies had a neutralizing effect, influencing the PK/PD parameters of the recombinant proteins. Furthermore, the neutralizing properties of these antibodies could be confirmed by the Bethesda inhibitor assay. The presence of neutralizing anti-human FIX antibodies was also reported in a study with hemophilia B dogs following treatment with rFIX or pdFIX [21]. Taken together, hemophilia B dogs seem to be particularly immunologically reactive towards various pd and rFIX preparations. Moreover, this reactivity most likely contributed to the differences in estimated PK values of rIX-FP between cynomolgus monkeys and hemophilia B dogs.
Besides rIX-FP, other FIX products are in development demonstrating extended half-lives in various animal species (e.g., N9-GP: 41 hours [FIX-deficient mouse], 76 hours [minipig] and 156 hours [hemophilia B dog] [8]; rFIXFc: 46 hours [FIX-deficient mouse], 35 hours [rat], 48 hours [hemophilia B dog] and 47 hours [cynomolgus monkey] [9]), and each product has a specific mode of half-life extension [26–28]. For rIX-FP, an albumin-mediated active recycling mechanism via the FcRn receptor has been suggested. In addition, using albumin as a genetic fusion partner has several further advantages, including a lack of intrinsic activity and the natural abundance of albumin in blood, potentially preventing problems with tolerability. Furthermore, due to the cleavable linker within rIX-FP, the albumin moiety is completely removed during activation of the FIX molecule, leaving an active clotting factor closely resembling the native activated human FIX.
Experiences with half-life extended molecules in hemophilia B dogs and cynomolgus monkeys suggest that animal PK data are predictive of a longer half-life in humans [7,8,11,29]. For example, whilst an albumin/interferon-α fusion protein was shown to have an enhanced PK profile versus interferon-α in cynomolgus monkeys [11], subsequent studies in patients with chronic hepatitis C confirmed the favorable PK properties [29]. A phase I trial to investigate the safety of rIX-FP and to confirm the enhanced PK profile of rIX-FP in patients with hemophilia B has been completed (clinicaltrials.gov; NCT01233440). Furthermore, a phase I/II trial to assess the pharmacokinetics, safety and efficacy of rIX-FP in patients with hemophilia B is currently ongoing (NCT01361126). If the results of the presented rIX-FP study in cynomolgus monkeys and hemophilia B dogs would directly translate to hemophilia B patients, a once-weekly prophylactic dosing regimen could be expected.
Conclusion
The presented data reveal that rIX-FP, with its cleavable linker, has a favorable PK profile with reduced clearance and extended half-life versus pdFIX and rFIX products, respectively, in cynomolgus monkeys and hemophilia B dogs. Furthermore, rIX-FP treatment was well tolerated and demonstrated a sustained PD effect. These data support and add to the existing preclinical PK/PD literature on rIX-FP. Ongoing clinical studies will show whether the observed improved kinetics translate into a significant half-life extension in patients with hemophilia B.
Acknowledgments
The authors would like to thank Elizabeth Southey and Jenny Geatrell of Watermeadow Medical for providing writing assistance. We thank Bärbel Dörr, Annette Feussner, Sabrina Schenk, Stefan Bliss and Elmar Raquet (all CSL Behring) for their excellent technical assistance.
Footnotes
Institution where work was carried out: CSL Behring GmbH, Marburg, Germany; University of North Carolina, Chapel Hill, NC, USA; Prolytic GmbH, Frankfurt, Germany
Authorship contributions: MWN designed and performed research, analyzed and interpreted data, and contributed to manuscript preparation. TCN and EPM performed research, analyzed and interpreted data, and contributed to manuscript preparation. JMC analyzed and interpreted data, and contributed to manuscript preparation. IP and GD designed research, interpreted data and contributed to manuscript preparation. SZ performed research, interpreted data and contributed to manuscript preparation.
Conflicts of Interest Disclosures: MWN, JMC, IP, SZ and GD are employees of CSL Behring. TCN and EPM received support for the costs of performing the experiments under a contractual arrangement with CSL Behring. All work was funded by CSL Behring.
References
- 1.Peyvandi F, Jayandharan G, Chandy M, Srivastava A, Nakaya SM, Johnson MJ, Thompson AR, Goodeve A, Garagiola I, Lavoretano S, Menegatti M, Palla R, Spreafico M, Tagliabue L, Asselta R, Duga S, Mannucci PM. Genetic diagnosis of haemophilia and other inherited bleeding disorders. Haemophilia. 2006;12(Suppl 3):82–9. doi: 10.1111/j.1365-2516.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 2.White GC, 2nd, Beebe A, Nielsen B. Recombinant factor IX. Thromb Haemost. 1997;78:261–5. [PubMed] [Google Scholar]
- 3.Ewenstein BM, Joist JH, Shapiro AD, Hofstra TC, Leissinger CA, Seremetis SV, Broder M, Mueller-Velten G, Schwartz BA Mononine Comparison Study Group. Pharmacokinetic analysis of plasma-derived and recombinant FIX concentrates in previously treated patients with moderate or severe hemophilia B. Transfusion. 2002;42:190–7. doi: 10.1046/j.1537-2995.2002.00039.x. [DOI] [PubMed] [Google Scholar]
- 4.Poon MC. Pharmacokinetics of factors IX, recombinant human activated factor VII and factor XIII. Haemophilia. 2006;12(Suppl 4):61–9. [Google Scholar]
- 5.Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S International Prophylaxis Study Group Pharmacokinetics Expert Working Group. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2–10. doi: 10.1111/j.1365-2516.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 6.Björkman S, Shapiro AD, Berntorp E. Pharmacokinetics of recombinant factor IX in relation to age of the patient: implications for dosing in prophylaxis. Haemophilia. 2001;7:133–9. doi: 10.1046/j.1365-2516.2001.00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Negrier C, Knobe K, Tiede A, Giangrande P, Møss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118:2695–701. doi: 10.1182/blood-2011-02-335596. [DOI] [PubMed] [Google Scholar]
- 8.Ostergaard H, Bjelke JR, Hansen L, Petersen LC, Pedersen AA, Elm T, Møller F, Hermit MB, Holm PK, Krogh TN, Petersen JM, Ezban M, Sørensen BB, Andersen MD, Agersø H, Ahmadian H, Balling KW, Christiansen ML, Knobe K, Nichols TC, et al. Prolonged half-life and preserved enzymatic properties of factor IX selectively PEGylated on native N-glycans in the activation peptide. Blood. 2011;118:2333–41. doi: 10.1182/blood-2011-02-336172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters RT, Low SC, Kamphaus GD, Dumont JA, Amari JV, Lu Q, Zarbis-Papastoitsis G, Reidy TJ, Merricks EP, Nichols TC, Bitonti AJ. Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115:2057–64. doi: 10.1182/blood-2009-08-239665. [DOI] [PubMed] [Google Scholar]
- 10.Metzner HJ, Weimer T, Kronthaler U, Lang W, Schulte S. Genetic fusion to albumin improves the pharmacokinetic properties of factor IX. Thromb Haemost. 2009;102:634–44. doi: 10.1160/TH09-04-0255. [DOI] [PubMed] [Google Scholar]
- 11.Osborn BL, Olsen HS, Nardelli B, Murray JH, Zhou JX, Garcia A, Moody G, Zaritskaya LS, Sung C. Pharmacokinetic and pharmacodynamic studies of a human serum albumin-interferon-α fusion protein in cynomolgus monkeys. J Pharmacol Exp Ther. 2002;303:540–8. doi: 10.1124/jpet.102.037002. [DOI] [PubMed] [Google Scholar]
- 12.Osborn BL, Sekut L, Corcoran M, Poortman C, Sturm B, Chen G, Mather D, Lin HL, Parry TJ. Albutropin: a growth hormone-albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur J Pharmacol. 2002;456:149–58. doi: 10.1016/s0014-2999(02)02644-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Ou Y, Shi Y. AlbuBNP, a recombinant B-type natriuretic peptide and human serum albumin fusion hormone, as a long-term therapy of congestive heart failure. Pharm Res. 2004;21:2105–11. doi: 10.1023/b:pham.0000048203.30568.81. [DOI] [PubMed] [Google Scholar]
- 14.Duttaroy A, Kanakaraj P, Osborn BL, Schneider H, Pickeral OK, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R, Crossan D, Bock J, Kaufman TE, Reavey P, Carey-Barber M, Krishnan SR, Garcia A, Murphy K, Siskind JK, McLean MA, et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54:251–8. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JE, Stewart MW, De Boever EH, bins RL, Hodge RJ, Walker SE, Holland MC, Bush MA Albiglutide Study Group. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:4810–17. doi: 10.1210/jc.2008-1518. [DOI] [PubMed] [Google Scholar]
- 16.Weimer T, Wormsbächer W, Kronthaler U, Lang W, Liebing U, Schulte S. Prolonged in vivo half-life of factor VIIa by fusion to albumin. Thromb Haemost. 2008;99:659–67. doi: 10.1160/TH07-08-0525. [DOI] [PubMed] [Google Scholar]
- 17.Kasper CK, Aledort L, Aronson D, Counts R, Edson JR, van Eys J, Fratantoni J, Green D, Hampton J, Hilgartner M, Levine P, Lazerson J, McMillan C, Penner J, Shapiro S, Shulman NR. Proceedings: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975;34:612. [PubMed] [Google Scholar]
- 18.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy K, Stewart P, Sigman J, Read M, Keith JC, Jr, Brinkhous KM, Nichols TC, Schaub RG. Pharmacokinetics of recombinant factor IX after intravenous and subcutaneous administration in dogs and cynomolgus monkeys. Thromb Haemost. 2002;87:824–30. [PubMed] [Google Scholar]
- 20.Nichols TC, Raymer RA, Franck HW, Merricks EP, Bellinger DA, DeFriess N, Margaritis P, Arruda VR, Kay MA, High KA. Prevention of spontaneous bleeding in dogs with haemophilia A and haemophilia B. Haemophilia. 2010;16(Suppl 3):19–23. doi: 10.1111/j.1365-2516.2010.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkhous KM, Sigman JL, Read MS, Stewart PF, McCarthy KP, Timony GA, Leppanen SD, Rup BJ, Keith JC, Jr, Garzone PD, Schaub RG. Recombinant human factor IX: replacement therapy, prophylaxis, and pharmacokinetics in canine hemophilia B. Blood. 1996;88:2603–10. [PubMed] [Google Scholar]
- 22.Poon MC, Lillicrap D, Hensman C, Card R, Scully MF. Recombinant factor IX recovery and inhibitor safety: a Canadian post-licensure surveillance study. Thromb Haemost. 2002;87:431–5. [PubMed] [Google Scholar]
- 23.Stern DM, Knitter G, Kisiel W, Naworth PP. In vivo evidence of intravascular binding sites for coagulation factor IX. Br J Haematol. 1987;66:227–32. doi: 10.1111/j.1365-2141.1987.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 24.Cheung WF, van den Born J, Kühn K, Kjellén L, Hudson BG, Stafford DW. Identification of the endothelial cell binding site for factor IX. Proc Natl Acad Sci USA. 1996;93:11068–73. doi: 10.1073/pnas.93.20.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillis S, Furie BC, Furie B, Patel H, Huberty MC, Switzer M, Foster WB, Scoble HA, Bond MD. Gamma-carboxyglutamic acids 36 and 40 do not contribute to human factor IX function. Protein Sci. 1997;6:185–96. doi: 10.1002/pro.5560060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–83. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 27.Andersen JT, Sandlie I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet. 2009;24:318–32. doi: 10.2133/dmpk.24.318. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–22. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balan V, Nelson DR, Sulkowski MS, Everson GT, Lambiase LR, Wiesner RH, Dickson RC, Post AB, Redfield RR, Davis GL, Neumann AU, Osborn BL, Freimuth WW, Subramanian GM. A Phase I/II study evaluating escalating doses of recombinant human albumin–interferon-α fusion protein in chronic hepatitis C patients who have failed previous interferon-α-based therapy. Antivir Ther. 2006;11:35–45. [PubMed] [Google Scholar]