Abstract
Chemotherapy for cancer causes significant gut toxicity, leading to severe clinical manifestations and an increased economic burden. Despite much research, many of the underlying mechanisms remain poorly understood hindering effective treatment options. Recently there has been renewed interest in the role tight junctions play in the pathogenesis of chemotherapy-induced gut toxicity. To delineate the underlying mechanisms of chemotherapy-induced gut toxicity, this study aimed to quantify the molecular changes in key tight junction proteins, ZO-1, claudin-1, and occludin, using a well-established preclinical model of gut toxicity. Female tumor-bearing dark agouti rats received irinotecan or vehicle control and were assessed for validated parameters of gut toxicity including diarrhea and weight loss. Rats were killed at 6, 24, 48, 72, 96, and 120 h post-chemotherapy. Tight junction protein and mRNA expression in the small and large intestines were assessed using semi-quantitative immunohistochemistry and RT-PCR. Significant changes in protein expression of tight junction proteins were seen in both the jejunum and colon, correlating with key histological changes and clinical features. mRNA levels of claudin-1 were significantly decreased early after irinotecan in the small and large intestines. ZO-1 and occludin mRNA levels remained stable across the time-course of gut toxicity. Findings strongly suggest irinotecan causes tight junction defects which lead to mucosal barrier dysfunction and the development of diarrhea. Detailed research is now warranted to investigate posttranslational regulation of tight junction proteins to delineate the underlying pathophysiology of gut toxicity and identify future therapeutic targets.
Keywords: tight junctions, occludin, zonular-occludens-1, claudin-1, gut toxicity, mucositis, chemotherapy, pro-inflammatory cytokines
Introduction
Chemotherapy for cancer can cause significant gut toxicity leading to severe clinical manifestations affecting the entirety of the gastrointestinal tract (GIT).1 Symptoms including but not limited to pain, ulceration, vomiting, and diarrhea, are a significant burden on patients’ quality of life, requiring greater resource utilization, and resulting in significant economic burden.2 Chemotherapy-induced gut toxicity (CIGT) is therefore a clinical and economic challenge to oncology practice. Currently there are limited treatment options for patients with CIGT and development of effective preventative therapies is hampered by a lack of understanding of the underlying pathobiological mechanisms.3
CIGT development is a multifactorial process characterized by dynamic biochemical interactions between chemotherapeutic agents and cellular constituents of the mucosa.4-6 Recent research has focused on the molecular mechanisms that underpin CIGT, highlighting roles for apoptosis,1 the immune system,7 the gut microbiome,8 and matrix metalloproteinases (MMPs).9 More recently, intestinal tight junctions were proposed to play important roles in the pathophysiology of CIGT.10
Dynamic regulation of tight junctions is fundamental to many physiological processes as disruption drastically alters mucosal barrier function and intestinal permeability, making these traits a hallmark of many pathological states.11,12 The molecular architecture of the tight junction exhibits a complex arrangement of interacting cytoplasmic and transmembrane proteins. Briefly, peripherally located zonular occludens (ZO) proteins interact to anchor tight junction membrane proteins to the cytoskeleton.13,14 These scaffolding proteins play important roles in tight junction formation and are crucial in barrier integrity.13 Claudins are essential components of the intercellular tight junction and major determinants of paracellular solute fluxes.15 The structural organization of claudin proteins varies and different claudin subtypes are responsible for different roles within the tight junction.13 Claudin-1 is of particular interest with regards to gastrointestinal inflammation and has been implicated in the pathophysiology of a number of inflammatory bowel disorders (IBDs).16 Further, recent research has identified roles for claudin-1 in apoptosis17 and cellular regeneration,18 both of which are key events in CIGT. Occludin is a key transmembrane protein integral to tight junction integrity. The importance of occludin to tight junction function has been conclusively demonstrated through numerous investigations.13,19,20 For example, knockout (occludin−/−) mice exhibit morphologically intact tight junctions,21 however, poor tight junction integrity and mucosal barrier dysfunction follow. These results indicate likely roles for occludin in tight junction stability and barrier function as opposed to tight junction assembly.22
There is substantial anecdotal evidence to suggest that tight junctions play key roles in the development of CIGT.10 In 1997, Keefe et al. identified a transient abnormality in intestinal permeability in patients receiving high-dose chemotherapy.23 Marked abnormalities in intestinal permeability have also been shown in patients receiving various myeloablative treatments24 indicating that intestinal function is compromized by various cytotoxic regimens. Further, morphological defects in tight junctions have been identified, with Keefe et al. (2000) demonstrating significant increases in the number of open intestinal tight junctions in patients receiving high-dose chemotherapy. Given the regulatory roles of tight junctions in maintaining mucosal barrier function, these data strongly suggest tight junction involvement in CIGT pathophysiology.
In addition to these clinical findings, recent in vivo research has identified elevated proinflammatory cytokines7 and pathogenic bacteria25 as hallmarks of CIGT. Importantly, proinflammatory cytokines26 and pathogenic bacteria27 exhibit modulatory effects on tight junction proteins and therefore may be responsible for changes in barrier integrity. Breakdown of the mucosal barrier enables noxious agents and pathogens to penetrate the epithelium. This phenomenon was recently reported, with bacteria found in the basolateral compartment and mesenteric lymph nodes of chemotherapy-treated rats.28 It is therefore hypothesized that bacteria- and proinflammatory cytokine-mediated tight junction disruption are pivotal in the development of gut toxicity.10
Results
Irinotecan causes significant gut toxicity
All rats receiving irinotecan developed gut toxicity represented by diarrhea and significant weight loss. Diarrhea occurred in a biphasic response, with symptoms first appearing at 6 h after irinotecan administration. There was an initial resolution of diarrhea before a second more severe diarrhea appeared, with maximal symptoms seen 72 h post-irinotecan. Rats receiving vehicle control did not develop diarrhea at any time point (Fig. 1A).
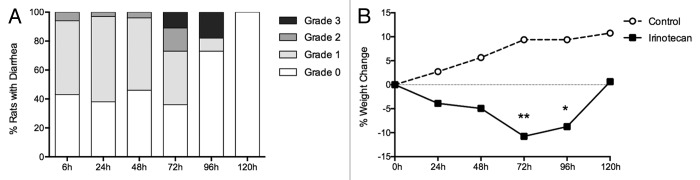
Figure 1. Measures of clinical toxicity. (A) Percentage of rats with grade 0, 1, 2, and 3 diarrhea between 6 and 120 h following irinotecan (175 mg/kg ip) administration. (B) Percentage change in weight from baseline to 120 h in rats following vehicle control (sorbitol/lactic acid buffer: 45 mg/mL sorbitol/0.9 mg/mL lactic acid, pH 3.4) or irinotecan (175 mg/kg ip). *P < 0.01 vs. 120 h, **P < 0.0001 vs. 24 h and 120 h. A one-way analysis of variance with Tukey’s post hoc was performed to determine significance (n = 39).
Rats receiving irinotecan had a peak weight loss compared with baseline at 72 h after chemotherapy (mean ± SD = 11.1 ± 6.6%, P < 0.0001) before recovery at 120 h (mean ± SD = −0.25 ± 6.7%). Rats receiving vehicle control continued to gain weight over the course of the experiment (Fig. 1B).
Irinotecan causes severe histological damage in the small and large intestines
Marked histological evidence of gut toxicity was observed in the jejunum and colon of irinotecan-treated rats (Fig. 2). Characteristic apoptotic bodies were observed at 6 h post-irinotecan in the crypt epithelium of the both regions of the gut. Gross architectural disturbances, including villous blunting and crypt degeneration throughout the mucosa, were particularly evident 48 h and 72 h after irinotecan administration (Fig. 2). Restoration of the mucosa was evident by 120 h, indicated by the return of architectural integrity and the presence of mitotically active cells.
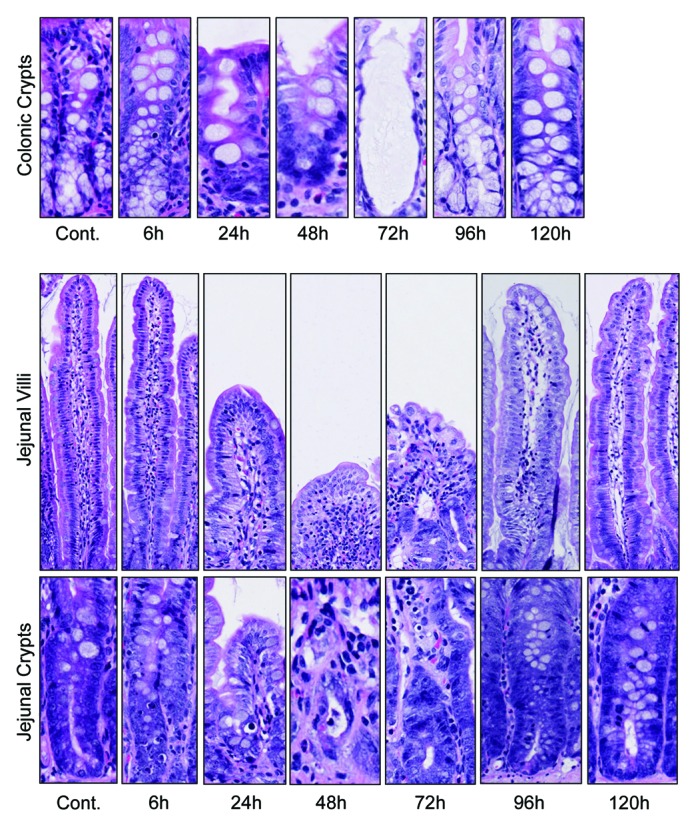
Figure 2. Irinotecan administration causes severe histological damage in the jejunum and colon of DA rats. Characteristic apoptotic bodies are visible at 6 and 96 h post-irinotecan. Gross architectural changes (villous blunting and crypt degeneration) are evident at 24 h, but are most severe at 48 and 72 h hours. Restoration of the epithelium is evident at 120 h, indicated by mitotically active cells. Original magnification 200× (villus) and 400× (crypt).
Irinotecan causes molecular defects in intestinal tight junction proteins
ZO-1
There were no significant changes in protein expression of ZO-1 in the jejunum at any time point investigated (P = ns) (Fig. 3). In contrast, there was a significant decrease in ZO-1 protein expression 96 h following administration of irinotecan in the colonic crypts (P < 0.05) (Figs. 4 and 5). ZO-1 mRNA expression remained stable across the time course of gut toxicity (P = ns) in the small and large intestine (data not shown).
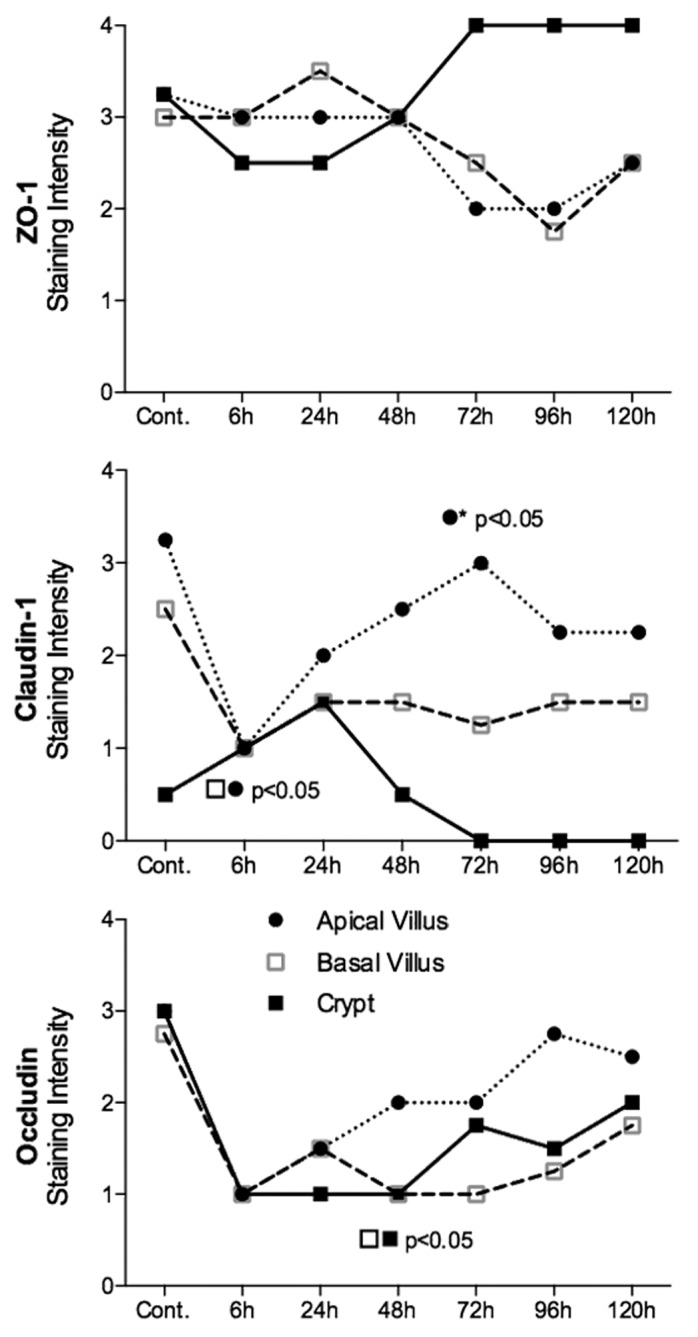
Figure 3. Irinotecan causes molecular defects in all claudin-1 and occludin in the jejunum of the DA rat. There was no statistically significant change in ZO-1 protein expression in the jejunum. Claudin-1 protein expression was significantly decreased 6 h following irinotecan administration, while occludin expression was downregulated in jejunal crypt epithelium 48 h following treatment. ZO-1, claudin-1, and occludin protein expression was analyzed apical villus, basal villus, and crypt epithelium of the jejunum. Staining intensity was analyzed in a blinded fashion (HR Wardill and RJ Gibson) using a validated semi-quantitative grading system.55 A Kruskall–Wallis with a Dunn multiple comparison was performed to determine significance. Data presented as median values (n = 39); ●■□ P < 0.05 vs. control, ●* P < 0.05 vs. 6 h.
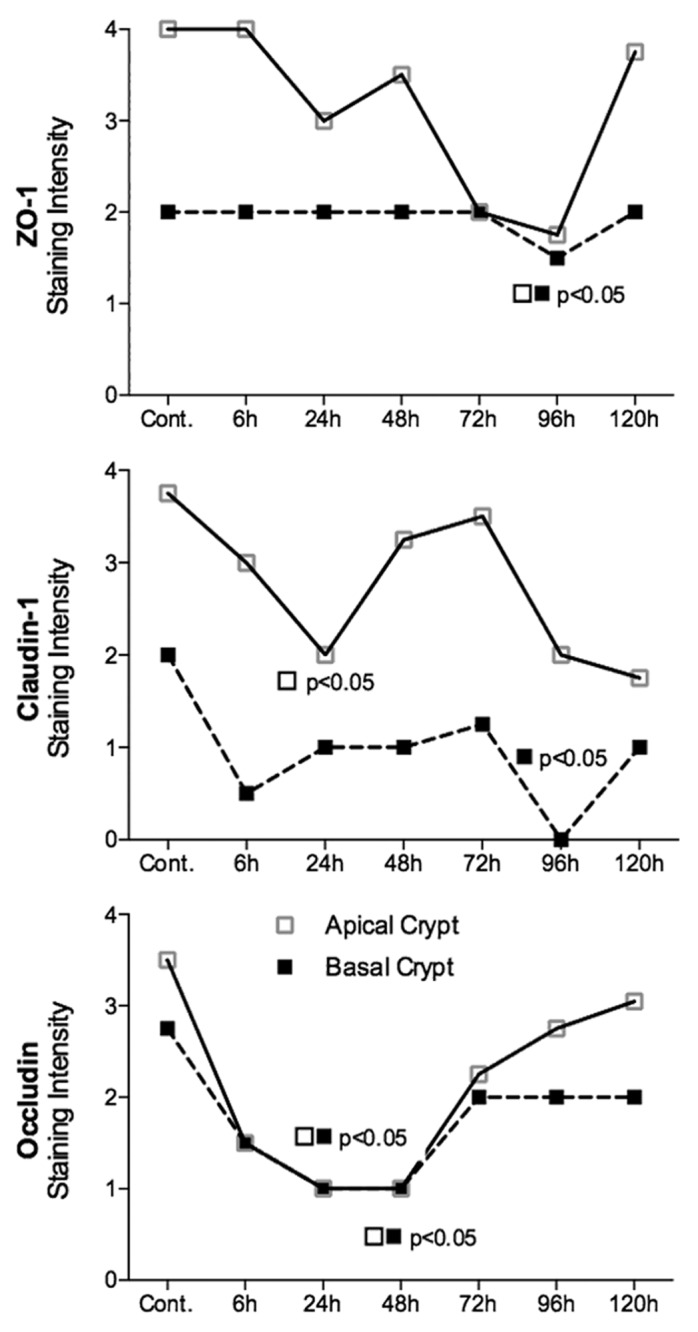
Figure 4. Irinotecan causes tight junction defects in the colon of DA rats. ZO-1 protein expression was significantly decreased in the apical crypt epithelium 96 h following chemotherapy. Irinotecan caused significant downregulation in claudin-1 protein expression in the apical crypt epithelium 24 h following treatment, while a significant decrease was observed 96 h following irinotecan in the crypt crypt epithelium of the colon. Significant downregulation in occludin protein expression was observed at 24 and 48 h following irinotecan administration in the apical and basal crypt epithelium. ZO-1, claudin-1, and occludin protein expression was analyzed in the basal and apical crypt epithelium of the colon. Staining intensity was analyzed in a blinded fashion (HR Wardill and RJ Gibson) using a validated semi-quantitative grading system.55 A Kruskall–Wallis with a Dunn multiple comparison was performed to determine significance. Data presented as median values (n = 39); ■□ P < 0.05 vs. control.
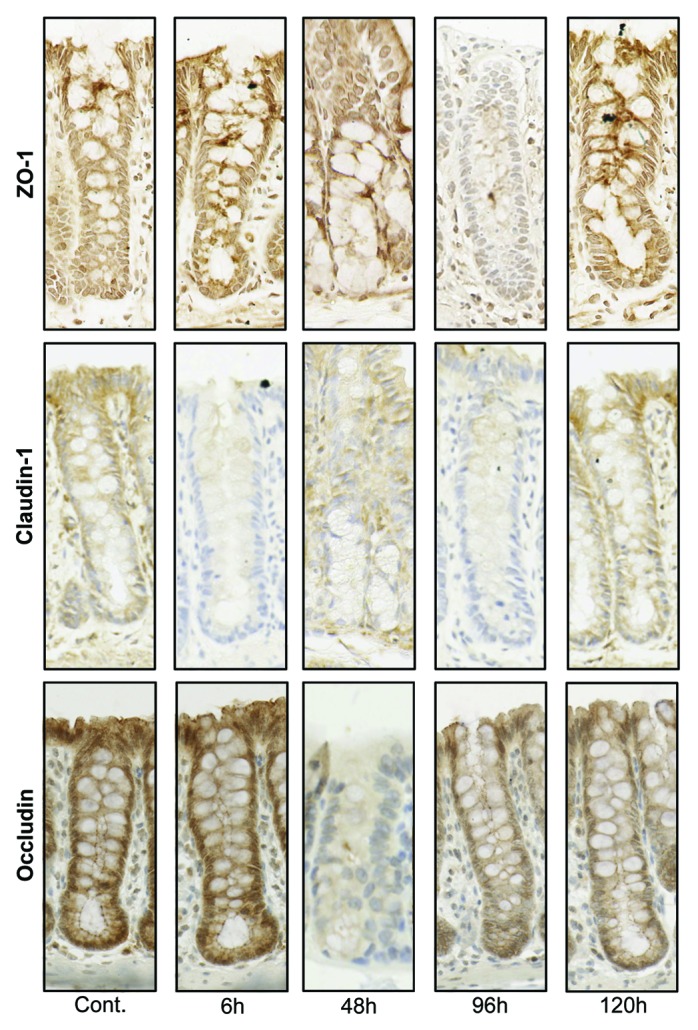
Figure 5. Tight junction protein (ZO-1, claudin-1, and occludin) immunostaining in the colon at selected time points following irinotecan (175 mg/kg ip) administration. Photomicrographs taken at original magnification 400×.
Claudin-1
Claudin-1 protein expression was significantly decreased 6 h after irinotecan administration in both the apical and basal villus regions of the jejunum (P < 0.05) (Fig. 3). Expression returned to baseline at 72 h. No significance difference was observed in jejunal crypts (P = ns). There was a significant decrease in claudin-1 protein expression at 24 and 96 h following irinotecan in the apical and basal colonic crypts, respectively (P < 0.05) (Figs. 4 and 5). Claudin-1 mRNA expression was significantly downregulated (P < 0.05) 6 h following chemotherapy in both the jejunum (−8.3-fold change) and colon (−1.8-fold change) of DA rats. Levels remained significantly (P < 0.05) low at 24 h in the colon only (−2.5-fold change). A 9.8-fold increase in claudin-1 mRNA expression was observed in the jejunum at 72 h following irinotecan (Fig. 6).
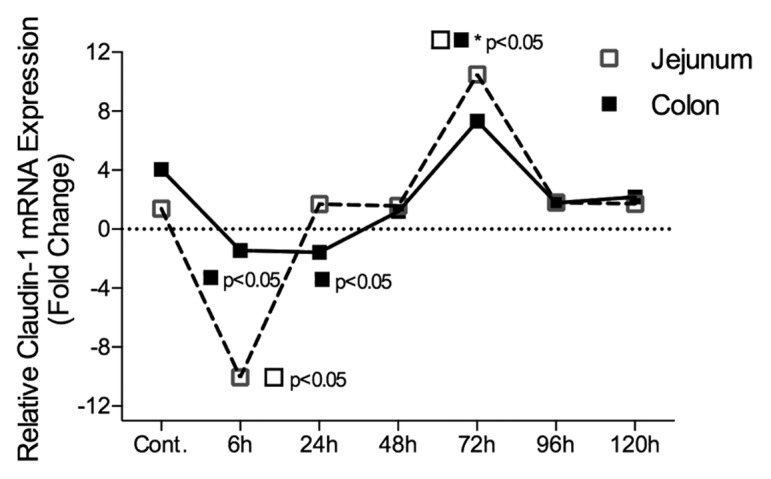
Figure 6. Claudin-1 mRNA expression in the jejunum and colon of irinotecan-treated DA rats. Irinotecan caused an −8.3-fold change in claudin-1 mRNA expression in the jejunum 6 h following irinotecan followed by a 9.8-fold increase at 72 h. A −1.8 and −2.5-fold change in claudin-1 mRNA expression was observed in the colon of irinotecan treated DA rats at 6 h and 24 h, respectively. The fold change in mRNA expression values were used to create the graphs. All data are relative to internal controls (untreated animals, n = 4) and a validated housekeeping gene (UBC). Relative mRNA expression was calculated using the Pfaffl method of relative quantification. A one-way analysis of variance with the Tukey post hoc was performed to determine significance. Data presented as median values (n = 39); ■□ P < 0.05 vs. control, ■□*P < 0.05 vs. 6 h.
Occludin
Occludin protein expression was significantly downregulated in the jejunal crypts 48 h following irinotecan administration (P < 0.05) (Fig. 3). This pattern of expression was mirrored in the colon, where occludin protein expression was significantly downregulated (P < 0.05) in both apical and basal crypt regions at 48 h following irinotecan treatment (Fig. 4 and 5). There was no change in occludin mRNA expression in the jejuna or colon of DA rats (P = ns) at any time point under investigation (data not shown).
RT-PCR efficiency
The amplification efficiencies for each set of primers were measured using serial dilutions of cDNA in triplicate. PCR efficiency and a standard curve were calculated by the Rotor Gene 6 program. Variations in cycle times and annealing temperatures did not yield comparable PCR efficiencies (ZO-1 = 1.01; claudin-1 = 1.1; occludin = 1.21; UBC = 1.31); therefore the Pfaffl method29 of relative quantification was employed.
Stability of housekeeping gene (UBC)
The fold change in UBC expression was calculated using the 2−ΔΔCt method where control rats were used as a baseline. UBC showed no differential mRNA expression in the jejunum or colon of DA rats across the time-course of irinotecan-induced gut toxicity (data not shown), indicating high stability.
Discussion
Chemotherapy-induced gut toxicity (CIGT) has become increasingly important as a dose-limiting factor of anti-cancer therapies, particularly with the advent of more aggressive chemotherapy regimens.2 Evidence exists for the involvement of tight junctions in the pathophysiology of CIGT,10 however, to date few studies have conducted detailed investigations of intestinal tight junctions following chemotherapy. The present study therefore aimed to characterize the expression of three key tight junctional proteins, ZO-1, claudin-1, and occludin, in both the small and large intestine using a well-established pre-clinical model of gut toxicity. Key findings from this study indicate that tight junction defects coincide with validated histolopathological and clinical markers of gut toxicity (diarrhea and weight loss), strongly implicating tight junctions in the development of CIGT.
Late-onset diarrhea is a key dose-limiting factor for many chemotherapy patients,30 however to date there are no clear mechanisms underpinning its development. The clinical and economic impact associated with chemotherapy-induced diarrhea has only recently been appreciated, prompting research efforts in an attempt to reveal the pathophysiology of this complication.31 Despite having vastly different pathogeneses, inflammatory bowel disorders (IBDs) are often used to identify potential mechanisms of CIGT due to highly comparable clinical manifestations.17 Recent research investigating the pathophysiology and symptomology of IBDs has shown that tight junction defects and barrier disturbances contribute to diarrhea development through leak-flux mechanisms.32-34 In line with this research, the present study identified tight junction defects which coincided with key events in the pathophysiology of CIGT and preceded the onset of clinical symptoms. Of particular importance was the significant decrease in ZO-1 protein expression in the colonic crypts at 96 h following insult, coinciding with the presence of severe late-onset diarrhea observed in our animal model. Previous research has also shown that chemotherapy induces significant derangement of intestinal electrolyte and water fluxes which correlate with overt treatment-induced diarrhea.35 Given that tight junctions are key regulators of mucosal barrier function, it is feasible that disruption to tight junction proteins, particularly ZO-1, may contribute to the development of late-onset diarrhea through leak-flux mechanisms. These findings highlight tight junctions as a potential therapeutic target in the clinical setting and detailed investigations are now required.
In addition to ZO-1 disruption, the current study identified significant changes in claudin-1 at both the protein and gene level. Previous research has identified emerging roles for claudin-1 in mediating apoptosis and aiding tissue regeneration.18,36 Liu and colleagues recently demonstrated this concept, reporting significantly increased levels of claudin-1 in human breast cancer MCF-7 cells—a cell line recognized for their low levels of apoptosis. In line with these findings, downregulation of claudin-1 by siRNA knockdown resulted in a significant increase in apoptosis markers such as caspase-8. Further, loss of claudin-1 was shown to increase the susceptibility of MCF-7 cells to TNF-induced apoptosis. In the current study, significantly reduced claudin-1 protein and mRNA expression was observed 6 h following irinotecan insult, coinciding with peak apoptosis levels1 thus supporting this novel role of claudin-1 as a potent anti-apoptotic mediator. Claudin-1 downregulation was also identified 24 h following chemotherapy, and this is likely due to the slower proliferative rate of the colon relative to the jejunum. In addition to apoptosis, our results also support a role for claudin-1 in aiding tissue regeneration, a newly identified function of claudin-1, as its overexpression coincides with the known time point of cellular regeneration in the epithelium of the jejunum and colon (72 h).4-6 Unexpectedly however, this was accompanied by a decrease in protein expression in the colonic crypts and which may be attributable to the second wave of apoptosis, as the body resets homeostasis.1
There is conflicting evidence regarding mRNA analysis of tight junction proteins following chemotherapy. Most recently, a preclinical study conducted by Nakao et al. (2012) investigated tight junction proteins in tumor-naïve rats and found tight junction changes were associated with gut barrier dysfunction. Specifically, a significant decrease in both occludin and claudin-1 mRNA expression was identified;28 however given their small sample size (n = 10) and single time-point evaluation, these conclusions should be interpreted with caution. In contrast, several other preclinical studies found no change in occludin and ZO-1 mRNA expression following administration of methotrexate (MTX), but demonstrated decreased claudin-1 mRNA expression.37,38 In accordance with these latter findings, our study did not identify any significant change in occludin or ZO-1 mRNA expression following irinotecan administration. These inconsistencies highlight the need for further research to clarify the effect of various cytotoxic agents on tight junction protein mRNA levels at time points relevant to clinical symptoms.
The disparate protein and mRNA expression identified in the present study suggest that irinotecan indirectly modulates both occludin and ZO-1 through posttranslational regulation. Posttranslational modification of occludin and ZO-1, in particular proteolytic degradation, is a well-documented phenomenon.12 Recent research has indicated proinflammatory cytokines are able to modulate tight junction proteins26,39 and it is therefore hypothesized that cytokine-mediated tight junction modulation may play a key role in the development of CIGT.10 In vitro application of tumor necrosis factor (TNF) has been shown to induce actin and tight junction rearrangement, resulting in a time-dependent decrease in tight junction protein expression and a parallel increase in intestinal permeability.26,40,41 Previous research has shown that elevated serum TNF and interleukin-1 are hallmarks of CIGT pathophysiology, with peaks occurring at 24–48 h post-chemotherapy.7 The present study has shown that occludin downregulation also occurs at these time-points, implying a correlation between the two. It is therefore suggested that proinflammatory cytokines not only contribute to histological damage in the gut, but also mediate mucosal barrier function through tight junction modulation.
Matrix metalloproteinases (MMPs) are extracellular matrix signaling molecules recently recognized for their proteolytic functions.12 Substantial in vivo evidence supports a role for MMP-mediated occludin proteolysis and membrane cleavage.42,43 This is of particular importance as MMPs have recently been identified as key mediators of intestinal toxicity induced by irinotecan.9 Al-Dasooqi and colleagues (2010) reported significantly increased small intestinal and serum levels of MMP-9 and MMP-12 following irinotecan. Given the proteolytic functions of MMPs this suggests MMP-mediated tight junction damage may occur. This correlation has previously been shown in the central nervous system, with MMP-9 levels in brain tissue correlating with reductions in occludin and ZO-1 protein levels.43,44 Further, MMP inhibition has been shown to ameliorate hypoxic-induced occludin proteolysis.45 Further studies are now warranted in order to establish the molecular mechanisms of these posttranslational regulations.
In addition to posttranslational modulation, reversible phosphorylation of tight junction proteins has recently been established as a vital aspect of tight junction integrity and barrier regulation.12,46 Despite the importance of tight junction phosphorylation for optimal tight junction assembly and function,12,46 few studies have investigated posttranslational phosphorylation of tight junction proteins in the gut. Hamada and colleagues38 reported decreased tyrosine phosphorylation of ZO-1 despite observing no change in ZO-1 protein and mRNA expression. Although not addressed in this study, dephosphorylation may be responsible for chemotherapy-induced intestinal barrier dysfunction and future investigations are now necessary.
Conclusion
Tight junction proteins can undergo a broad array of regulatory modification in response to various pathological cues, highlighting the plasticity of these dynamic signaling complexes. These molecular events are likely to contribute to mucosal barrier dysfunction, increased intestinal permeability and the development of diarrhea; all characteristic of CIGT. It is likely that ZO-1 primarily is a late-onset and symptom-associated protein, whereas claudin-1 and occludin have more potential as prognostic markers as their modulation precedes barrier dysfunction and symptomology. Detailed research is now required to investigate posttranslational modulation of tight junction proteins to elucidate the molecular mechanisms that underpin the development of gut toxicity. This may lead to a greater understanding of how tight junction modifications affect gastrointestinal homeostasis, thus revealing their therapeutic potential in chemotherapy-induced gut toxicity and other diseases characterized by barrier dysfunction.
Methods
Animals and ethics
Female Dark Agouti (DA) rats, weighing between 150 and 170 g were used for this study. Rats were housed in Perspex cages at a temperature of 22 ± 1 °C and subject to a 14 h light/10 h dark cycle. Animals had ad libitum access to autoclaved chow and water. Experimental design was approved by the Animal Ethics Committees of the Institute of Medical and Veterinary Science (IMVS), and The University of Adelaide, and complied with the National Health and Medical Research Council (Australia) Code of Practice for Animal Care in Research and Teaching.47
Experimental design
The dark agouti mammary adenocarcinoma (DAMA) rat model of irinotecan-induced gut toxicity was used to conduct this study. DA rats are tumor-bearing as our previous research has shown that the response to irinotecan is more pronounced in tumor-bearing rats compared with naïve rats.48 Forty rats were randomly assigned to receive either irinotecan (n = 5–9 per time point) or vehicle control (n = 6). All rats received breast cancer inoculum as described previously.49,50 Briefly, mammary adenocarcinoma tumors syngeneic with the DA rat were diced, homogenized and filtered through sterile gauze. A viable cell count was conducted using 0.4% w/v trypan blue before 150 μl of cells were implanted subcutaneously into both right and left flanks of the rat at a concentration of 2.0 × 107 cells/mL. Tumors were allowed to grow for one week prior to administration of chemotherapy and did not exceed 15% of their total body weight at study end point. All rats received 0.01 mg/kg subcutaneous atropine (to reduce the cholinergic reaction) immediately prior to administration of either 175 mg/kg irinotecan (intraperitoneal) (kindly supplied by Pfizer, administered in a sorbitol/lactic acid buffer: 45 mg/mL sorbitol/0.9 mg/mL lactic acid, pH 3.4), or vehicle control (sorbitol/lactic acid buffer). This buffer has previously been shown to have no gut toxicity effects.51 Groups of rats were killed by exsanguination and cervical dislocation while under 3% isoflourane in 100% O2 anesthesia at times 6, 24, 48, 72, 96, and 120 h post-irinotecan treatment. The entire gastrointestinal tract (from the pyloric sphincter to the rectum) was dissected out and separated into the small intestine (pyloric sphincter to ileocecal sphincter) and colon (ascending colon to rectum). The small intestines and colons were flushed with chilled, sterile saline (Baxter Healthcare), and 1 cm samples dissected from 50% of the length of each, and fixed in 10% neutral buffered formalin, processed, and embedded in paraffin for immunohistological analyses.
Gut toxicity assessment
Gut toxicity assessment, including measures of weight loss and diarrhea, was recorded on a daily basis including baseline. Animals were weighed daily at the same time, and total weight loss/gain recorded. Further, diarrhea occurrence and severity was recorded 4× daily according to previous grading:48,51 0, no diarrhea; 1, mild diarrhea (staining of anus); 2, moderate diarrhea (staining spreading over top of legs); and 3, severe diarrhea (staining over legs and abdomen, often with continual anal leakage). All gut toxicity assessments were conducted in a blinded fashion (RJ Gibson and M Sultani).
Tissue preparation
Samples of small (jejunum) and large (colon) intestine were collected, snap frozen in liquid nitrogen and stored in RNAlater (Ambion) for molecular analysis. All tissues were stored at −80 °C until required. Additional samples (1 cm in length) of jejunum and colon were fixed in 10% neutral buffered formalin, processed, and embedded in paraffin wax for histological and immunohistochemical analyses.
Histological analysis
Sections of jejunum and colon were cut from paraffin blocks at 5 μm and mounted onto glass microscope slides. Slides were dewaxed in xylene, stained with Lilli-Mayer hematoxylin for 10 min and counterstained in eosin for 3 min. Slides were dehydrated through graded ethanols, cleared in xylene and coverslipped. Slides were scanned using the NanoZoomer (Hamamatsu Photonics) and assessed with NanoZoomer Digital Pathology software (Histalim, Montpellier). Validated histopathological markers of intestinal mucosal injury were used to examine histological damage.52
Immunohistochemistry
Immunohistochemical analysis was performed using the Level 2 USATM Ultra Streptavidin Detection System kit (Signet Laboratories) as per manufacturer’s instructions. Antigen retrieval was conducted using either a 10 mM/l citrate buffer (occludin and claudin-1) or protease pretreatment (Sigma P5147). To reduce nonspecific staining sections were blocked in 3% hydrogen peroxidase (H2O2)/methanol solution and 5% normal blocking serum in 0.01 M of PBS containing 0.1% sodium azide (NaN3). Sections were incubated with avidin/biotin blocking solution (Signet Laboratories) for 15 min, before the primary antibody was applied for overnight a 4 °C (rabbit polyclonnal anti-claudin-1 (10 μg/μl) and anti-ZO-1 (10 μg/μl); mouse monoclonal anti-occludin (2.5 μg/μl). Negative control sections had the primary antibody omitted and liver was used as a positive control in all experiments. Both linking and labeling reagents were applied (Signet Laboratories) followed by diaminobenzidine (DAB) chromogen in 0.03% hydrogen peroxidase. Slides were counterstained in Harris Haematoxylin, before being dehydrated, cleared, and coverslipped. Liver was used as a positive control in all immunohistochemistry runs. Slides were assessed using NanoZoomer Digital Pathology software (Histalim) and analyzed using a validated semi-quantitative grading system:9 0 = no staining, 1 = weak, 2 = mild, 3 = moderate, and 4 = intense. All samples were analyzed in a blinded fashion by two investigators (HR Wardill and RJ Gibson). As the focus of tight junction staining is epithelial, this was the only tissue region analyzed.
Real-time polymerase chain reaction
RNA extraction
RNA extraction was performed on jejunum and colon samples as per manufacturer’s instructions (NucleoSpin RNA Isolation Kit, Macherey-Nagel). Briefly, tissue samples were homogenized in 250 μl and 500 μl of TRIzol® Reagent (Invitrogen), respectively. Samples were centrifuged for 15 min at 4 °C and the upper aqueous layer removed. Following a sequence of filtration steps, RNA-binding conditions were adjusted and DNA digestion performed. A series of washing steps was performed before highly pure mRNA was eluted in RNase-free water. Once eluted, RNA was stored at −80 °C.
Assessment of RNA quantity and quality
RNA was quantified for yield (ng/μl) and purity using the Thermo Scientific Nanodrop 1000 spectrophotometer. RNA integrity was assessed at the Adelaide Microarray Facility (SA Pathology) using the Agilent 2100 Bioanalyser RNA 6000 Nano Chip (Series II) kit.
Reverse transcription and RT-PCR
One microgram of RNA was reverse transcribed using the iScriptTM cDNA Synthesis Kit (BioRad) as per manufacturer’s instructions. RT-PCR was performed using the Rotor-Gene 3000 (Corbett Research). Amplification mixes contained 1 μl of cDNA sample (100 ng/μl), 5 μl of SYBR green fluorescence dye, 3 μl of RNase-free water and 0.5 μl of each forward and reverse primer (prediluted to 50 pmol/μl) to make a total volume of 10 μl. Thermal cycling conditions included a denaturing step at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 56 °C for 15 s, and extension at 72 °C for 20 s. All samples were run in triplicate. Primer efficiency was evaluated using standard curves and cycle threshold (Ct) values were calculated by Rotor Gene 6 analysis software. Ct values were used to quantify occludin gene expression, relative to untreated control calibrator and a validated housekeeping gene using the Pfaffl method of relative quantification.29 Ubiquitin C (UBC), a validated housekeeping gene,53 was used as a reference gene in all RT-PCR runs. Housekeeping gene stability was validated using the 2−ΔΔCt method.54
Statistical analysis
Data were compared using Prism version 6.0 (GraphPad® Software). A Kolmogorov–Smirnov test was used to assess normality and a Bartlett test was employed to assess equal variance. When normality and equal variance were confirmed, a one-way analysis of variance with a Tukey post hoc test was used to identify statistical significance between groups. In other cases, a Kruskall–Wallis with a Dunn multiple comparison was used to identify statistical significance. A P value < 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts were disclosed.
Acknowledgments
The authors would like to thank Mrs Emily Schneider, Mrs Nadia Gagliardi, and Mr Chris Leigh from the School of Medical Sciences for technical assistance with immunohistochemistry staining and Mr Tavik Morgenstern for technical assistance with figure layouts. J.B. is the recipient of an NHMRC Post-Doctoral Training Fellowship, N.A.-D. is the recipient of a Clinical Centre of Research Excellence Post-Doctoral Training Fellowship. Funding for this project was provided by a Cure Cancer/Cancer Australia Research Grant and a South Australian Professional Development Scholarship awarded to R.J.G.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/27222
References
- 1.Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–7. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531–9. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 3.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE, Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–31. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 4.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2(Suppl 3):3–8. [PubMed] [Google Scholar]
- 5.Sonis ST. A biological approach to mucositis. J Support Oncol. 2004;2:21–32, discussion 35-6. [PubMed] [Google Scholar]
- 6.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 7.Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther. 2008;7:1139–45. doi: 10.4161/cbt.7.7.6207. [DOI] [PubMed] [Google Scholar]
- 8.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Keefe DM. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther. 2008;7:1919–25. doi: 10.4161/cbt.7.12.6940. [DOI] [PubMed] [Google Scholar]
- 9.Al-Dasooqi N, Gibson RJ, Bowen JM, Logan RM, Stringer AM, Keefe DM. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp Biol Med (Maywood) 2010;235:1244–56. doi: 10.1258/ebm.2010.010082. [DOI] [PubMed] [Google Scholar]
- 10.Wardill HR, Bowen JM, Gibson RJ. Chemotherapy-induced gut toxicity: are alterations to intestinal tight junctions pivotal? Cancer Chemother Pharmacol. 2012;70:627–35. doi: 10.1007/s00280-012-1989-5. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–81. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 12.Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32:242–50. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–56. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Shin K, Margolis B. ZOning out tight junctions. Cell. 2006;126:647–9. doi: 10.1016/j.cell.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Will C, Fromm M, Müller D. Claudin tight junction proteins: novel aspects in paracellular transport. Perit Dial Int. 2008;28:577–84. [PubMed] [Google Scholar]
- 16.Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–73. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 17.Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009;1165:294–300. doi: 10.1111/j.1749-6632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 18.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–98. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 20.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–76. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 21.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 1997;92:385–9. doi: 10.1042/cs0920385. [DOI] [PubMed] [Google Scholar]
- 24.Blijlevens NM, Donnelly JP, de Pauw BE. Prospective evaluation of gut mucosal barrier injury following various myeloablative regimens for haematopoietic stem cell transplant. Bone Marrow Transplant. 2005;35:707–11. doi: 10.1038/sj.bmt.1704863. [DOI] [PubMed] [Google Scholar]
- 25.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Burns J, Keefe DM. Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the DA rat. Exp Biol Med (Maywood) 2007;232:96–106. [PubMed] [Google Scholar]
- 26.Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–8. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–93. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, Utusnomiya T, Shimada M. Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res. 2012;173:341–7. doi: 10.1016/j.jss.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson RJ, Keefe DM. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer. 2006;14:890–900. doi: 10.1007/s00520-006-0040-y. [DOI] [PubMed] [Google Scholar]
- 31.Bowen JM, Elad S, Hutchins RD, Lalla RV, Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) Methodology for the MASCC/ISOO Mucositis Clinical Practice Guidelines Update. Support Care Cancer. 2013;21:303–8. doi: 10.1007/s00520-012-1592-7. [DOI] [PubMed] [Google Scholar]
- 32.Amasheh M, Grotjohann I, Amasheh S, Fromm A, Söderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009;44:1226–35. doi: 10.1080/00365520903131973. [DOI] [PubMed] [Google Scholar]
- 33.Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–43. doi: 10.1053/gast.2002.34784. [DOI] [PubMed] [Google Scholar]
- 34.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 35.Carneiro-Filho BA, Lima IP, Araujo DH, Cavalcante MC, Carvalho GH, Brito GA, Lima V, Monteiro SM, Santos FN, Ribeiro RA, et al. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci. 2004;49:65–72. doi: 10.1023/B:DDAS.0000011604.45531.2c. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Wang L, Lin XY, Wang J, Yu JH, Miao Y, Wang EH. Anti-apoptotic effect of claudin-1 on TNF-α-induced apoptosis in human breast cancer MCF-7 cells. Tumour Biol. 2012;33:2307–15. doi: 10.1007/s13277-012-0493-1. [DOI] [PubMed] [Google Scholar]
- 37.Beutheu Youmba S, Belmonte L, Galas L, Boukhettala N, Bôle-Feysot C, Déchelotte P, Coëffier M. Methotrexate modulates tight junctions through NF-κB, MEK, and JNK pathways. J Pediatr Gastroenterol Nutr. 2012;54:463–70. doi: 10.1097/MPG.0b013e318247240d. [DOI] [PubMed] [Google Scholar]
- 38.Hamada K, Shitara Y, Sekine S, Horie T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother Pharmacol. 2010;66:1031–8. doi: 10.1007/s00280-010-1253-9. [DOI] [PubMed] [Google Scholar]
- 39.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–61. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–6. [PubMed] [Google Scholar]
- 41.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. doi: 10.1016/S0002-9440(10)62264-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casas E, Barron C, Francis SA, McCormack JM, McCarthy KM, Schneeberger EE, Lynch RD. Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments. Exp Cell Res. 2010;316:353–65. doi: 10.1016/j.yexcr.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beauchesne E, Desjardins P, Hazell AS, Butterworth RF. Altered expression of tight junction proteins and matrix metalloproteinases in thiamine-deficient mouse brain. Neurochem Int. 2009;55:275–81. doi: 10.1016/j.neuint.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–27. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 45.Bauer AT, Bürgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010;30:837–48. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Council NHMRC. Australian code of practice for the care and use of animals for scientific purposes. Canberra: National Health and Medical Research Council, 2004. [Google Scholar]
- 48.Gibson RJ, Bowen JM, Alvarez E, Finnie J, Keefe DM. Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy. 2007;53:360–9. doi: 10.1159/000107458. [DOI] [PubMed] [Google Scholar]
- 49.Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG. The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol. 2002;50:53–8. doi: 10.1007/s00280-002-0460-4. [DOI] [PubMed] [Google Scholar]
- 50.Gibson RJ, Keefe DM, Thompson FM, Clarke JM, Goland GJ, Cummins AG. Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci. 2002;47:2751–7. doi: 10.1023/A:1021061306913. [DOI] [PubMed] [Google Scholar]
- 51.Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol. 2003;18:1095–100. doi: 10.1046/j.1440-1746.2003.03136.x. [DOI] [PubMed] [Google Scholar]
- 52.Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DM. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol. 2008;62:33–41. doi: 10.1007/s00280-007-0570-0. [DOI] [PubMed] [Google Scholar]
- 53.Al-Dasooqi N, Bowen JM, Gibson RJ, Logan RM, Stringer AM, Keefe DM. Selection of housekeeping genes for gene expression studies in a rat model of irinotecan-induced mucositis. Chemotherapy. 2011;57:43–53. doi: 10.1159/000321477. [DOI] [PubMed] [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 55.Bowen JM, Gibson RJ, Keefe DM, Cummins AG. Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology. 2005;37:56–62. doi: 10.1080/00313020400023461. [DOI] [PubMed] [Google Scholar]


