Abstract
Helicobacter pylori infections can induce pathologies ranging from chronic gastritis, peptic ulceration to gastric cancer. Bacterial isolates harbor numerous well-known adhesins, vacuolating cytotoxin VacA, protease HtrA, urease, peptidoglycan, and type IV secretion systems (T4SS). It appears that H. pylori targets more than 40 known host protein receptors on epithelial or immune cells. A series of T4SS components such as CagL, CagI, CagY, and CagA can bind to the integrin α5β1 receptor. Other targeted membrane-based receptors include the integrins αvβ3, αvβ5, and β2 (CD18), RPTP-α/β, GP130, E-cadherin, fibronectin, laminin, CD46, CD74, ICAM1/LFA1, T-cell receptor, Toll-like receptors, and receptor tyrosine kinases EGFR, ErbB2, ErbB3, and c-Met. In addition, H. pylori is able to activate the intracellular receptors NOD1, NOD2, and NLRP3 with important roles in innate immunity. Here we review the interplay of various bacterial factors with host protein receptors. The contribution of these interactions to signal transduction and pathogenesis is discussed.
Keywords: c-Met, E-cadherin, EGF receptor, integrins, molecular pathogenesis, virulence
Introduction
H. pylori is a predominant extracellular pathogen colonizing the stomach of about half of the human world population. These infections are associated with chronic, often asymptomatic gastritis in all infected individuals, while less often more severe gastric diseases can arise such as peptic ulcer disease and gastric cancer.1,2 Gastric adenocarcinoma represent the second leading cause of cancer-related death worldwide with about 700 000 people dying by this malignancy each year.3 The clinical outcome of infections with H. pylori is dependent on a very complex scenario of host-pathogen interactions. Disease progression is controlled by various key factors including the genetic predisposition of the host, the bacterial genotype, and environmental parameters.1-4 The cellular and molecular mechanisms developed by H. pylori to undermine host defense strategies are subject of intense investigation. H. pylori strains are highly diverse both in their genetic polymorphisms and potential to induce pathogenicity. Dozens of bacterial factors have been reported to influence the pathogenesis of H. pylori infections. There are two major virulence determinants expressed by the bacteria, the CagA protein encoded by the cytotoxin-associated genes pathogenicity island (cagPAI) and the vacuolating cytotoxin (VacA). While the cagPAI encodes a type IV secretion system (T4SS) for direct injection of CagA into the host cytoplasm followed by tyrosine phosphorylation at its EPIYA-motifs, VacA is secreted in the culture supernatant and can trigger various responses including pore formation in the host cell membrane, modification of endo-lysosomal trafficking, cellular vacuolation, immune cell inhibition, and apoptosis.1-3,5-8 Other well-described pathogenicity-associated mechanisms include flagella-driven bacterial motility, urease-mediated neutralization of pH, secretion of proteases (such as HtrA) into the extracellular space, shedding of outer-membrane vesicles, and peptidoglycan-dependent immune responses.1,2,9-13
H. pylori expresses several classical surface adhesins which allow tight binding of the bacteria to gastric epithelial cells. H. pylori genomes encode more than 30 outer membrane proteins (OMPs) which can be divided into the Helicobacter outer membrane porins (Hop) and Hop-related (Hor) families.14 The Hop group of proteins includes several well described cell adhesion factors such as BabA, SabA, AlpA/B, HopI, HopQ, HopZ, and OipA. It was shown that considerable diversity can be found among the OMPs of clinical H. pylori strains. This feature possibly reflects a selective evolutionary pressure for bacterial adhesion which may differ both across and within infected individuals during the lifelong infection process. H. pylori encodes some well-known factors targeting host carbohydrates. BabA and SabA, the two first discovered H. pylori adhesins, are typical examples.15 While BabA binds to blood group antigens specific for ABO and Lewis b (Leb) antigens, SabA interacts specifically with sialylated Lewis x (sLex), and sialylated Lewis a (sLea) antigens. It has been found that some of the adhesion factors discussed below act in conjunction with other proteins, e.g., from the cagPAI, in order to hijack several host cell processes including varied transcription, opening of cell-to-cell junctions, cytoskeletal rearrangements, onset of inflammation, and others.14-17 In addition to the above carbohydrate binders, intensive research in recent years has demonstrated that H. pylori also targets host cholesterol, heparan sulfate, phosphatidylserine, sphingomyelin, and other lipids1,7,13,18,19 as well as a broad variety of host protein receptors. For 25 of these protein receptors the corresponding bacterial factor has been identified (summarized in Table 1), while for at least 19 others the matching bacterial factors remain unknown (Table S1). There is also a growing list of H. pylori factors for which a host receptor has been proposed, but its identity has not been unraveled. Here we review the various molecular strategies of known H. pylori pathogenicity factors which facilitate binding to and signaling through host protein receptors of gastric epithelial and immune cells. We focus on the identified host cell surface proteins receptors, but also discuss several intracellular receptors which are crucial part of the host immune system. An overall model for major activities and signaling strategies exploited by H. pylori is presented in Figure 1.
Table 1. Targeted host cell protein receptors interacting with known ligands of H. pylori.
| Host protein receptor | Bacterial factor | Host cells used | H. pylori strains used | Applied methods | Proposed role during infection and pathogenesis | References |
|---|---|---|---|---|---|---|
| β1 integrin | CagL | AGS, GD25 vs. GD25β1, knockout MEFs | P1, P12 | BBS, PCA, CAA, CD, ABB, CSLM, FESEM, RPT, PIS, siRNA, shRNA ASPAB, WB | Host cell docking of T4SS | 52 |
| AGS | 7.13, P12 | LRGA, siRNA, ELISA, WB, IP, RPT, RT-PCR, PCA | Inhibition of H,Kα -mediated gastric acid secretion | 67,68 | ||
| AGS, MKN45, HeLa, knockout MEFs | P12, purified proteins in vitro | RPT, CAA, CSA, IFM, LCI, FESEM, ASPAB, PIS, WB, PC | Cell attachment, signaling and spreading | 55,56 | ||
| AGS, WM-115 | P12 | RPT, CAA, PCA, CD, ASPAB, PAH, WB | Host cell docking of T4SS | 57 | ||
| CagA, CagI, CagY | GE11 vs. GE11β1, CHO vs. CHOβ1, HL60 vs. dHL60, AGS | P12, P145, P217 | Y2H, RPT, BBS, ABB, PDA, FACS, FESEM, PIS, WB, PC | Host cell docking of T4SS | 59,60 | |
| CagY | AGS, MKN45, HeLa, knockout MEFs | Purified proteins in vitro |
RPT, CAA, CSA | Cell attachment, signaling and spreading | 56 | |
| β3 integrin | CagL | WM-115 | Purified proteins in vitro |
CAA, PCA, NMR | Host cell docking of T4SS | 92 |
| β5 integrin | CagL | AGS, AMO, AM4, Mongolian gerbils | P1, P12, B8, 7.13, 26695, J99, Tx30a, Ka125, 1061 | BBS, RT-PCR, LRGA, siRNA, PIS, WB, IHC | Host cell docking of T4SS, gastrin expression | 91 |
| CD18 (β2 integrin) | VacA | Primary T cells, Jurkat T cells, PBMCs, SKW3 T cells, SK-b2.7 (β2-minus), EL4 T cells | P12, 60190 | FACS, CLSM, IFM, LCI, ELISA, TCPA, WB | Receptor for VacA, deregulation of NFAT and T-cell proliferation | 104 |
| CD46 | Urease A, AhpC | AGS, Kato-III | J99, 26695, HPAG1, 67:21 | RPT, FACS, IFM, CAA, PCA, ELISA, MS | CD46 inhibits urease and is bactericidal to H. pylori | 42 |
| CD74 | Urease B | N87, Kato-III, HS738, P3HR1-B | LC11, 26695, 43504, 51B | FACS, IP, CAA, WB | NFκB activation, changed antigen presentation by MHCII | 39,40 |
| c-Met | T4SS, CagA | AGS, HeLa | P1 | ASPAB, IFM, IP, WB, siRNA, PIS, CMA | Epithelial cell motility | 79 |
| T4SS, CagA | AGS | 26695, 60190 | PIS, CMIA, siRNA, IHC, CSLM, IP, CZG, WB | Epithelial cell invasion | 80,81 | |
| CagA | AGS, EAGS, 293T, BING, Kato-III, CIH28, Mongolian gerbils | 11637, 43504, 43579, 26695, B128 | IP, PDA, siRNA, CSLM, IHC, LRGA, RT-PCR, ELISA, WB | Epithelial cell proliferation and inflammation | 82 | |
| E-cadherin | HtrA | MKN28 | P12 | CSLM, CZG, ICA, TWA, PIS, WB | Epithelial barrier disruption | 12 |
| CagA | MKN28, MKN45 | No infections, CagA transfection | CSLM, IP, LRGA, cDNA-MA, WB | β-catenin activation | 101 | |
| EGF receptor (ErbB1) | VacA | HeLa | 43504 | ABB, IP, PIS, WB | Internalization of VacA by endocytosis | 151 |
| T4SS | AGS | 60190, J44, 43504, J166, J238, J68, 107A | LRGA, PIS, ASPAB, RGAA, ELISA, RT-PCR, EMSA, WB | IL-8 secretion, EGR1 activation | 72,75 | |
| T4SS | AGS, ImPC, kinase-defective EGFR mice | 7.13 | ABB, TUNEL, FACS, ASPAB, PIS, IHC, IFM, WB | Anti-apoptotic signaling | 76 | |
| CagL | AGS, HeLa, MKN28, knockout MEFs | 7.13, P12 | IP, RPT, CAA, CSA, IFM, LCI, ASPAB, PIS, LRGA, siRNA, ELISA, RT-PCR, PCA, WB | Activation of NFκB, cell attachment, signaling and spreading | 56,68 | |
| ErbB2 (Her2-NEU) | T4SS | AGS, HeLa | P1 | ASPAB, WB, PIS, CMA | unknown | 79 |
| T4SS, VacA | AGS | P1, P12, 60190, P310, 26695, P277, 11637 | CMA, IFM, ASPAB, siRNA, WB | Cell signaling and motility, VacA exhibits counteracting activities | 78 | |
| ErbB3 | CagL | AGS, MKN45, HeLa, knockout MEFs | Purified proteins in vitro |
RPT, CAA, CSA, IFM, FESEM, ASPAB, WB | Cell attachment, signaling and spreading | 56 |
| Fibronectin | VacA | HeLa, AGS | 49503 | CAA, IFM, PIS | Cell signaling | 31 |
| HtrA | MKN28 | P12 | CSLM, CZG, ICA, TWA, PIS, WB | Epithelial barrier disruption | 12 | |
| GP130 | CagA | AGS | 60190, 147C, 147A | CMA, CSLM, ASPAB, IP, PIS, RT-PCR, EMSA, WB | Phosphorylated CagA affects SHP2/ERK and JAK/STAT signaling | 90 |
| ICAM1 and LFA1 | T4SS | U937, JoskM | P1, P12, G27 | FACS, IFM, ABB, WB | Homotypic aggregation of APCs, CD4+ T cell deregulation | 127 |
| Laminin | AlpA/B | AGS, Mongolian Gerbils | 26695, SS1 | FACS, BBS, CAA, IHC | Enhanced binding and inflammation | 25 |
| MHC II | Urease B | Kato-III, N87, COS-1, B cell lines |
Purified proteins in vitro |
FACS, ELISA, bead assays, capture assays, CAA | Altered antigen presentation through MHC II | 121 |
| NOD1 | Peptido-glycan | AGS, AGS NOD1KD cells, HEK293, NOD1−/− mice, primary cells | 251, 26695, 189, 249 | LRGA, siRNA, PGN labeling, HPLC, IFM, IHC, GPA,ELISA, RT-PCR, PIS, WB | Activation of NFκB (pro- inflammatory responses) | 9,152 |
| Peptido-glycan-peptide | AGS, HT29, NOD1−/− knockout mice, primary cells, knockout MEFs | TN2-GF4 | ASPAB, LRGA, ELISA, siRNA, IP, PIS, WB | Interferon γ and ISGF3 induction | 51 | |
| NOD2 | T4SS | BMDCs from NOD2−/− and other knockout mice | 26695, P1, P12, G27, SPM326 | ELISA, GPA, RT-PCR, WB | Activation of inflammasome | 150 |
| RPTP-α | VacA | G401, Cos-7, AZ-251 | Purified VacA | IP, CAA, FACS, MS, PCA, WB | VacA receptor | 30 |
| G401, Cos-7, AZ-251 | Purified VacA | IP, CAA, FACS, WB | VacA receptor | 153 | ||
| RPTP-β | VacA | AZ-521 | Purified VacA | IP, FACS, PS, WB | VacA receptor | 28 |
| AZ-521, HL60, BHK-21 | Purified VacA | IP, CAA, FACS, PIS, WB | VacA receptor | 32 | ||
| RPTP-β−/− mice, primary cells, BHK-21 | Purified VacA | IHC, IFM, BBS, ASPAB, RT-PCR, WB | VacA receptor | 29 | ||
| TCR | Lpp20 | Mouse splenocytes, CD4+ T cells |
Synthetic peptides | FACS, TCPA, MHC restriction assay | Characterization of 2 CD4+ T cell epitopes of Lpp20 | 122 |
| HpaA | PBMCs, T cells, B cells, DCs | Synthetic peptides | FACS, TCPA, ELISPOT | Associated with reduced risk of severe gastric diseases | 123 | |
| UreB | PBMCs, T cells, B cells, DCs | Synthetic peptides | FACS, TCPA | Promising peptides for vaccine design | 124 | |
| TLR2 | LPS | Primary monocytes, HEK293, PECs | 43504, SS1, Astra 244 | Protein Array, ELISA | Activation of pro-inflammatory responses | 139 |
| MKN28, MKN48, THP1, T24, HEK293 | Clinical isolates | ELISA, FACS, LRGA, RT-PCR, WB | Activation of pro-inflammatory responses | 131 | ||
| HEK293, HEK-TLR2, HEK-TLR4 | Purified LPS samples | LGRA, ELISA, cDNA-MA,WB | Activation of pro-inflammatory responses | 138 | ||
| HSP-60 | NOMO1, U937 | 43504 | RPT, ASPAB, FACS, siRNA, ELISA, PIS, RT-PCR, WB | Activation of pro-inflammatory responses | 133 | |
| TLR4 | LPS | Guinea pig primary cells | 11637 | RT-PCR, ASPAB, WB | Activation of TLR4 and mitogen oxidase 1 | 154 |
| C57BL/6 mice, MKN1, human PBMCs | Purified LPS samples | TUA, ELISA | Low activity of LPS on TLR4 | 155 | ||
| HEK293, HEK-TLR2, HEK-TLR4 | J99, B128, X47 | MS, TLR-SA, MCA | Modification of LPS is crucial for host colonisation | 156 | ||
| TLR9 | DNA 5‘TTTAGGG |
BMDCs | Purified DNA oligos | ELISA | Importance of TLR-9 in the pathogenesis of IBD |
143 |
Abbreviations: AB, antibody; ABB, antibody blocking; ADAM, A disintegrin and metalloprotease; AhpC, Alkyl hydroperoxide reductase; AlpA/B, adherence-associated lipoproteins A/B; APCs, antigen presenting cells; ASPAB, activation specific phospho-antibody; BBS, biacore binding studies using surface plasmon resonance; BMDCs, bone marrow-derived cells; CAA, cell adhesion assay; Cag, proteins encoded by the cytotoxin-associated genes; CD, circular dichroism spectroscopy; CD18, cluster of differentiation 18; cDNA-MA, cDNA microarray; CMA, cell motility assay; CMIA, collagen type I and Matrigel invasion assays; CSA, cell spreading assay; CSLM, confocal laser scanning microscopy; CZG, casein zymography gel; DCs, dendritic cells; ErbB1–3, erythroblastic leukemia viral oncogene homologs in the EGFR family; EGFR, epidermal growth factor receptor; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunosorbent spot; EMSA, electrophoretic mobility shift assay; FACS, fluorescence-activated cell sorting; FESEM, field emission scanning electron microscopy; GP130, glycoprotein 130; GPA, gentamicin protection assay; H,Kα, H, K–ATPAse α-subunit; HSP, eat shock protein; HtrA, high temperature requirement A; ICAM1, intracellular adhesion molecule 1; HPLC, high-pressure liquid chromatography; IBD, inflammatory bowel disease; ICA, in vitro cleavage assay; IFM, immunofluorescence microscopy; IHC, immunohistochemistry; ISGF3, interferon-stimulated gene factor 3; ImPC, immortalized primary cells; IP, immunoprecipitation; ISGF3, IFN-stimulated gene factor 3; JAK, janus kinase; KD, knockdown of genes; LCI, live cell imaging; LFA1, lymphocyte function-associated antigen 1; Lpp20, lipoprotein 20; LPS, lipopolysaccharide; LRGA, luciferase reporter gene assay; MCA, mouse colonization assay; MEF, mouse embryonic fibroblast; MHC-II, major histocompatibility group II; MS, mass spectrometry; NFAT, nuclear factor of activated T-cells; NMR, nuclear magnetic resonance spectroscopy; NOD, nucleotide-binding oligomerization domain; PAH, peptide array hybridization; PC, protein crystallization; PCA, peptide competition assays; PBMCs, peripheral blood mononuclear cells; PDA, pulldown assay; PECs, peritoneal exudate cells; PIS, pharmacological inhibitor studies; PS, peptide sequencing; RGAA, ras GTPase activation assay; RPT, recombinant protein techniques; RPTP, receptor protein tyrosine phosphatase; RT-PCR, real-time/reverse transcriptase PCR; Shp-2, SH2-containing protein phosphatase; shRNA, small hairpin RNA; siRNA, small interfering RNA; STAT3, signal transducer and activation of transcription 3; T4SS, type IV secretion system; TCPA, T-cell proliferation assay; TCR, T cell receptor; TLR, toll-like receptor; TLR-SA, TLR signaling assay; TUA, thymidine uptake assay; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; TWA, transwell assay; VacA, vacuolating cytotoxin A; WB, western blotting; Y2H, yeast two hybrid screening.
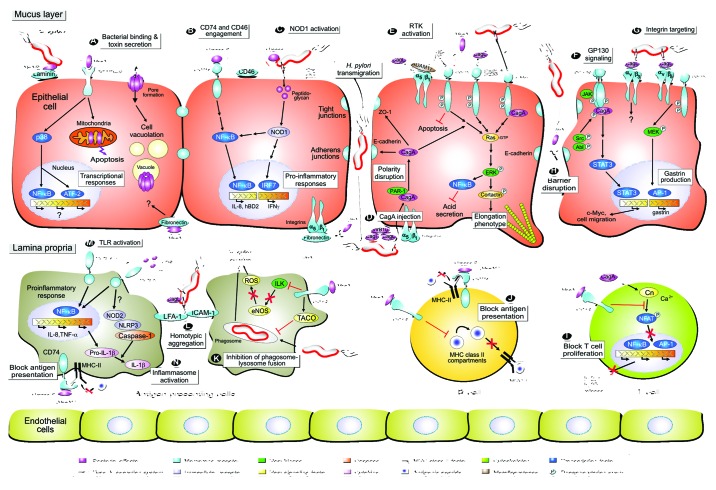
Figure 1. Model of Helicobacter pylori-mediated contact with host protein receptors on epithelial and immune cells to trigger bacterial binding and/or downstream signal transduction events. The interplay between gastric epithelial and various types of immune cells with bacterial pathogenicity factors modulates multiple host responses during the course of infection as indicated. (A) H. pylori expresses several adhesins, some of which can bind to a host protein receptor. One example is the AlpA and AlpB adhesins binding to the matrix protein laminin. Attached H. pylori or those swimming in the mucus can secrete virulence factors into the medium including VacA and urease. VacA is a pore-forming toxin and can bind to various host surface receptors such as the RPTP tyrosine phosphatases. Internalization of VacA into cells leads to the formation of large vacuoles and gastric damage, a hallmark of the ulceration process. VacA can also trigger p38 MAP kinase activation, nuclear responses and mitochondria-associated apoptosis. (B) The H. pylori urease complex has an important function in buffering the acidic pH in the human stomach. However, urease B can also bind directly to the CD74 [MHC-II (class II major histocompatibility complex)-associated invariant chain] receptor on host cells, possibly activating the pro-inflammatory transcription factor NFκB and IL-8 release. Another receptor, CD46, acts as a bactericidal factor as it can bind to the urease A subunit and inhibits H. pylori urease activity. (C) After adherence, H. pylori can translocate effector molecules, such as CagA and peptidoglycan, into the host cell using a type IV secretion system (T4SS)-dependent process. Peptidoglycan binds to the intracellular receptor NOD1, activating transcription factors NFκB or IRF7 to stimulate the secretion of IL-8 or interferon-γ (IFNγ), respectively. (D) Injection of CagA requires various indicated T4SS pilus components and a host protein receptor, integrin β1. Since integrins are normally basolateral receptors, it is not yet clear if injection of CagA appears at apical or basolateral surfaces. However, injected CagA can interact with a number of host cell signaling molecules to trigger several signaling cascades as shown. For example, CagA can bind to PAR-1 and E-cadherin, possibly affecting cell polarity. CagA also contributes to sustained NFκB activity, inhibition of gastric acid production and cell elongation by targeting the actin-binding protein cortactin. (E) The H. pylori T4SS can also activate a number of receptor tyrosine kinases (RTKs) including EGFR, ErbB2, ErbB3 and c-Met which play various roles as indicated. Some yet undefined inhibitory activities on EGFR activation and wound healing have been attributed to VacA. (F) H. pylori targets the glycoprotein receptor GP130 using CagA (phosphorylated by Src and Abl kinases) to activate signal transducer and activation of transcription (STAT) signaling. (G) A novel CagL→integrin αvβ5 signaling complex was characterized to trigger gastrin expression. CagL can also bind to another integrin member, αvβ3, but the resulting downstream signaling is not yet clear. (H) Targeting of tight junctions and E-cadherin-based adherens junctions by the serine protease HtrA and CagA contribute to the disruption of the epithelial barrier. These events may cause leakage of nutrients into the gastric lumen and the ability of H. pylori to cross the epithelial layer by a paracellular pathway. H. pylori and bacterial antigens reach the lamina propria. For example, VacA can bind here to fibronectin in the extracellular matrix. (I) VacA also exhibits suppressive effects on immune cell function in vitro. VacA can interact with the integrin member β2 (CD18) on T-cells, which inhibits the transcription factor NFAT and IL-2 secretion, resulting in a blockade of T-cell activation and proliferation. Interestingly, it seems that CagA has some counteracting activities by activating NFAT via Ca2+-dependent calcineurin (Cn) signaling. (J) Urease B and VacA can also inhibit antigen presentation in B cells, possibly by interfering with antigen loading. (K) VacA was also reported to prevent phagosome-lysosome fusion in macrophages by recruiting the coat protein TACO (coronin 1) and can block integrin-linked kinase (ILK) to prevent the production of reactive oxygen species (ROS), thus supporting bacterial survival. (L) In addition, infection with H. pylori is accompanied by the formation of large homotypic aggregates of macrophages in a T4SS-dependent manner. This occurs through upregulation and recruitment of the intracellular adhesion molecule ICAM-1 to the cell surface, which then mediates aggregation via its ligand LFA-1, a signaling pathway that may regulate cell-cell interactions, inflammatory responses or inhibits bacterial uptake. (M) There are also various reports showing that H. pylori lipopolysaccharide (LPS) can activate the toll-like receptors TLR2 and/or TLR4 to stimulate NFκB and innate immune responses. (N) Recent data suggest that the H. pylori T4SS can also induce the host inflammasome in mice, which is regulating important innate immune functions. This requires the cooperative interaction among host innate immune receptors TLR2, NOD2, and NLRP3 as important regulators of caspase-1 and IL-1β activation in dendritic cells as indicated. For more details and references, see text and Table 1.
Interactions of H. pylori with Protein Receptors of Epithelial Cells
The AlpA/B proteins can bind to laminin
During screening of a transposon-based library in H. pylori strain P1, two homologous genes (alpA and alpB) were identified to encode for integral OMPs involved in bacterial adhesion to cultured AGS epithelial cells and gastric mucosa.20 Further knockout studies have then shown that both genes are involved in adherence of H. pylori to the gastric mucosa of patients, however, the actual host cell binding partner(s) remained unknown for long time.20 The alpA/B locus was found to be present in many H. pylori strains and expressed both during mouse and human infections.21,22 Expression profiling of various OMPs in clinical H. pylori isolates was then performed and revealed that 200 strains expressed the alpA/B gene products while many other OMPs exhibited highly variable expression patterns.22 Reduced numbers of colonizing bacteria have been noted in guinea pigs when infected with alpA and alpB mutants of strain GP15.23 Inactivation of alpA/B genes in strain TN resulted in H. pylori unable to colonize mice while the same mutation in 43505 strain background reduced but not eliminated colonization density.24 In vitro binding studies have then shown that the AlpA/B proteins can bind to mouse laminin, a well-known extracellular matrix (ECM) protein.25 Further experiments indicated that plasmid-based expression of alpA resulted in attachment of Escherichia coli to mouse laminin.25 This could suggest that mouse laminin maybe a receptor for AlpA/B proteins (Fig. 1A). However, if human laminin can bind to AlpA/B with similar high affinity and by which mechanism awaits future investigation. Moreover, the alpA/B locus has also been shown to influence host cell signaling and cytokine production during infection, probably by an indirect effect through enhanced binding. In fact, deletion of alpA/B genes reduced interleukin-8 (IL-8) induction during infection of AGS cells with East Asian but not with Western H. pylori strains.24 In addition, the alpA/B mutants of two Western strains poorly colonized the stomachs of C57BL/6 mice and were associated with reduced mucosal levels of induced IL-8 and IL-6.24 In contrast to these results, in another recent study alpA/B gene mutants of H. pylori strain SS1 induced more severe inflammation than the parental strain in infected gerbils.25 The reason for these different results are yet unclear, but could be explained by polymorphic AlpA/B proteins in H. pylori strains and/or species-specific differences in receptor binding.
VacA interactions with RPTP-α/β and fibronectin
VacA is a pore-forming toxin and present in virtually all known H. pylori strains. The protoxin is expressed as a 135–140 kDa full-length protein, which undergoes processing into the 98 kDa toxin (p98) that is secreted into the extracellular milieu by an autotransporter route.8,18 Members of this autotransporter family are also called type V secretion systems (T5SS) and facilitate their own secretion without the assistance of other bacterial proteins. Secreted VacA can be further processed into the mature toxin (p88) and α-protein (p10). The mature, secreted p88 subunit has been described to undergo additional proteolytic processing to generate the p33 and p55 fragments, which are considered to represent the two functional subunits of VacA.2,8,18,26 Like many other bacterial toxins, VacA can interact with the plasma membrane of epithelial cells followed by internalization using a pinocytic-like mechanism and intracellular trafficking.2,18,19 The 2.4-Å crystal structure of the VacA p55 domain has been determined and docking experiments led to a model for how VacA monomers assemble into oligomeric structures capable of membrane channel formation.27 A remarkable characteristic of VacA is that it can bind various cell surface molecules in vitro or on epithelial cells. Elucidating which of these factors is necessary for VacA internalization and subversion of cellular signaling has been a challenging topic in recent years, and is still not fully understood.8 Besides other factors, VacA can interact with multiple host protein receptors, including the receptor protein tyrosine phosphatases RPTP-β28,29 and RPTP-α,30 and the ECM protein fibronectin31 (Fig. 1A). While the role of VacA binding to fibronectin is not fully elucidated, the function of RPTPs for VacA-mediated signaling has been studied in great detail. VacA induces gastric mucosal damage in wild-type mice but not in RPTP-β knockout (RPTP-β–/–) mice, suggesting that interaction of VacA with RPTP-β on the surface of epithelial cells in the stomach is crucial for VacA-triggered gastric ulceration.29 Remarkably, VacA can still induce vacuole formation in cultured RPTPβ–/– cells and in G401 human kidney cells, which do not express RPTP-β,29,30 but downregulation of RPTP-β by silencing RNA (siRNA) in HL-60 cells abrogated VacA-dependent vacuolation.32 These observations implicate that RPTP-β has a crucial function in VacA-mediated signaling but is not absolutely necessary for vacuole formation in all cell lines. VacA has also been shown to induce tyrosine phosphorylation of G-protein-coupled receptor-kinase-interacting protein-1 (Git-1) and lipid raft dependent signaling.29,33 In line with these observations, VacA was reported to bind to a QTTQP-motif in the extracellular domain of RPTP-β (at amino acid positions 747–751), which was also necessary for inducing Git-1 phosphorylation.30 Another reported consequence of VacA-binding to RPTP-β is the detachment of primary murine gastric epithelial cells from the ECM.29
Besides the VacA-induced activities described above, several other features have been reported. Yeast two-hybrid studies and other experiments have indicated that VacA can specifically interact with two intracellular factors, the intermediate filament binding protein VIP5434 or receptor for activated C-kinase (Rack1),35 but their importance for the infection process remains unknown. VacA can be also targeted to the mitochondria and induce apoptosis by releasing cytochrome c.8 The effect of VacA on endosomal and lysosomal functions has also been studied by following procathepsin D maturation and epidermal growth factor (EGF) degradation exposed to the toxin. VacA inhibited the conversion of procathepsin D (53 kDa) into both the intermediate (47 kDa) and the mature (31 kDa) form in HeLa cells, and intracellular degradation of EGF was also suppressed.36 This suggests that VacA can also dampen the degradative power of late endosomes and lysosomes. Further studies have shown that addition of VacA to AZ-521 cells activates two classes of mitogen activated protein kinases (MAPKs), p38 and ERK1/2, and the activating transcription factor 2 (ATF2) signaling pathway.37 VacA has also been reported to induce activation of p38 in several other types of cells.38 Pharmacological inhibition of p38 kinase activity, however, did not block the vacuolation phenotype or mitochondrial damage, indicating that VacA-mediated stimulation of the p38/ATF-2 signaling pathway is independent of endosomal and mitochondrial pathways of VacA.37 Thus, VacA is a remarkable bacterial virulence factor with multiple activities on epithelial cells.
Binding of H. pylori urease subunits to CD74 and CD46 receptors
H. pylori utilize different strategies for attachment to the gastric epithelium and downstream signaling during the course of infection. Expression of cluster of differentiation 74 (CD74), a polypeptide involved in the formation and transport of major histocompatibility (MHC complex) class II protein, was reported to be highly upregulated in infected gastric epithelial cells.39 Bacterial binding to the cells was enhanced when CD74 surface expression increased by treatment with interferon gamma (IFN-γ) or by fibroblast cells transfected with CD74 constructs, while binding was decreased in the presence of CD74 blocking antibodies, enzyme cleavage of CD74, or CD74-coated bacteria. H. pylori was also shown to bind directly to affinity-purified CD74 protein even in the absence of MHC-II.39 Crosslinking of CD74 and the engagement of CD74 were verified to stimulate IL-8 production by various unrelated cell lines. It was then reported that urease from a pool of H. pylori surface proteins was co-precipitated with CD74.40 Using recombinant urease A and B protein subunits (UreA and UreB) in immunoprecipitation and flow cytometry studies, it was shown that UreB can bind directly to CD74. By utilizing both recombinant urease subunits and ΔureB knockout H. pylori, the UreB-CD74 interaction was shown to activate the pro-inflammatory transcription factor NFκB and IL-8 release (Fig. 1B). Further confirmation of the interaction of UreB with CD74 was obtained using specific function-blocking antibodies and fibroblast cells transfected with CD74 that also responded with elevated NFκB activation and IL-8 production.40 Binding of UreB to CD74 expressed on gastric epithelial cells presented novel insights into H. pylori signaling that may contribute to upregulation of pro-inflammatory responses during infection. However, the role of UreB is not yet fully clear because other studies using different H. pylori strains excluded factors such as urease, CagA or VacA from being involved in IL-8 induction.41 Thus, more studies are necessary to investigate the role of UreB and CD74 in host cell signaling.
In addition, H. pylori can interact with other CD-type receptors. For example, H. pylori infection caused shedding of CD46 (a C3b/C4b binding complement regulator and a receptor for several human pathogens) into the extracellular environment. A soluble form of CD46 bound to H. pylori and inhibited bacterial growth, in a dose- and time-dependent manner, by interacting with UreA and alkyl hydroperoxide reductase (AhpC), which are essential bacterial pathogenicity-associated factors.42 Intriguingly, binding of CD46 or CD46-derived synthetic peptides blocked the urease activity and ability of H. pylori to survive in acidic environments. Oral administration of a CD46 peptide eradicated H. pylori from infected mice, suggesting that CD46 is an antimicrobial compound.42 Thus, CD46-derived peptides might be developed in future to treat H. pylori infections.
T4SS-dependent signaling to intracellular NOD1 and downstream pathways
Very early reports from many labs have demonstrated that the cagPAI is the major source of NFκB and IL-8 induction by H. pylori.5,41,43 Systematic mutagenesis of all genes in the cagPAI revealed that 15 of a total of 27 genes were required for IL-8 production.44 This and other studies argued against an injected IL-8-inducing effector protein encoded within the cagPAI, suggesting that either the T4SS pilus itself or a factor encoded outside of the cagPAI may induce NFκB.5,44,45 A possible solution of this dilemma was reported by Viala and coworkers showing that pro-inflammatory signaling by H. pylori to NFκB and AP-1 required NOD1 (Fig. 1C), an intracellular pathogen-recognition protein with specificity for peptidoglycan from Gram-negative bacteria.9,46,47 These observations suggested that delivery of peptidoglycan by the T4SS is involved in triggering NOD1→NFκB-mediated pro-inflammatory activities such as secretion of β-defensin-248 or IL-8.9 The function of the NOD1 pathway in H. pylori sensing, was verified by transfection of siRNA and expression of dominant-negative NOD1 in the presence of NFκB luciferase reporter plasmids.9 A specific motif of peptidoglycan binding to NOD1 was identified by investigating various H. pylori muropeptide fractions in NFκB reporter assays. Reversed-phase HPLC of muramidase-digested peptidoglycan produced a single fraction with pronounced NFκB-activating capability for host cells. Mass spectrometry of this fraction exhibited a mass/charge ratio of 893, that corresponds to the meso-diaminopimelate (mDAP)–containing N-acetylglucosamine-N-acetylmuramic acid (GM-tripeptide) motif specifically recognized by NOD1.9,49 To investigate this further, an H. pylori mutant deficient in lytic transglycosylase activity (Δslt), which is involved in bacterial muropeptide release, was shown to be defective in NFκB activation. Moreover, consistent with importance of NOD1 in host defense, NOD1-deficient mice were more susceptible to infection by T4SS-positive H. pylori as compared with wild-type mice.9 It was therefore proposed that sensing of H. pylori by NOD1 may represent a model system for host recognition of non-invasive pathogens. However, the work by Viala and coworkers has been challenged by a series of other studies. For example, during H. pylori infection of NOD1 knockdown cells, three crucial events necessary to activate NFκB were not downregulated including NFκB inhibitor-α (IκBα) degradation, IκBα phosphorylation and c-Jun N-terminal kinase (JNK) phosphorylation.50,51 In addition, it was shown that H. pylori induces NOD1 activation, which stimulates a RICK→TRAF3→TBK1→IKKε→IRF7 cascade, but not NFκB, leading to the synthesis of type I interferon.51 Thus, more work is necessary to understand the role of peptidoglycan and NOD1 in H. pylori pathogenesis.
Injection of CagA by the T4SS requires binding to integrin α5β1
Since the original discovery of T4SS-mediated transport of CagA it was assumed for many years that CagA can be randomly injected in any mammalian cell type. However, systematic studies of cultured cells derived from different tissues and hosts have shown that CagA can be injected and tyrosine-phosphorylated in only 31 of 35 tested cell lines.14 These observations are in agreement with the idea that the T4SS requires a specific host cell receptor for proper function which may be missing in some cell types, hypothesizing a sophisticated control mechanism by which H. pylori delivers CagA.10 The first identified host receptor for the H. pylori T4SS was the integrin member β1 (Fig. 1D).52 Integrins are well-known cell adhesion receptors, which are evolutionarily old and play crucial roles in many developmental and pathological processes.53 The integrin family comprises 24 heterodimeric members composed of α and β chains that mediate the attachment of cells to the ECM, but can also take part in sophisticated cell-cell interactions of healthy tissues.53 Integrin binding of the H. pylori T4SS was verified by a series of studies such as the application of integrin β1−/− knockout cells (GD25 vs. GD25 re-expressing integrin β1A), integrin function-blocking antibodies, small hairpin RNA (shRNA)-mediated stable knockdown of integrin β1 and competition with InvA from Yersinia (a well-known bacterial adhesin for integrin β1) during infection.52 Thus, comprehensive proof was provided that expression of integrin β1 is essential for injection of CagA.52 The first identified H. pylori interaction partner of integrin β1 was the T4SS pilus protein CagL. Like the human ECM protein fibronectin, which is the natural ligand of integrin β1,53 CagL carries an RGD-motif (-Arg-Gly-Asp-) shown to be important for interaction with integrin β1 on host cells.52,54 Several crystal structures of CagL revealed an elongated four-helix bundle of the protein that appears to mimic proposed VirB5 orthologs, but is evolutionary unrelated to VirB5.55 Remarkably, the RGD-motif is surface exposed but located within a long α-helix. This is highly unexpected because previously characterized integrin-binding RGD-motifs in mammalian proteins are located within extended or flexible loops.53 In addition, surface plasmon resonance binding studies have demonstrated that recombinant wild-type CagL can strongly interact with purified integrin α5β1 (dissociation constant Kd = 0.09) and with an affinity higher than that of CagLRGA mutant (Kd = 0.36) or fibronectin (Kd = 0.8).52,56 While CagL binding to host cells is mediated primarily by the RGD-motif, an auxiliary binding site for CagL–integrin interaction was also identified in peptide arrays.57 The surface exposed FEANE (-Phe-Glu-Ala-Asn-Glu-) motif in CagL was found in spatial proximity to the RGD sequence.57 The presence of this motif enhanced the interaction of CagL with integrins, and was thus named RGD helper sequence (RHS). The injection defect for CagA by H. pylori carrying the CagLRGA mutant has been confirmed in two studies,52,58 while mutation of the RGD-motif in CagL revealed no reduction of phosphorylated CagA in another report.59 However, in line with the observation that CagL can interact with integrins, various other structural T4SS proteins (including CagA, CagI, and CagY) have also been demonstrated to bind to integrin β1 in yeast-two-hybrid studies or in vitro.59 This study has also shown by surface plasmon resonance that CagA itself can bind to purified integrin α5β1 with high affinity (dissociation constant Kd = 0.15) and the binding site has been mapped to the N-terminus of CagA.59,60 Using pharmacological inhibitors and transfection of integrin deletion mutants, it was shown that integrin clustering is essential for CagA translocation, but not signaling via the integrin β1 cytoplasmic tail.59 Using mass spectrometry, it was further reported that surface exposed CagL associates with two other cagPAI proteins, CagI and CagH.61 Scanning electron microscopy revealed that not only CagL is involved in the formation of T4SS pili,52 but also CagI and CagH.61 The ΔcagI and ΔcagL mutants failed to form T4SS pili, while the ΔcagH mutant revealed a hyperpiliated phenotype and produced pili that were elongated and thickened as compared with those of wild-type H. pylori.61 This led to the suggestion that T4SS pilus dimensions may be regulated by CagH. Taken together, the above results implicate that CagL, CagH, and CagI are components of a T4SS subassembly complex involved in the biogenesis of pili that interact with integrin α5β1. Whether CagY is involved in pilus generation is under debate because CagY deletion mutants still produced T4SS pili like wild-type bacteria,62 while this is not the case in another report.59 Interestingly, CagY has an unusual domain structure, in which an extraordinary number of direct DNA repeats is predicted to cause rearrangements that invariably yield in-frame insertions or deletions.63 Recent infection studies in murine and non-human primate models have shown that these rearrangements in CagY are sufficient to cause gain or loss of function in the H. pylori T4SS and are driven by the host immune system.62 It was therefore proposed that CagY may function as a sort of molecular switch or perhaps a rheostat that alters the function of the T4SS and “tunes” CagA injection and host pro-inflammatory responses so as to maximize persistent infection.62 However, although it seems clear that each of the above H. pylori factors exhibits a crucial role for T4SS function and injection of CagA, the exact co-operation of the various integrin-targeting T4SS proteins is not yet clear and needs to be investigated in future.
CagL is involved in integrin α5β1-dependent NFκB activation and gastric acid suppression
H. pylori infection represses the expression of gastric H, K-ATPase α subunit (HKα), the parietal cell enzyme mediating acid secretion, which is considered to contribute to transient hypochlorhydria in the stomach in vivo.64 In line with these observations, infection of gastric biopsies or cultivated epithelial cells with H. pylori in vitro inhibits the activity of endogenous HKα promoter or transfected HKα-luciferase reporter constructs.65,66 The mechanism of H. pylori-triggered suppression of HKα and ensuing hypochlorhydria have been explored in some detail. It appears that signaling by H. pylori leading to NFκB activation and HKα promoter downregulation correlate tightly, while other factors such as activated IL-1β are not involved.66 T4SS-expressing H. pylori isolates are known to activate NFκB as described above. The NFκB-binding regions in the HKα promoter sequence were identified and shown to repress its transcriptional activation.65 In particular, binding experiments have shown that although the phosphorylated, activated p65 subunit of NFκB is generated upon infection with H. pylori, the p50/p65 heterodimeric complex of NFκB fails to bind to the HKα promoter region. Insertion of point mutations at positions -159 and -161 within the NFκB binding site in the HKα promoter sequence abrogated binding of NFκB p50 and prevented H. pylori downregulation of HKα promoter activity of these constructs.65 Investigation of the involved bacterial factors have shown that the T4SS proteins CagE, CagL, CagM, and possibly injected CagA and peptidoglycan, are mechanistically implicated in NFκB induction and transcriptional HKα repression.67,68 During acute H. pylori infection, it was shown that CagL binding to integrin α5β1 leads to activation of the EGF receptor (EGFR) resulting in MAPK stimulation (predominantly ERK1/2) and NFκB-mediated repression of HKα.68 Collectively, these studies provide evidence that cagPAI-positive H. pylori inhibits HKα gene expression by an integrin α5β1→EGFR→ERK1/2→NFκB signaling cascade mediating NFκB p50 homodimer binding to the HKα promoter (Fig. 1E). These findings contribute significantly to our current understanding of H. pylori gastric acid suppression. Interestingly, there obviously exists a second pathway of CagL-mediated NFκB activation leading to IL-8 release, which is integrin α5β1-dependent, but obviously proceeds in a manner independently of NOD1 engagement.58 Thus, it will be highly interesting to dissect these various signal transduction pathways in further detail.
Signaling of the T4SS to receptor tyrosine kinase EGFR, anti-apoptosis, and wound healing
EGFR is a 170-kDa transmembrane glycoprotein belonging to the ErbB group of receptor tyrosine kinases (RTKs), which include the ErbB1, ErbB2, ErbB3, and ErbB4 members. 3The EGFR family plays an important role in cell growth, proliferation, differentiation, and wound healing.69 However, overexpression of EGFR genes was described for various human tumors and has been implicated in the development and prognosis of malignancies.3,69 There have also been various reports of elevated EGFR levels both in gastritis and in association with H. pylori infection in vivo.70,71 In previous in vitro studies it has been shown that infection of cultured gastric epithelial cells with H. pylori results in the rapid transactivation of EGFR via metalloproteinase-dependent cleavage of heparin-binding EGF (HB-EGF).72,73 It was also demonstrated that prolonged infection with cagPAI-positive H. pylori strains lead to an enhanced surface expression of EGFR due to inhibited EGFR endocytosis, implicated in the suppression of human β-defensin 3, and improved bacterial survival.74 Further studies have shown that binding of the T4SS pilus component CagL to integrin plays a crucial mechanistic role in EGFR activation.68 During acute H. pylori infection, CagL binding to integrin α5β1 leads to activation of a disintegrin and metalloprotease 17 (ADAM-17) (Fig. 1E). ADAM-17 is normally bound to integrin α5β1 and inactive, but CagL interference during infection dissociates ADAM-17 from the integrin complex, thereby stimulating the proteolytic activity of ADAM-17 and ADAM-17-mediated release of HB-EGF as confirmed by co-immunoprecipitation, siRNA, and ELISA experiments.68 HB-EGF can then activate EGFR leading to activation of the small GTP-binding protein Ras and MAPK stimulation (predominantly ERK1/2) resulting in NFκB-mediated repression of HKα68,72 or upregulation of early growth response gene 1 (Egr-1).75 Because EGFR signaling also regulates cell survival,69 studies were performed to investigate whether H. pylori-induced EGFR activation may promote gastric epithelial survival.76 Inhibiting EGFR kinase activity or decreasing EGFR expression increased H. pylori-induced apoptosis in conditionally immortalized stomach (ImSt) epithelial cells of mice. Blocking H. pylori-induced EGFR activation with a HB-EGF neutralizing antibody or abrogating ADAM-17 expression increased apoptosis of H. pylori-infected AGS and ImSt cells, respectively.76 Moreover, H. pylori infection stimulated gastric epithelial cell apoptosis in kinase-defective EGFR (EGFRwa2) mice, but not in wild-type mice. Furthermore, H. pylori-induced EGFR phosphorylation stimulated phosphotidylinositol-3-kinase (PI3-K)-dependent activation of the anti-apoptotic factor Akt, increased expression of the anti-apoptotic factor Bcl-2, and decreased expression of the pro-apoptotic factor Bax.76 Interestingly, activation of EGFR by H. pylori is not a one-way street. Depending on the used strain, purified VacA or VacA-containing culture supernatants exhibited suppressive effects on proliferation, migration or wound healing of gastric epithelial cells in vitro.77 In fact, highly active VacA preparations or infection with VacA-expressing H. pylori exhibited inhibitory capabilities on phosphorylation of EGFR78 and ErbB2 (also called HER2/Neu), another member of the EGFR family78 (Fig. 1E). Immunofluorescence data showed internalized EGFR, possibly due to endocytosis, in infections with VacA-expressing H. pylori, providing a possible mechanism for how significant portions of EGFR are inactivated by VacA.78 Interestingly, RPTP-α but not vacuolation per se played a role in this process. These findings provide important insights into crosstalk of the bacterial virulence factors T4SS, CagA, and VacA in the deregulation of EGFR signaling by H. pylori, possibly involved in development of gastric tumorigenesis.
Interference of H. pylori with the receptor tyrosine kinase c-Met
The hepatocyte growth factor (HGF) receptor, c-Met, is strongly implicated in late-stage cancer progression and poor patient prognosis. H. pylori was previously proposed to stimulate c-Met phosphorylation dependent upon interaction of c-Met with the bacterial CagA protein, which is required for H. pylori-induced cancer cell motility, invasion, and nuclear signaling.79-82 However, in another recent study it was suggested that the observation of c-Met phosphorylation is an artifact caused by cross-reactivity of commercial phospho-c-Met antibodies.83 First, it was reported that H. pylori activates c-Met, which is involved in invasive growth of tumor cells.79 The injected H. pylori effector protein CagA may interact with the c-Met receptor as determined by immunoprecipitation, which could promote cellular signaling leading to a forceful motogenic response of infected AGS cells. The H. pylori-induced motogenic response is suppressed by pharmacological inhibition of phospholipase C-γ and MAPKs (Fig. 1E), but not growth factor receptor-bound protein 2 (Grb-2) or Grb2-associated binder 1 (Gab-1), respectively.79 Second, injected or transfected CagA has been shown to activate Ras→Raf→MEK→ERK1/2 signaling pathway to enhance peptidoglycan-mediated NFκB stimulation,84 but the actual cell interaction partner of CagA remained unknown. Suzuki and coworkers reported that the interaction of CagA with c-Met is involved in NFκB activation.82 They reported on a conserved FPLKRHDKVDDLSKVG (Phe-Pro-Leu-Lys-Arg-His-Asp-Lys-Val-Asp-Asp-Leu-Ser-Lys-Val-Gly) motif at the C-terminus of CagA for binding to c-Met, which is distinct from the EPIYA (Asp-Pro-Ile-Tyr-Ala) motifs, and was designated CRPIA (conserved repeat responsible for phosphorylation-independent activity). It was proposed that the CRPIA motif in non-phosphorylated CagA was involved in interacting with activated c-Met, leading to the sustained downstream activation of PI3-K/Akt and the transcription factors β-catenin and NFκB, which promoted proliferative and inflammatory responses during H. pylori infection.82 Remarkably, the CRPIA region described to interact with activated c-Met was identical to the described CagA multimerization sequence85 and interaction site of CagA to partitioning defective 1b (Par1b) kinase inhibiting its activity86 by binding to a pocket in the active site. Thus, it will be interesting to investigate whether and how the CRPIA motif fits into the active site of c-Met. In a third study, the role of H. pylori on c-Met and cell invasion was investigated.80 The authors found that H. pylori induces AGS cell invasion in collagen type I and in matrigel invasion assays. The abrogation of the c-Met receptor activity using the inhibitor NK4 or siRNA-mediated silencing of c-Met expression suppressed both cell invasion and elevated matrix metalloprotease (MMP) activity, respectively. Studies with different H. pylori mutants revealed that cell invasion, c-Met tyrosine phosphorylation, and increased MMP-2 and MMP-9 activity were all dependent on the presence of the functional T4SS, but not on VacA.80 In a fourth report, the phosphorylation of the 135 kDa c-Met receptor upon H. pylori infection as described above was called into question.83 The data showed that shRNA-mediated c-Met knockdown did not reduce H. pylori-induced cell motility, suggesting that c-Met was not required for motility. This study also showed that the anti-phospho-Met antibody did not recognize the 135 kDa c-Met protein but a 10 kDa smaller cross-reacting protein which was identified as phosphorylated CagA itself.83 The authors concluded that c-Met is not phosphorylated upon infection with H. pylori and the above studies misinterpreted phosphorylated CagA as the c-Met phosphoprotein.83 Thus, the role of c-Met in H. pylori-induced signaling is not fully clear and needs to be investigated more thoroughly in future studies.
Targeting of GP130 and JAK/STAT signaling by injected CagA
Engagement of the glycoprotein-130 (GP130) receptor and associated downstream signaling has been shown to play a crucial role in the development of gastric cancer.87 GP130 is the signal transducer of the IL-6 receptor complex, which can bind to tyrosine phosphatase SHP2 as well as the signal transducer and activation of transcription (STAT) members STAT1 and STAT3 through differential targeting of phospho-tyrosine residues in its cytoplasmic domain. It has been demonstrated that GP130Y757F mice, carrying a phosphorylation-resistant “knock-in” mutation abrogating SHP2→Ras→ERK signaling, developed gastric adenomas by three months of age.88 In contrast, mice expressing the reciprocal mutation ablating STAT1/3 signaling (GP130ΔSTAT), or deficient in IL-6-mediated GP130 signaling (IL-6–/– mice), exhibited impaired colonic mucosal wound healing capabilities.88 The cellular factors that are upregulated in response to H. pylori infection have been shown to modulate SHP2/ERK or Janus kinase (JAK)/STAT signaling pathways, suggesting that H. pylori persistence and its consequent pathologies could be influenced by GP130-mediated signal transduction. In particular, c-Src and c-Abl kinases control hierarchic tyrosine phosphorylation events and function of the injected CagA effector protein which can then interact with the SH2 domain of SHP2.89 Further studies investigated the role of injected phospho-CagA in the activation of the SHP2/ERK and JAK/STAT pathways downstream of the GP130 receptor.90 In this report, the authors showed that in the presence of phosphorylated CagA, SHP2 was recruited to GP130 (Fig. 1F). Phosphorylated CagA showed enhanced SHP2 binding activity and ERK1/2 phosphorylation, while injection of the phospho-resistant CagA mutant revealed preferential STAT3 activation.90 These findings indicate that the phosphorylation status of injected CagA affects a signaling switch between the SHP2/ERK and JAK/STAT3 pathways through GP130, providing a novel mechanism to explain how H. pylori hijacks disease-related signaling cascades in epithelial cells.
T4SS interactions with the integrin members αvβ3 and αvβ5
One of the most important hormones in the stomach of humans is gastrin. Gastrin is a peptide and mainly required for the regulation of gastric pH, but is also involved in other processes such as growth and differentiation of gastric epithelial cells. In H. pylori infected patients, gastrin secretion can be upregulated by the pathogen, resulting in hypergastrinaemia which is known to represent a major risk factor for developing gastric carcinoma. Very recently, the upstream host protein receptor complex and bacterial factors involved in H. pylori-triggered gastrin gene expression were investigated.91 The presence of an intact T4SS, integrin linked kinase (ILK), and integrin member αvβ5, but not integrin α5β1, were necessary for gastrin promoter induction by H. pylori (Fig. 1G). Using gastric epithelial cells which were stably transfected with a gastrin promoter luciferase reporter construct and various signal transduction approaches, a novel CagL→integrin αvβ5→ILK signaling complex was characterized as being crucial for H. pylori-induced gastrin expression.91 Interestingly, the interaction of CagL with integrin αvβ5 obviously proceeds in an RGD-independent fashion, because in surface plasmon resonance binding studies using the same settings as described above52 wild-type CagL bound to purified integrin αvβ5 with about the same high affinity (Kd = 0.20) as compared with the CagLRGA point mutant (Kd = 0.35). To verify an RGD-independent binding of CagL with integrin αvβ5, AGS cells were infected with H. pylori expressing either wild-type CagL or CagLRGA point mutant followed by immunoprecipitation with α-CagL antibodies. In each experiment, equal amounts of CagL were precipitated, and western blot analyses with α-integrin β1 and β5 antibodies showed a significantly weaker and an equally intense band compared with the wild-type CagL infected cells, respectively.91 Further studies have then demonstrated that upon engagement of integrin αvβ5 and ILK by CagL, H. pylori induces the EGFR→Raf→MEK (mitogen activated protein kinase kinase)→ERK1/2 downstream signaling cascade which also played an important role in the upregulation of gastrin expression.91 In addition to the above findings, another study has shown that recombinant wild-type CagL can also bind to a third integrin member, αvβ3 (Fig. 1G). This interaction can be inhibited with conformational designed cyclic RGD peptides derived from the CagL epitope ALRGDLA (-Ala-Leu-Arg-Gly-Asp-Leu-Ala-).92 Inhibition of the CagL-αvβ3 interaction by different RGD peptides verified the importance of the RGD motif for CagL binding capabilities. In agreement with these findings, CagLRAD and CagLRGA point mutants exhibited decreased affinity to integrin αvβ3.92 Moreover, structure–activity relationship studies with cyclic RGD peptides in a spatial screening approach revealed a distinct influence of the three-dimensional arrangement in the RGD motif on the ability to interfere with this interaction, however, an H. pylori-triggered downstream signaling cascade is yet unknown for integrin αvβ3. Nevertheless, the above studies strongly suggest that H. pylori can target an array of at least three integrin members on epithelial cells for different purposes. However, because integrin heterodimers are commonly expressed at basolateral membranes, and not at apical surfaces,53 it will be important to investigate in future how H. pylori can achieve integrin contact during infection of polarized epithelial cells.
Targeting of E-cadherin by two H. pylori factors
E-cadherin is a 125 kDa cell surface protein present in adherens junctions of polarized epithelial cells, where it has essential functions in cell-to-cell adhesion and tumor suppression.93 E-cadherin contains a large extracellular domain of 90 kDa (NTF domain for N-terminal fragment), a transmembrane domain and a short intracellular domain (CTF domain for C-terminal fragment). Ectodomain cleavage of E-cadherin occurs frequently and is an important step in the pathogenesis of inflammatory responses or neoplastic transformation, a phenomenon that was attributed to various MMPs and ADAM-10.93 Infection of polarized epithelial cells has shown that H. pylori can target E-cadherin in two different ways, extracellularly by secreted HtrA (Fig. 1H) and intracellularly by injected CagA (Fig. 1D). First, HtrA is a chaperone and serine protease in the periplasm, which has often been described as a crucial factor contributing to the pathogenesis of a wide range of bacteria94 and delivery of HtrA into the extracellular space has been discovered by mass spectrometry of secreted H. pylori proteins.95 In contrast to many other bacteria where the htrA genes can be mutated,94 generation of htrA knockout mutants in H. pylori seems challenging and was not yet successful.12,96 The unavailability of such mutant in H. pylori hampered rapid progress of functional studies. So it was unknown for long time whether HtrA can target host cell proteins combined with a functional role during H. pylori infection. Recently, an extracellular function of HtrA has been unraveled by demonstrating that it is secreted as an active serine protease, where it can cleave-off the extracellular NTF domain of E-cadherin in vitro and in vivo.12 In following studies, E-cadherin cleavage has also been shown for HtrAs of other enteric pathogens such as Campylobacter jejuni, pathogenic Escherichia coli, and Shigella flexneri, but not Neisseria gonorrhoeae, a pathogen of the urogenital tract.97,98 This indicates that HtrA-mediated E-cadherin cleavage is not unique for H. pylori, but may represent an entire novel mechanism how various bacterial pathogens can promote their own transmigration across polarized epithelial cells. Thus, this represents a novel mechanism of E-cadherin ectodomain shedding, besides the known MMP-dependent cleavage events.12,99 However, whether HtrA-mediated E-cadherin cleavage has an influence on the integrity and tumor-suppressive function of the E-cadherin complex composed of β-catenin and the Armadillo-domain protein p120 catenin is not yet clear. Remarkably, E-cadherin shedding results in the locally disruption of adherens junctions in polarized gastric epithelial cells allowing H. pylori entry into the intercellular space,12 which is in agreement with the detection of H. pylori between neighboring epithelial cells and in underlying tissues of stomach biopsies obtained from gastric cancer patients.100 In a second set of reports, transfected CagA was reported to interact with E-cadherin.101 It was proposed that this interaction can disrupt the adherens junctions between polarized epithelial cells and releases β-catenin from this junctional complexes and increases cytoplasmic and nuclear β-catenin pools leading to transcriptional upregulation of mitogenic genes implicated in carcinogenesis.101,102 A very recent study reported that CagA forms a complex not only with E-cadherin, but a trimeric complex also including c-Met and p120 catenin.81 These interactions by immunoprecipitation were not seen in the widely used non-polarized AGS cells, which are E-cadherin deficient. However, the multiprotein complex was observed during infection of polarized NCI-N87 cells which express endogenous E-cadherin or in AGS cells stably transfected with E-cadherin. It seems that when the CagA⁄p120catenin⁄E-cadherin/c-Met complex is present, the cell invasive phenotype induced by H. pylori can be suppressed.81 The molecular basis for this observation is not yet fully clear, but it may be that injected CagA counteracts the activities of extracellular HtrA on E-cadherin. Future studies should investigate this signaling in more detail and also the possible crosstalk between HtrA and CagA activities during infection.
Interactions of H. pylori with Protein Receptors of Immune Cells
VacA targets CD18 receptor to suppress T-cell proliferation
Research in the last ten years has provided compelling evidence that H. pylori has accumulated multiple activities during evolution to suppress immune cell functions for establishing persistent infections. Some of these functions have been attributed to VacA that does not only interact with gastric epithelial cells as described above, but also with immune cells including T-cells, B-cells, and antigen-presenting cells (APCs) such as macrophages or dendritic cells (DCs). A very early study demonstrated that VacA can inhibit processing and presentation of antigenic peptides to human CD4+ T-cells and suggested for the first time that VacA may contribute to the persistence of H. pylori by interfering with protective immunity.103 For example, it was shown that VacA can efficiently enter activated, migrating primary human T-cells by binding to the CD18 receptor (also called integrin β2) and exploiting the recycling of the lymphocyte function-associated antigen and adhesion molecule LFA-1.104 However, non-activated human primary T-cells do not endocytose VacA and the activated cell uptake of VacA was regulated by protein kinase C (PKC) isoforms PKCη and PKCζ through phosphorylation of threonine residue T758 in the cytoplasmic tail of CD18.105 LFA-1-deficient Jurkat T-cells were also resistant to vacuolation, and genetic complementation with wild-type LFA-1 restored sensitivity to VacA. Interestingly, VacA targeted human, but not murine CD18 for cell entry, consistent with the species-specific adaptation concept of H. pylori.104
Infection of T-cells with H. pylori or co-incubation with purified VacA yielded multiple signaling responses. VacA can specifically block the proliferation of T-cells by interfering with IL-2-mediated signaling.38,106 After CD18-dependent entry into cultured human T-cells, VacA inhibits Ca2+ mobilization, and subsequently, induces the downregulation of Ca2+-dependent phosphatase calcineurin activities.104 This in turn inhibits the activity of transcription factor NFAT (nuclear factor of activated T-cells). Consequently, NFAT target genes such as IL-2 and the high-affinity IL-2 receptor (IL-2Rα) are not expressed any longer. However, in another study it appears that VacA exhibits a different effect on primary human CD4+ T-cells, whose proliferation is inhibited through the T-cell receptor (TCR), CD28, and eventually by other VacA activities.107 In this study, VacA was shown to suppress IL-2-induced cell-cycle progression and proliferation of primary T-cells without affecting NFAT. Therefore, VacA may suppress the clonal expansion of T-cells that have been activated by bacterial antigens, thereby allowing H. pylori to evade the adaptive immune response, resulting in chronic infection. Using epithelial cells, it has also been shown that transfected CagA can induce a counteracting effect by activating NFAT through a PLCγ→Ca2+→calcineurin pathway allowing its nuclear translocation (Fig. 1I).108 However, this activation was abrogated by the effects of VacA as discussed above. These findings highlight some remarkable opposing effects exerted by two important virulent factors of H. pylori on dealing with epithelial cells and cells of the immune system. If these effects influence each other during infection is not clear and should be investigated.
Receptor-dependent activities of VacA on DCs and macrophages
VacA was also reported to affect the maturation of DCs. Exposure of DCs to VacA with LPS have changed the maturation characteristics and downregulated the expression of some marker molecules. This was achieved through the restoration of transcription factor E2F1 signaling involved in the regulation of DC maturation.109 In infected human THP-1 and mouse RAW 264.7 macrophage cell lines, wild-type H. pylori displayed an enhanced survival as compared with ΔvacA deletion mutants.110 Thus, it was proposed that VacA arrests phagosome maturation in macrophages by recruiting and retaining tryptophane aspartate-containing coat protein or coronin-1 (TACO) (Fig. 1K). However, increased survival of isogenic vacA mutants was not seen in two other reports when freshly isolated human monocytes were infected with H. pylori.111,112 These differences may be due to different vacA alleles, infective dose and/or cell types used in the different studies. VacA deficiency has also increased the expression of endothelial nitric oxide synthase (eNOS) resulting in enhanced production of reactive oxygen species (ROS) in infected U-937 macrophages as compared with wild-type H. pylori.113 A very recent report has shown that conditionally ILK−/− mice increased eNOS-dependent generation of superoxide during infection.114 Therefore, VacA interaction with CD18 could be also a prerequisite for inhibition of ILK and subsequent decrease of ROS to increase the survivability of H. pylori in phagosomes (Fig. 1K). In addition, VacA treatment stimulated the expression of cyclooxygenase-2 (Cox-2), a pro-inflammatory enzyme in neutrophils and macrophages.38 On the other hand, in a different cell culture model system using RBL-2H3 mast cells, binding of VacA results in a rapid change of intracellular cytosolic Ca2+ concentrations,115 which was accompanied by stimulation of pro-inflammatory cytokines, including TNFα and IL-6, resulting in chemotaxis and degranulation of these mast cells.115,116 These differences of action by a major virulence factor might influence the progression of associated pathologies in the chronic infection strategies of this special bacterium.
H. pylori virulence factor action on antigen presentation and bacterial recognition
CD74 or invariant chain is a very important receptor involved in MHC-II processing, antigenic peptide loading in B cells and presentation to the TCRs. As discussed above, the H. pylori protein UreB was found to interact with CD74 on epithelial cells, but also B-cells.40 Thus, a role of this interaction on the antigen presenting capacity of professional APCs is predictable. This interaction may therefore have an influence on the antigenic peptide loading and presentation to the TCRs (Fig. 1J). It was also shown that, in addition to the known role in antigen presentation, CD74 could also regulate the migration of DCs.117 T-cell mediated immunity against H. pylori is reported to be suppressed during infections both under in vitro and in vivo experimental conditions and that is supported by the presence of increased numbers of CD4+-CD25+-FOXP3+ T-regulatory cell populations at the sites of H. pylori colonization.118 As discussed above, VacA of H. pylori efficiently enters activated and migrating T-cells through the binding of CD18 and this leads to vacuolation and suppression of IL-2 production.104 The binding of VacA to CD18 on APCs could also be related to the inhibition of phagosome-lysosome fusion and disruption of antigen presentation capacity by this bacterium (Fig. 1J and K). In contrast, some earlier reports have also shown a Th1 response during H. pylori infection.119 Recent studies have indicated that the balance between different T-cell types determines the outcome of infection mediated pathologies.4 It appears that H. pylori is trying to behave as an adapted commensal bacterial friend toward its human host in asymptomatic individuals with the presence of more regulatory T-cells at the sites of infection, however, gastric ulcer patients have increased Th1 and Th2 type responses by comparison.118,120 This could be attributed to both the different aspects of T-cell responses and host genetic factors. Hence, the prominent T-cell epitope containing antigens of H. pylori and their loading on different MHC genotypes and the strength of further interaction with TCR could be a strong factor on making these differences in response. In addition to the binding partner for CD74, urease is an abundant antigen encountered by the host at the sites of infection.121 An immunodominant T-cell response can be able to clear an infection effectively and keep a memory status to prevent further infection of the same microbe. The binding of bacterial proteins to MHC or accessory molecules on T-cells can induce functional changes and deregulate the effector mechanisms of this important cell population of the adaptive immune system. Thus, the identification of H. pylori urease and other proteins’ ability to activate T-cell effector mechanisms would be of great importance to develop protective vaccines against this pathogen. Recent studies using synthetic peptides have identified novel T-cell epitopes from H. pylori specific major antigenic proteins such as UreB, outer membrane lipoprotein (Lpp20), and neuraminyllactose-binding hemagglutinin precursor (HpaA). The epitopes are identified as amino acids 73–385 and 438–452 on UreB, amino acids 83–97 and 58–72 on Lpp20, and amino acids 88–100 on HpaA.122-124 Among these identified epitopes, the MHCII molecule HLA-DRB1*1501 restricted immunodominant CD4+ T-cell response to HpaA88-100 is associated with reduced risk of severe gastric diseases.123 These findings indicate that efficient antigen presentation and recognition of certain H. pylori antigens by TCRs could influence the outcome of associated pathologies. Therefore, further studies on antigen processing, presentation, and recognition during H. pylori infection are required to understand the deregulation of immune responses against this pathogen.
H. pylori induces T4SS-dependent aggregation of macrophages through receptor engagement
ICAM1 (also known as CD54) is a cell surface integrin molecule mainly expressed on endothelial cells and immune cell populations. It is a member of the immunoglobulin superfamily that contains five extracellular immunoglobulin domains, a transmembrane domain, and a short cytoplasmic domain that connects with cytoskeletal proteins. This molecule has been studied well on their role in the transmigration processes of immune cells. In addition to the critical roles in leukocyte recruitment and T-cell development, some recent findings also support their role in signal transduction pathways of the cells and in viral, bacterial, and protozoal infections as well as tumor metastasis.125,126 H. pylori virulence and pathogenicity-associated factors are known to be manipulating the immune mechanisms of the host in many different ways and thereby preventing an effective immune defense to clear the infection. Infection with cagPAI-positive H. pylori strains induced the strong expression and surface recruitment of ICAM1 and LFA1 in macrophage like cell lines, which resulted in large homotypic aggregations.127 The formation of homotypic aggregations of these phagocytic cells was dependent on the integrity of the T4SS, but was independent of injected CagA. The antibody-mediated blocking of ICAM1 or LFA1 completely abolished the aggregation phenotype of infected cells.127 ICAM1 is actually the natural ligand for LFA1, suggesting that ICAM1-LFA1 interaction between neighboring macrophages facilitates the cell aggregate formation phenotype induced by H. pylori (Fig. 1L). What could this mean for the infection process? ICAM1 expression on endothelial cells and their interaction with LFA1 expressed on phagocytes and T-cells is well-known to facilitate the movement of immune cells to the sites of inflammation. APCs expressing ICAM1 on their surface can interact with LFA1 on T-cells during immunological synapse formation and the strength of this bonding profoundly affects the antigen specific stimulation of T-cells.128 Therefore, changes in the expression of these important interacting molecules of the immune system could have many implications on the immune processes such as immune cell recruitment, antigen presentation, and development of adaptive immunity during H. pylori infection.
TLR signaling in immune cells induced by H. pylori
Toll-like receptors (TLRs) form the integral part of the pattern recognition receptors (PRRs), which can detect pathogens and mount pro-inflammatory changes at the site of infection to the development of adaptive immune responses. The involvement of TLRs in H. pylori recognition and subsequent signaling could play a major role in inflammatory processes at the gastric mucosal surfaces and deeper tissues (Fig. 1M). The studies on the direct involvement of TLRs in immune cells during H. pylori infection, however, are sparsely present in the literature. In one of the early reports, immunhistochemistry has shown the upregulation of TLR4 in the lamina propria mononuclear cells of H. pylori infected patients.129 The same study noted that H. pylori LPS significantly stimulated the NFκB activation in THP1 macrophages through a reporter assay. H. pylori LPS induced NFκB activation in THP1 cells was then found to be mediated through TLR4.130,131 Recently, the upregulated expression and activation of TLR2 and TLR5 in THP1 cells leading to the production of cytokines IL-8 and TNFα during H. pylori infection was also demonstrated.132 In addition, H. pylori heat shock protein-60 (HSP-60) induced the activation of NFκB through a MAPK pathway leading to the production of IL-8 in human NOMO1 monocytic cell lines. This response was reduced when the cells were pretreated with TLR2 antibodies or siRNA, respectively.133 However, HSP-60 induced IL-6 production in mouse macrophages was reported to be independent of a TLR2-TLR4-MyD88 (myeloid differentiation primary response-88) signaling mechanism.134 MyD88 is a major adaptor protein in TLR signaling, and the use of B cells and macrophages derived from MyD88−/− mice abrogated the ability to mount pro-inflammatory changes during H. pylori infection. This was also reflected in vivo by reduced inflammation and increased colonization in MyD88−/− mice.135 In another study, a cancer related micro RNA (miR155) known to be regulated by TLR ligands in monocyte-derived cells was found to be upregulated during infection with cagPAI-positive H. pylori, but the signaling was independent of TLR2/4 and NOD1/2.136 However, many studies have utilized HEK293, an embryonic kidney epithelial cell line which does not express most of the TLRs,9 and these cells were stably transfected with certain TLRs to be used for H. pylori infection studies.131,132,137,138 In addition, various studies with epithelial cell lines have described different activities of TLRs during H. pylori infection and most of them reported controversial roles of these receptors, which includes the detection of LPS by TLR4 vs. TLR2 (Fig. 1M).131,138-141 Interestingly, H. pylori flagellin was found to be evading recognition by TLR5 and that was attributed to the amino acid sequences 89–96 of the N-terminal D1 domain.142 Genetic swapping experiments of the corresponding motif in flagellin of Salmonella abolished its recognition by TLR5.142 Recent reports have also shown that H. pylori genomic DNA contains a specific immune-regulatory sequence (5′TTTAGGG) which induces an anti-inflammatory response through the inhibition of TLR9-mediated signaling and this associated with the inverse relation between inflammatory bowel disease (IBD) and H. pylori.143 However, the search for H. pylori ligands to TLRs has not yielded reproducible results yet. As an important class of immune receptors, the role of TLRs must be studied in more detail to get a clearer picture about their involvement in shaping the chronic pathogenicity of this bacterium.
H. pylori activates the inflammasome through TLR2 and NOD2 signaling
Nod-like receptors (NLRs) including NOD1 and NOD2 represent a large group of proteins involved in innate immunity. The role of some of these intracellular PRRs for sensing bacterial components and inducing pro-inflammatory changes have been studied in detail since their discovery about 10 y ago.144 Other NLR members such as NLRC4, NLRP1, and NLRP3 are well-known to form multimeric protein structures known as the “inflammasome” to activate pro-caspase-1, which leads to the maturation of pro-forms of IL-1β and IL-18 into their active forms. Sometimes, the inflammasome can also induce pro-inflammatory cell death or pyroptosis. In addition, NLRs like NLRP6, NLRP7, and NLRP12 have been recently implicated in inflammasome formation.145 However, the role of inflammasome activation during H. pylori infection is largely unknown in the literature. In one of the earlier studies, it was shown that H. pylori induced an increased IL-18 mRNA expression in the gastric mucosa of infected patients with no significant difference in caspase-1 activation and IL-18 mature form.146 Another study has found that IL-18 mRNA and protein expression in H. pylori-infected epithelial cells and monocytes were dependent on the cagPAI status and OipA protein expression.147 Apart from these studies, the use of a mouse infection model for H. pylori has demonstrated the activation of caspase-1 and processing of pro-IL-1β and pro-IL-18 into active forms. But these studies also highlighted the differential roles of these pro-inflammatory cytokines on T-cell driven immunopathologies during H. pylori infection.148,149 The only study on the involvement of the inflammasome during H. pylori infection has elucidated a role of cagPAI and the TLR2→NOD2→NLRP3 axis for maturation of IL-1β in mouse DCs.150 The induction of NLRP3 expression and activation of caspase-1 for processing of pro-IL-1β by H. pylori was mediated through TLR2 and NOD2 receptors (Fig. 1N). However, mouse deficient in IL-1β, IL-1 receptor, and caspase-1 have shown more bacterial load during infection with H. pylori in comparison with the NLRP3−/− and wild-type mice.150 More studies should be performed in the future in order to better understand the sensing of bacterial components by other NLRs, inflammasome formation, and to get an overall mechanism of action of this important process in innate immunity against H. pylori infection.
Conclusions
H. pylori is one of the most successful pathogens infecting about half of the human world population and is responsible for a considerable global health burden, including peptic ulcer disease and gastric cancer. Studies of host-bacterial interactions using their fundamental adhesins, surface exposed, secreted or even injected pathogenicity- and virulence-associated factors have provided us with remarkable insights into H. pylori biology. Here we have reviewed the interaction of a multitude of bacterial factors with a broad variety of host protein receptors. Currently, altogether 25 protein receptors have been reported to be exploited by 17 known bacterial factors (Table 1; Figure 1). There is also a growing list of at least 19 other targeted protein receptors including important molecules such as CD44, PAR, uPAR, PPAR, A2A adenosine receptor, NMDA receptors, mucins, caveolin, and IL17 receptors for which the corresponding bacterial factors still remain to be identified (Table S1). The current data suggest a model in which the major H. pylori adhesins establish the initial and sustained host cell contact. Once intimate contact is established, other bacterial factors can start interacting with specific host cell protein receptors to facilitate bacterial binding and/or signaling. However, not only host protein receptors are targeted by H. pylori, but also non-proteinaceous factors including carbohydrates or lipids, which are reviewed elsewhere.15,18,19 The T4SS is such an example which requires host integrins but also other molecules such as phosphatidylserine and cholesterol to facilitate injection of CagA, probably in cholesterol-rich lipid rafts.14,63 This indicates that at least three known host factors are involved in T4SS-mediated CagA injection. However, very recent studies also suggest that even some non-cagPAI encoded factors such as HopI, HopQ, and LPS biosynthesis genes are also involved in host cell interaction and injection of CagA.16,17 If these bacterial factors can bind to other host receptors assisting in T4SS function is yet unclear and awaits further investigation. However, it can be assumed that the T4SS injection mechanism is much more complicated than originally proposed52 and requires even multiple cagPAI-independent factors, probably acting cooperatively. In addition, we have reviewed our current knowledge on other bacterial factors including VacA, HtrA, UreA, UreB, HSP-60, Lpp20, HpaA, LPS or peptidoglycan, and how they target host protein receptors and downstream signaling cascades. It can be therefore assumed that there is a highly dynamic system of extensive crosstalk between host protein receptors and their bacterial ligands to make up a scenario of complex host cellular processes leading to persistent colonization and chronic pathogenicity. In the future, it will be also important to unravel if other important H. pylori pathogenicity factors such as secreted γ-glutamyltranspeptidase (GGT)4 target a host cell receptor for uptake and signaling. In addition, it should be mentioned that genetic differences (e.g., single-nucleotide polymorphisms, called SNPs) were found in various host protein receptors and immunoregulatory proteins such as IL-1 receptor, IL-1 receptor antagonist, TLRs, E-cadherin, IL-1β or TNFα, which are known to crucially influence the clinical outcome of H. pylori infections.1 These factors also appear to play a key role in the pathophysiology of gastric disease development and their importance has been investigated in animal models which mimic human gastric neoplasia.3,4 It can be expected that more genetic polymorphisms both in the host and in H. pylori will be uncovered with advancing technologies, so that there is every prospect of defining full genetic risk profiles in near future. This will aid in improving the current testing and treatment strategies of H. pylori infections. Thus, it appears that H. pylori-host receptor interactions will continue to be a fascinating and rewarding research topic in future studies.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
The work of Backert S is supported through a DFG grant (project B10 of CRC-796).
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/27001
References
- 1.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–23. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119:2475–87. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polk DB, Peek RM., Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385–99. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J Exp Med. 2000;191:587–92. doi: 10.1084/jem.191.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backert S, Meyer TF. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol. 2006;9:207–17. doi: 10.1016/j.mib.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163–76. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 8.Boquet P, Ricci V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012;20:165–74. doi: 10.1016/j.tim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 10.Wessler S, Backert S. Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol. 2008;16:397–405. doi: 10.1016/j.tim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G, et al. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol. 2010;77:1539–55. doi: 10.1111/j.1365-2958.2010.07307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoy B, Löwer M, Weydig C, Carra G, Tegtmeyer N, Geppert T, Schröder P, Sewald N, Backert S, Schneider G, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin EJ, Trent MS. Colonize, evade, flourish: How glyco-conjugates promote virulence of Helicobacter pylori. [Epub ahead of print]. Gut Microbes 2013; 4:4; PMID:23859890; 10.4161/gmic.25721 [DOI] [PMC free article] [PubMed]
- 14.Backert S, Clyne M, Tegtmeyer N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal. 2011;9:28. doi: 10.1186/1478-811X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aspholm M, Kalia A, Ruhl S, Schedin S, Arnqvist A, Lindén S, Sjöström R, Gerhard M, Semino-Mora C, Dubois A, et al. Helicobacter pylori adhesion to carbohydrates. Methods Enzymol. 2006;417:293–339. doi: 10.1016/S0076-6879(06)17020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiménez-Soto LF, Clausen S, Sprenger A, Ertl C, Haas R. Dynamics of the Cag-type IV secretion system of Helicobacter pylori as studied by bacterial co-infections. Cell Microbiol. 2013;15:1924–37. doi: 10.1111/cmi.12166. [DOI] [PubMed] [Google Scholar]
- 17.Belogolova E, Bauer B, Pompaiah M, Asakura H, Brinkman V, Ertl C, Bartfeld S, Nechitaylo TY, Haas R, Machuy N, et al. Helicobacter pylori outer membrane protein HopQ identified as a novel T4SS-associated virulence factor. Cell Microbiol. 2013;15:1896–912. doi: 10.1111/cmi.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320–32. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 19.Backert S, Tegtmeyer N. the versatility of the Helicobacter pylori vacuolating cytotoxin vacA in signal transduction and molecular crosstalk. Toxins (Basel) 2010;2:69–92. doi: 10.3390/toxins2010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol. 1999;31:1537–48. doi: 10.1046/j.1365-2958.1999.01300.x. [DOI] [PubMed] [Google Scholar]
- 21.Rokbi B, Seguin D, Guy B, Mazarin V, Vidor E, Mion F, Cadoz M, Quentin-Millet MJ. Assessment of Helicobacter pylori gene expression within mouse and human gastric mucosae by real-time reverse transcriptase PCR. Infect Immun. 2001;69:4759–66. doi: 10.1128/IAI.69.8.4759-4766.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odenbreit S, Swoboda K, Barwig I, Ruhl S, Borén T, Koletzko S, Haas R. Outer membrane protein expression profile in Helicobacter pylori clinical isolates. Infect Immun. 2009;77:3782–90. doi: 10.1128/IAI.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jonge R, Durrani Z, Rijpkema SG, Kuipers EJ, van Vliet AH, Kusters JG. Role of the Helicobacter pylori outer-membrane proteins AlpA and AlpB in colonization of the guinea pig stomach. J Med Microbiol. 2004;53:375–9. doi: 10.1099/jmm.0.45551-0. [DOI] [PubMed] [Google Scholar]
- 24.Lu H, Wu JY, Beswick EJ, Ohno T, Odenbreit S, Haas R, Reyes VE, Kita M, Graham DY, Yamaoka Y. Functional and intracellular signaling differences associated with the Helicobacter pylori AlpAB adhesin from Western and East Asian strains. J Biol Chem. 2007;282:6242–54. doi: 10.1074/jbc.M611178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senkovich OA, Yin J, Ekshyyan V, Conant C, Traylor J, Adegboyega P, McGee DJ, Rhoads RE, Slepenkov S, Testerman TL. Helicobacter pylori AlpA and AlpB bind host laminin and influence gastric inflammation in gerbils. Infect Immun. 2011;79:3106–16. doi: 10.1128/IAI.01275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telford JL, Ghiara P, Dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce MF, Censini S, Covacci A, Xiang Z, et al. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–8. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangwer KA, Mushrush DJ, Stauff DL, Spiller B, McClain MS, Cover TL, Lacy DB. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc Natl Acad Sci U S A. 2007;104:16293–8. doi: 10.1073/pnas.0707447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahiro K, Niidome T, Kimura M, Hatakeyama T, Aoyagi H, Kurazono H, Imagawa Ki, Wada A, Moss J, Hirayama T. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase β. J Biol Chem. 1999;274:36693–9. doi: 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 29.Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T, et al. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375–81. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- 30.Yahiro K, Wada A, Nakayama M, Kimura T, Ogushi K, Niidome T, Aoyagi H, Yoshino K, Yonezawa K, Moss J, et al. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J Biol Chem. 2003;278:19183–9. doi: 10.1074/jbc.M300117200. [DOI] [PubMed] [Google Scholar]
- 31.Hennig EE, Godlewski MM, Butruk E, Ostrowski J. Helicobacter pylori VacA cytotoxin interacts with fibronectin and alters HeLa cell adhesion and cytoskeletal organization in vitro. FEMS Immunol Med Microbiol. 2005;44:143–50. doi: 10.1016/j.femsim.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Padilla PI, Wada A, Yahiro K, Kimura M, Niidome T, Aoyagi H, Kumatori A, Anami M, Hayashi T, Fujisawa J, et al. Morphologic differentiation of HL-60 cells is associated with appearance of RPTPbeta and induction of Helicobacter pylori VacA sensitivity. J Biol Chem. 2000;275:15200–6. doi: 10.1074/jbc.275.20.15200. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama M, Hisatsune J, Yamasaki E, Nishi Y, Wada A, Kurazono H, Sap J, Yahiro K, Moss J, Hirayama T. Clustering of Helicobacter pylori VacA in lipid rafts, mediated by its receptor, receptor-like protein tyrosine phosphatase β, is required for intoxication in AZ-521 Cells. Infect Immun. 2006;74:6571–80. doi: 10.1128/IAI.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bernard M, Moschioni M, Napolitani G, Rappuoli R, Montecucco C. The VacA toxin of Helicobacter pylori identifies a new intermediate filament-interacting protein. EMBO J. 2000;19:48–56. doi: 10.1093/emboj/19.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennig EE, Butruk E, Ostrowski J. RACK1 protein interacts with Helicobacter pylori VacA cytotoxin: the yeast two-hybrid approach. Biochem Biophys Res Commun. 2001;289:103–10. doi: 10.1006/bbrc.2001.5950. [DOI] [PubMed] [Google Scholar]
- 36.Satin B, Norais N, Telford J, Rappuoli R, Murgia M, Montecucco C, Papini E. Effect of helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J Biol Chem. 1997;272:25022–8. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama M, Kimura M, Wada A, Yahiro K, Ogushi K, Niidome T, Fujikawa A, Shirasaka D, Aoyama N, Kurazono H, et al. Helicobacter pylori VacA activates the p38/activating transcription factor 2-mediated signal pathway in AZ-521 cells. J Biol Chem. 2004;279:7024–8. doi: 10.1074/jbc.M308898200. [DOI] [PubMed] [Google Scholar]
- 38.Boncristiano M, Paccani SR, Barone S, Ulivieri C, Patrussi L, Ilver D, Amedei A, D’Elios MM, Telford JL, Baldari CT. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J Exp Med. 2003;198:1887–97. doi: 10.1084/jem.20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beswick EJ, Bland DA, Suarez G, Barrera CA, Fan X, Reyes VE. Helicobacter pylori binds to CD74 on gastric epithelial cells and stimulates interleukin-8 production. Infect Immun. 2005;73:2736–43. doi: 10.1128/IAI.73.5.2736-2743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beswick EJ, Pinchuk IV, Minch K, Suarez G, Sierra JC, Yamaoka Y, Reyes VE. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect Immun. 2006;74:1148–55. doi: 10.1128/IAI.74.2.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma SA, Tummuru MK, Miller GG, Blaser MJ. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–7. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basmarke-Wehelie R, Sjölinder H, Jurkowski W, Elofsson A, Arnqvist A, Engstrand L, Hagner M, Wallin E, Guan N, Kuranasekera H, et al. The complement regulator CD46 is bactericidal to Helicobacter pylori and blocks urease activity. Gastroenterology. 2011;141:918–28. doi: 10.1053/j.gastro.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479–86. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Fischer W, Püls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–48. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 45.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–81. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 46.Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol. 2009;183:8099–109. doi: 10.4049/jimmunol.0900664. [DOI] [PubMed] [Google Scholar]
- 47.Hutton ML, Kaparakis-Liaskos M, Turner L, Cardona A, Kwok T, Ferrero RL. Helicobacter pylori exploits cholesterol-rich microdomains for induction of NF-kappaB-dependent responses and peptidoglycan delivery in epithelial cells. Infect Immun. 2010;78:4523–31. doi: 10.1128/IAI.00439-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boughan PK, Argent RH, Body-Malapel M, Park JH, Ewings KE, Bowie AG, Ong SJ, Cook SJ, Sorensen OE, Manzo BA, et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infection. J Biol Chem. 2006;281:11637–48. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 49.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jéhanno M, Viala J, Tedin K, Taha MK, Labigne A, Zähringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 50.Hirata Y, Ohmae T, Shibata W, Maeda S, Ogura K, Yoshida H, Kawabe T, Omata M. MyD88 and TNF receptor-associated factor 6 are critical signal transducers in Helicobacter pylori-infected human epithelial cells. J Immunol. 2006;176:3796–803. doi: 10.4049/jimmunol.176.6.3796. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645–62. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, König W, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–6. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 53.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Backert S, Fronzes R, Waksman G. VirB2 and VirB5 proteins: specialized adhesins in bacterial type-IV secretion systems? Trends Microbiol. 2008;16:409–13. doi: 10.1016/j.tim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Barden S, Lange S, Tegtmeyer N, Conradi J, Sewald N, Backert S, Niemann HH. A Helical RGD Motif Promoting Cell Adhesion: Crystal Structures of the Helicobacter pylori Type IV Secretion System Pilus Protein CagL. Structure. 2013;21:1931–41. doi: 10.1016/j.str.2013.08.018. [DOI] [PubMed] [Google Scholar]; PMID:24076404; 10.1016/j.str.2013.08.018 [DOI]
- 56.Tegtmeyer N, Hartig R, Delahay RM, Rohde M, Brandt S, Conradi J, Takahashi S, Smolka AJ, Sewald N, Backert S. A small fibronectin-mimicking protein from bacteria induces cell spreading and focal adhesion formation. J Biol Chem. 2010;285:23515–26. doi: 10.1074/jbc.M109.096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conradi J, Tegtmeyer N, Woźna M, Wissbrock M, Michalek C, Gagell C, Cover TL, Frank R, Sewald N, Backert S. An RGD helper sequence in CagL of Helicobacter pylori assists in interactions with integrins and injection of CagA. Front Cell Infect Microbiol. 2012;2:70. doi: 10.3389/fcimb.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorrell RJ, Guan J, Xin Y, Tafreshi MA, Hutton ML, McGuckin MA, Ferrero RL, Kwok T. A novel NOD1- and CagA-independent pathway of interleukin-8 induction mediated by the Helicobacter pylori type IV secretion system. Cell Microbiol. 2012;15:554–70. doi: 10.1111/cmi.12055. [DOI] [PubMed] [Google Scholar]
- 59.Jiménez-Soto LF, Kutter S, Sewald X, Ertl C, Weiss E, Kapp U, Rohde M, Pirch T, Jung K, Retta SF, et al. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog. 2009;5:e1000684. doi: 10.1371/journal.ppat.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaplan-Türköz B, Jiménez-Soto LF, Dian C, Ertl C, Remaut H, Louche A, Tosi T, Haas R, Terradot L. Structural insights into Helicobacter pylori oncoprotein CagA interaction with β1 integrin. Proc Natl Acad Sci U S A. 2012;109:14640–5. doi: 10.1073/pnas.1206098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaffer CL, Gaddy JA, Loh JT, Johnson EM, Hill S, Hennig EE, McClain MS, McDonald WH, Cover TL. Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathog. 2011;7:e1002237. doi: 10.1371/journal.ppat.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrozo RM, Cooke CL, Hansen LM, Lam AM, Gaddy JA, Johnson EM, Cariaga TA, Suarez G, Peek RM, Jr., Cover TL, et al. Functional plasticity in the type IV secretion system of Helicobacter pylori. PLoS Pathog. 2013;9:e1003189. doi: 10.1371/journal.ppat.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190–202. doi: 10.1111/j.1742-4658.2011.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. 2012;47:609–18. doi: 10.1007/s00535-012-0592-1. [DOI] [PubMed] [Google Scholar]
- 65.Saha A, Hammond CE, Trojanowska M, Smolka AJ. Helicobacter pylori-induced H,K-ATPase alpha-subunit gene repression is mediated by NF-kappaB p50 homodimer promoter binding. Am J Physiol Gastrointest Liver Physiol. 2008;294:G795–807. doi: 10.1152/ajpgi.00431.2007. a. [DOI] [PubMed] [Google Scholar]
- 66.Saha A, Hammond CE, Gooz M, Smolka AJ. The role of Sp1 in IL-1beta and H. pylori-mediated regulation of H,K-ATPase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2008;295:G977–86. doi: 10.1152/ajpgi.90338.2008. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saha A, Hammond CE, Beeson C, Peek RM, Jr., Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut. 2010;59:874–81. doi: 10.1136/gut.2009.194795. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saha A, Backert S, Hammond CE, Gooz M, Smolka AJ. Helicobacter pylori CagL activates ADAM17 to induce repression of the gastric H, K-ATPase alpha subunit. Gastroenterology. 2010;139:239–48. doi: 10.1053/j.gastro.2010.03.036. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–8. doi: 10.1042/BST0311203. [DOI] [PubMed] [Google Scholar]
- 70.Coyle WJ, Sedlack RE, Nemec R, Peterson R, Duntemann T, Murphy M, Lawson JM. Eradication of Helicobacter pylori normalizes elevated mucosal levels of epidermal growth factor and its receptor. Am J Gastroenterol. 1999;94:2885–9. doi: 10.1111/j.1572-0241.1999.01432.x. [DOI] [PubMed] [Google Scholar]
- 71.Wong BC, Wang WP, So WH, Shin VY, Wong WM, Fung FM, Liu ES, Hiu WM, Lam SK, Cho CH. Epidermal growth factor and its receptor in chronic active gastritis and gastroduodenal ulcer before and after Helicobacter pylori eradication. Aliment Pharmacol Ther. 2001;15:1459–65. doi: 10.1046/j.1365-2036.2001.01051.x. [DOI] [PubMed] [Google Scholar]
- 72.Keates S, Sougioultzis S, Keates AC, Zhao D, Peek RM, Jr., Shaw LM, Kelly CP. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem. 2001;276:48127–34. doi: 10.1074/jbc.M107630200. [DOI] [PubMed] [Google Scholar]
- 73.Wallasch C, Crabtree JE, Bevec D, Robinson PA, Wagner H, Ullrich A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun. 2002;295:695–701. doi: 10.1016/S0006-291X(02)00740-4. [DOI] [PubMed] [Google Scholar]
- 74.Bauer B, Pang E, Holland C, Kessler M, Bartfeld S, Meyer TF. The Helicobacter pylori virulence effector CagA abrogates human β-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe. 2012;11:576–86. doi: 10.1016/j.chom.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 75.Keates S, Keates AC, Nath S, Peek RM, Jr., Kelly CP. Transactivation of the epidermal growth factor receptor by cag+ Helicobacter pylori induces upregulation of the early growth response gene Egr-1 in gastric epithelial cells. Gut. 2005;54:1363–9. doi: 10.1136/gut.2005.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, Dempsey PJ, Peek RM Jr., Cover TL, Washington MK, Wilson KT, et al. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology 2009; 136:1297-307, e1-3; 10.1053/j.gastro.2008.12.059; PMID:19250983 [DOI] [PMC free article] [PubMed]
- 77.Tabel G, Hoa NT, Tarnawski A, Chen J, Domek M, Ma TY. Helicobacter pylori infection inhibits healing of the wounded duodenal epithelium in vitro. J Lab Clin Med. 2003;142:421–30. doi: 10.1016/j.lab.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Tegtmeyer N, Zabler D, Schmidt D, Hartig R, Brandt S, Backert S. Importance of EGF receptor, HER2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: antagonistic effects of the vacuolating cytotoxin VacA. Cell Microbiol. 2009;11:488–505. doi: 10.1111/j.1462-5822.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 79.Churin Y, Al-Ghoul L, Kepp O, Meyer TF, Birchmeier W, Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249–55. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliveira MJ, Costa AC, Costa AM, Henriques L, Suriano G, Atherton JC, Machado JC, Carneiro F, Seruca R, Mareel M, et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem. 2006;281:34888–96. doi: 10.1074/jbc.M607067200. [DOI] [PubMed] [Google Scholar]
- 81.Oliveira MJ, Costa AM, Costa AC, Ferreira RM, Sampaio P, Machado JC, Seruca R, Mareel M, Figueiredo C. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J Infect Dis. 2009;200:745–55. doi: 10.1086/604727. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 83.Snider JL, Cardelli JA. Helicobacter pylori induces cancer cell motility independent of the c-Met receptor. J Carcinog. 2009;8:7. doi: 10.4103/1477-3163.50892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102:9300–5. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. J Biol Chem. 2006;281:32344–52. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 86.Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–3. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 87.Wang TC, Goldenring JR. Inflammation intersection: gp130 balances gut irritation and stomach cancer. Nat Med. 2002;8:1080–2. doi: 10.1038/nm1002-1080. [DOI] [PubMed] [Google Scholar]
- 88.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–97. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 89.Mueller D, Tegtmeyer N, Brandt S, Yamaoka Y, De Poire E, Sgouras D, Wessler S, Torres J, Smolka A, Backert S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553–66. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, Blaser MJ, Lee YC. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem. 2010;285:16042–50. doi: 10.1074/jbc.M110.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiedemann T, Hofbaur S, Tegtmeyer N, Huber S, Sewald N, Wessler S, Backert S, Rieder G. Helicobacter pylori CagL dependent induction of gastrin expression via a novel αvβ5-integrin-integrin linked kinase signalling complex. Gut. 2012;61:986–96. doi: 10.1136/gutjnl-2011-300525. l. [DOI] [PubMed] [Google Scholar]
- 92.Conradi J, Huber S, Gaus K, Mertink F, Royo Gracia S, Strijowski U, Backert S, Sewald N. Cyclic RGD peptides interfere with binding of the Helicobacter pylori protein CagL to integrins αVβ3 and α5β1. Amino Acids. 2012;43:219–32. doi: 10.1007/s00726-011-1066-0. b. [DOI] [PubMed] [Google Scholar]
- 93.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–97. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 94.Frees D, Brøndsted L, Ingmer H. Bacterial proteases and virulence. Subcell Biochem. 2013;66:161–92. doi: 10.1007/978-94-007-5940-4_7. [DOI] [PubMed] [Google Scholar]
- 95.Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, Sabarth N, Meyer TF, Jungblut PR. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 2002;70:3396–403. doi: 10.1128/IAI.70.7.3396-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salama NR, Shepherd B, Falkow S. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J Bacteriol. 2004;186:7926–35. doi: 10.1128/JB.186.23.7926-7935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoy B, Geppert T, Boehm M, Reisen F, Plattner P, Gadermaier G, Sewald N, Ferreira F, Briza P, Schneider G, et al. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J Biol Chem. 2012;287:10115–20. doi: 10.1074/jbc.C111.333419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boehm M, Hoy B, Rohde M, Tegtmeyer N, Bæk KT, Oyarzabal OA, Brøndsted L, Wessler S, Backert S. Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog. 2012;4:3. doi: 10.1186/1757-4749-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schirrmeister W, Gnad T, Wex T, Higashiyama S, Wolke C, Naumann M, Lendeckel U. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp Cell Res. 2009;315:3500–8. doi: 10.1016/j.yexcr.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 100.Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–23. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 101.Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Jr., Azuma T, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–26. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 102.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–51. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187:135–40. doi: 10.1084/jem.187.1.135. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sewald X, Gebert-Vogl B, Prassl S, Barwig I, Weiss E, Fabbri M, Osicka R, Schiemann M, Busch DH, Semmrich M, et al. Integrin subunit CD18 Is the T-lymphocyte receptor for the Helicobacter pylori vacuolating cytotoxin. Cell Host Microbe. 2008;3:20–9. doi: 10.1016/j.chom.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Sewald X, Jiménez-Soto L, Haas R. PKC-dependent endocytosis of the Helicobacter pylori vacuolating cytotoxin in primary T lymphocytes. Cell Microbiol. 2011;13:482–96. doi: 10.1111/j.1462-5822.2010.01551.x. [DOI] [PubMed] [Google Scholar]
- 106.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 107.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci U S A. 2004;101:7727–32. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yokoyama K, Higashi H, Ishikawa S, Fujii Y, Kondo S, Kato H, Azuma T, Wada A, Hirayama T, Aburatani H, et al. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc Natl Acad Sci U S A. 2005;102:9661–6. doi: 10.1073/pnas.0502529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim JM, Kim JS, Yoo DY, Ko SH, Kim N, Kim H, Kim YJ. Stimulation of dendritic cells with Helicobacter pylori vacuolating cytotoxin negatively regulates their maturation via the restoration of E2F1. Clin Exp Immunol. 2011;166:34–45. doi: 10.1111/j.1365-2249.2011.04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng PY, Jones NL. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol. 2003;5:25–40. doi: 10.1046/j.1462-5822.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 111.Ramarao N, Gray-Owen SD, Backert S, Meyer TF. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol Microbiol. 2000;37:1389–404. doi: 10.1046/j.1365-2958.2000.02089.x. [DOI] [PubMed] [Google Scholar]
- 112.Rittig MG, Shaw B, Letley DP, Thomas RJ, Argent RH, Atherton JC. Helicobacter pylori-induced homotypic phagosome fusion in human monocytes is independent of the bacterial vacA and cag status. Cell Microbiol. 2003;5:887–99. doi: 10.1046/j.1462-5822.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 113.Yuan J, Li P, Tao J, Shi X, Hu B, Chen H, Guo X. H. pylori escape host immunoreaction through inhibiting ILK expression by VacA. Cell Mol Immunol. 2009;6:191–7. doi: 10.1038/cmi.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herranz B, Marquez S, Guijarro B, Aracil E, Aicart-Ramos C, Rodriguez-Crespo I, Serrano I, Rodríguez-Puyol M, Zaragoza C, Saura M. Integrin-linked kinase regulates vasomotor function by preventing endothelial nitric oxide synthase uncoupling: role in atherosclerosis. Circ Res. 2012;110:439–49. doi: 10.1161/CIRCRESAHA.111.253948. [DOI] [PubMed] [Google Scholar]
- 115.de Bernard M, Cappon A, Pancotto L, Ruggiero P, Rivera J, Del Giudice G, Montecucco C. The Helicobacter pylori VacA cytotoxin activates RBL-2H3 cells by inducing cytosolic calcium oscillations. Cell Microbiol. 2005;7:191–8. doi: 10.1111/j.1462-5822.2004.00446.x. [DOI] [PubMed] [Google Scholar]
- 116.Supajatura V, Ushio H, Wada A, Yahiro K, Okumura K, Ogawa H, Hirayama T, Ra C. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J Immunol. 2002;168:2603–7. doi: 10.4049/jimmunol.168.6.2603. [DOI] [PubMed] [Google Scholar]
- 117.Faure-André G, Vargas P, Yuseff MI, Heuzé M, Diaz J, Lankar D, Steri V, Manry J, Hugues S, Vascotto F, et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–10. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- 118.Mitchell PJ, Afzali B, Fazekasova H, Chen D, Ali N, Powell N, Lord GM, Lechler RI, Lombardi G. Helicobacter pylori induces in-vivo expansion of human regulatory T cells through stimulating interleukin-1β production by dendritic cells. Clin Exp Immunol. 2012;170:300–9. doi: 10.1111/j.1365-2249.2012.04659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Enarsson K, Brisslert M, Backert S, Quiding-Järbrink M. Helicobacter pylori induces transendothelial migration of activated memory T cells. Infect Immun. 2005;73:761–9. doi: 10.1128/IAI.73.2.761-769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, Zaitoun AM, Atherton JC. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57:1375–85. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 121.Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918–24. doi: 10.4049/jimmunol.165.4.1918. [DOI] [PubMed] [Google Scholar]
- 122.Li Y, Jiang Y, Xi Y, Zhang L, Luo J, He D, Zeng S, Ning Y. Identification and characterization of H-2d restricted CD4+ T cell epitopes on Lpp20 of Helicobacter pylori. BMC Immunol. 2012;13:68. doi: 10.1186/1471-2172-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen L, Li B, Yang WC, He JL, Li NY, Hu J, He YF, Yu S, Zhao Z, Luo P, et al. A dominant CD4(+) T-cell response to Helicobacter pylori reduces risk for gastric disease in humans. Gastroenterology. 2013;144:591–600. doi: 10.1053/j.gastro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Yang WC, Chen L, Li HB, Li B, Hu J, Zhang JY, Yang SM, Zou QM, Guo H, Wu C. Identification of two novel immunodominant UreB CD4(+) T cell epitopes in Helicobacter pylori infected subjects. Vaccine. 2013;31:1204–9. doi: 10.1016/j.vaccine.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 125.Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun. 2006;74:830–8. doi: 10.1128/IAI.74.2.830-838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hayes SH, Seigel GM. Immunoreactivity of ICAM-1 in human tumors, metastases and normal tissues. Int J Clin Exp Pathol. 2009;2:553–60. [PMC free article] [PubMed] [Google Scholar]
- 127.Moese S, Selbach M, Meyer TF, Backert S. cag+ Helicobacter pylori induces homotypic aggregation of macrophage-like cells by up-regulation and recruitment of intracellular adhesion molecule 1 to the cell surface. Infect Immun. 2002;70:4687–91. doi: 10.1128/IAI.70.8.4687-4691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–26. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 129.Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, Miyaoka Y, Kazumori H, Ishimura N, Amano Y, et al. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol. 2004;173:1406–16. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- 130.Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, Shiratori Y, Omata M. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J Biol Chem. 2001;276:44856–64. doi: 10.1074/jbc.M105381200. [DOI] [PubMed] [Google Scholar]
- 131.Yokota S, Ohnishi T, Muroi M, Tanamoto K, Fujii N, Amano K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol Med Microbiol. 2007;51:140–8. doi: 10.1111/j.1574-695X.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 132.Kumar Pachathundikandi S, Brandt S, Madassery J, Backert S. Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS One. 2011;6:e19614. doi: 10.1371/journal.pone.0019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Y, Yokota K, Ayada K, Yamamoto Y, Okada T, Shen L, Oguma K. Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes. J Med Microbiol. 2007;56:154–64. doi: 10.1099/jmm.0.46882-0. [DOI] [PubMed] [Google Scholar]
- 134.Gobert AP, Bambou JC, Werts C, Balloy V, Chignard M, Moran AP, Ferrero RL. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J Biol Chem. 2004;279:245–50. doi: 10.1074/jbc.M307858200. [DOI] [PubMed] [Google Scholar]
- 135.Rad R, Brenner L, Krug A, Voland P, Mages J, Lang R, et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology 2007; 133:150-163.e3. [DOI] [PubMed]
- 136.Koch M, Mollenkopf HJ, Klemm U, Meyer TF. Induction of microRNA-155 is TLR- and type IV secretion system-dependent in macrophages and inhibits DNA-damage induced apoptosis. Proc Natl Acad Sci U S A. 2012;109:E1153–62. doi: 10.1073/pnas.1116125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith MF, Jr., Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552–60. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 138.Smith SM, Moran AP, Duggan SP, Ahmed SE, Mohamed AS, Windle HJ, O’Neill LA, Kelleher DP. Tribbles 3: a novel regulator of TLR2-mediated signaling in response to Helicobacter pylori lipopolysaccharide. J Immunol. 2011;186:2462–71. doi: 10.4049/jimmunol.1000864. [DOI] [PubMed] [Google Scholar]
- 139.Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446–54. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lepper PM, Triantafilou M, Schumann C, Schneider EM, Triantafilou K. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell Microbiol. 2005;7:519–28. doi: 10.1111/j.1462-5822.2005.00482.x. [DOI] [PubMed] [Google Scholar]
- 141.Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H, Ohara S, Kotake Y, Shimosegawa T, Yoshimura T. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1004–12. doi: 10.1152/ajpgi.00096.2007. [DOI] [PubMed] [Google Scholar]
- 142.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A. 2005;102:9247–52. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Owyang SY, Luther J, Owyang CC, Zhang M, Kao JY. Helicobacter pylori DNA’s anti-inflammatory effect on experimental colitis. Gut Microbes. 2012;3:168–71. doi: 10.4161/gmic.19181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 145.Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:551–65. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 146.Tomita T, Jackson AM, Hida N, Hayat M, Dixon MF, Shimoyama T, Axon AT, Robinson PA, Crabtree JE. Expression of Interleukin-18, a Th1 cytokine, in human gastric mucosa is increased in Helicobacter pylori infection. J Infect Dis. 2001;183:620–7. doi: 10.1086/318541. [DOI] [PubMed] [Google Scholar]
- 147.Yamauchi K, Choi IJ, Lu H, Ogiwara H, Graham DY, Yamaoka Y. Regulation of IL-18 in Helicobacter pylori infection. J Immunol. 2008;180:1207–16. doi: 10.4049/jimmunol.180.2.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Müller A. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol. 2012;188:3594–602. doi: 10.4049/jimmunol.1103212. [DOI] [PubMed] [Google Scholar]
- 149.Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Järbrink M, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–96. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim DJ, Park JH, Franchi L, Backert S, Núñez G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1β production in Helicobacter pylori infected dendritic cells. Eur J Immunol. 2013;43:2650–8. doi: 10.1002/eji.201243281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.