Abstract
Helicobacter pylori-associated gastric cancer is a major cause of morbidity and mortality worldwide, and is predicted to become even more common in developing countries as the population ages. Since gastric cancer develops slowly over years to decades, and typically progresses though a series of well-defined histologic stages, cancer biomarkers have potential to identify asymptomatic individuals in whom surgery might be curative, or even those for whom antibiotics to eradicate H. pylori could prevent neoplastic transformation. Here we describe some of the challenges of biomarker discovery, summarize current approaches to biomarkers of gastric cancer, and explore some recent novel strategies.
Keywords: gastric cancer, helicobacter pylori, biomarkers
Gastric Cancer and Helicobacter pylori
Gastric cancer is the fourth most common malignancy and the second most common cause of cancer-related death worldwide.1-3 If gastric cancer is detected at an early stage, the 5-y survival is approximately 90%.4 However, because there are no specific symptoms at the early stages when the disease is surgically curable, most cases present with locally advanced or metastatic disease, which has a median survival of only 24 mo and a 5-y survival of less than 15%.3 Thus, gastric cancer is a disease in need of early detection, and the current strategies have not been successful in decreasing the global burden of disease.
According to the 2008 GLOBOCAN estimates of the worldwide burden of cancer,5 the majority of gastric cancer cases occur in developing countries, with an age-standardized incidence rate that is twice as high in men as in women. Half of the cases occur in Eastern Asia, where the highest mortality rates are observed. High mortality rates are also observed in Central and Eastern Europe, and in Central and South America. In Latin America, the higher mortality rates are concentrated in nations along the Pacific rim, particularly in mountainous areas.6 Gastric cancer is projected to rise from fourteenth to eighth in all-cause mortality in the near term, due to the growing and aging populations in eastern Asia and Latin America.7
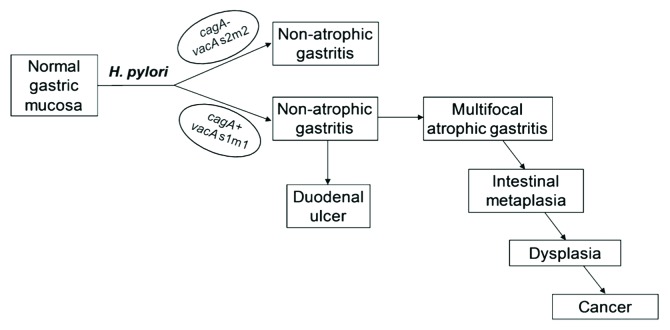
Pathologically, there are two histological types of gastric cancer: the diffuse type and the intestinal (well-differentiated) type.8 The diffuse type of gastric cancer is most often sporadic, but may also be hereditary, which is associated with loss of cell-cell interactions due to germ-line mutations in genes encoding epithelial junction proteins, particularly E-cadherin.9,10 Sporadic diffuse gastric cancer has been associated with H. pylori infection, which induces promoter methylation and silencing of the E-cadherin gene that is reversible upon H. pylori eradication.11,12 The intestinal type of gastric cancer is the most common histology and is associated with chronic gastritis. It was first proposed by Pelayo Correa that the intestinal type of gastric cancer follows a prolonged progression of pathologic changes that leads from chronic non-atrophic gastritis, to atrophic gastritis, intestinal metaplasia, dysplasia, and finally gastric adenocarcinoma. This “precancerous cascade” is shown schematically in Figure 1 and thoroughly discussed in a recent review.13 The initial gastric inflammatory response was first attributed to environmental factors such as excessive dietary salt and lack of fresh fruits and vegetables. However, the discovery of Helicobacter pylori in 1982 by Marshall and Warren, who in 2005 were awarded the Nobel Prize in Physiology or Medicine, led to the current understanding that chronic H. pylori infection initiates gastritis that can progress down the pathologic cascade to intestinal-type gastric cancer. While diet and host genetics continue to be recognized as important determinants of gastric cancer, approximately 60% of distal gastric cancer is attributable to H. pylori infection, prompting the designation of H. pylori as a Type I (definite) carcinogen by the WHO.
Figure 1. Schematic representation of the main clinical outcomes of H. pylori infection. The right side of the figure shows the sequential steps of the precancerous cascade. Reproduced from reference 13 with permission from John Wiley and Sons.
Approximately half of the world’s population is infected with H. pylori, with prevalence reaching more than 80% in many developing countries.14,15 However, infected patients have only a 1–3% lifetime risk of developing gastric cancer. Patients with H. pylori also have an approximately 10% lifetime risk of developing peptic ulcer disease, but those who develop duodenal ulcer generally do not develop gastric cancer.16 Since gastric cancer can only be treated successfully if it is identified early, screening approaches are needed to identify and treat those most at risk. There may be a role for endoscopic surveillance to detect precancerous lesions—much like is performed to detect colon cancer—or even mass screening and treatment of H. pylori to prevent gastric cancer.17-19 However, cost and the high likelihood of recurrent H. pylori infection after large-scale treatment20 must be addressed for these approaches to be practical. Here we consider another approach, the development of biomarkers to identify the subpopulation of those infected with H. pylori that are most at risk for development of gastric cancer.
The Challenge of Biomarker Discovery
It is widely believed by physicians and patients alike that early detection of cancer is critical to effective prevention and treatment, a view that has been fueled by rapid technological advances in high throughput detection, and is the foundation for the Early Detection Research Network (EDRN) initiative of the National Cancer Institute (http://edrn.nci.nih.gov). But the reality is that few cancer biomarkers are used in clinical practice today, and many of those that are used have come under increasing scrutiny as to whether they really deliver on the promise of improved cancer outcomes. Here we briefly address some of the challenges of biomarker discovery. For a more thorough discussion, we refer the reader to recent reviews.21,22
What do we want a biomarker to do?
It is important to be clear about exactly what we want a biomarker to do for prevention of gastric cancer. Probably the most desirable, but also most difficult to achieve, would be a biomarker that can identify those infected with H. pylori who are more likely to develop gastric cancer, so that they might be targeted for antibiotic therapy to eliminate infection, and perhaps also endoscopic surveillance to prevent the development of cancer. This will be challenging because only about 1% of those infected will develop gastric cancer, and because studies in humans and rodent models suggest that for antibiotics to be effective, they will have to be administered before preneoplastic changes have occurred.23-25 Also useful, though perhaps equally challenging, would be a biomarker that detects disease in those at an early stage without signs or symptoms, and who would benefit from surgery and/or chemotherapy. Gastric cancer, like most cancers, is much more amenable to cure when it is detected early, ideally before it has crossed anatomic barriers that make surgical resection impossible. Biomarkers may also be useful to predict the natural history of disease, predict the optimal therapy, and monitor disease activity during treatment. The latter is probably the least challenging hurdle for biomarker discovery, and several are currently in use, such as PSA and CA125.
The problem of overdiagnosis
Overdiagnosis is simply the identification of cancer in an asymptomatic person that would have never produced signs or symptoms had it not been discovered.26 Overdiagnosis can occur when a stable or very slow growing cancer is detected inadvertently or by an early detection screening test. The most familiar example is prostate cancer, which is estimated to be present in 30–70% of men over the age of 60 y, yet only a small fraction of them will ever have clinical sequelae.26 Less appreciated is the overdiagnosis of breast cancer, which is estimated to have a disease reservoir of 7–39% in asymptomatic women aged 40 to 70 y.26 The dilemma of course is that when cancer is diagnosed by a screening test, it is impossible to know whether it represents life-saving early detection or overdiagnosis, which can only be determined by long-term follow up studies. In one such study, it was estimated that in 2008, breast cancer was overdiagnosed in more than 70,000 women, which accounted for 31% of all breast cancers diagnosed.27 The solutions to this thorny problem will not be simple, all the more so because of political and emotional hyperbole, but an essential first step is recognition of the problem by physicians and patients alike.
Measurement considerations
Apparent successes in biomarker discovery have often not fulfilled their initial promise, owing in part to three major sources of error that have been thoughtfully discussed elsewhere in more detail28-30: bias, overfitting, and generalizability. Bias, or internal validity, refers to errors resulting from differences among patients other than the disease for which a biomarker is sought, or unequal assessment of results that may occur if investigators are not blinded. Specific inclusion and exclusion criteria, or stratification, are often used to minimize heterogeneity between groups. Bias can also result if data are collected or interpreted differently in the comparison groups. For example, if patient samples are collected differently or are stored for different lengths of time than controls, or have been thawed a different number of times, the quality of the material may differ among groups and result in biased results. Similarly, bias may result if different assays or versions of the assays, or even different reagent lots, are used to examine the samples. Neither large sample size nor reproducibility of the assays can mitigate bias. Overfitting refers to the apparent discrimination between cases and controls that is actually caused by chance. It is particularly problematic in discovery-based research that measures a large number of variables and then seeks to find a pattern that can discriminate among groups. The problem is accentuated if the sample size is small. Put differently, if enough possible predictors are examined, a pattern is almost certain to emerge that appears to distinguish the groups, but it would not likely stand up to the scrutiny of an independent validation set. The ideal method to address overfitting is to have completely independent testing and validation cohorts. However, since samples are often limited, a common alternative is to randomly split the sample into a training set that is used to derive the prediction rule, and a validation set that is used to independently evaluate it. Generalizability, also called external validity, concerns the question of to whom the results of a study can be reasonably applied. The generalizability of a study depends upon the careful definition of characteristics of the study participants. Thoughtful attention to these measurement issues, as well as standardized recommendations for reporting results,31 will be essential if recent technologic advances in biomarker measurement are to be translated into clinically meaningful assays.
Current Approaches to Biomarkers for the Detection of Gastric Cancer
Pepsinogen
The strongest known risk factor for the development of distal, non-cardia gastric cancer is H. pylori-induced atrophic gastritis,32-35 in which the normal gastric glands are absent and replaced by fibrosis and occasionally intestinal metaplasia. As the gastric glands atrophy, there is loss of acid production, but also loss of pepsinogen, which is found in two distinct forms. Pepsinogen I (PGI) is produced exclusively by chief and mucus neck cells in the fundic glands, while pepsinogen II (PGII) is secreted widely throughout the stomach. As corpus atrophy develops, PGI levels decline proportionally, while PGII remains relatively stable or declines but to a lesser extent, leading to a decreased PGI/PGII ratio.
Both PGI and the PGI/PGII ratio have been studied extensively as biomarkers for atrophic gastritis, particularly in Japan and some other parts of Asia. Despite problems with identification of the optimal cutoffs, regional variation in performance, and the influence of age, gender, and H. pylori infection on pepsinogen levels,36 PGI and PGI/PGII are specific measures of gastric atrophy,37-39 though in some studies the sensitivity is low.40 But while abnormal PGI or PGI/PGII are indications of gastric corpus atrophy, they are very poor predictors of gastric cancer. This is best illustrated by a meta-analysis of 42 individual studies involving nearly 300,000 participants in population based screening, which showed that the positive predictive value of PGI/PGII was only 0.77–1.25%, so that 600 individuals would have to be screened to detect one case of gastric cancer.41 Nevertheless, the negative predictive value of PGI/PGII was > 99%, so a negative result is a strong indication of a healthy gastric mucosa. For this reason, the primary utility of pepsinogen is to distinguish those individuals with atrophy who merit periodic endoscopic screening, from those with a healthy gastric mucosa who do not. Large population-based studies in Europe and in Asia have validated the usefulness of this approach.42,43 But in areas of the world where atrophic gastritis and gastric cancer are common, positive results will be very frequent. Therefore, additional biomarkers are required to avoid the impractical and expensive strategy of performing endoscopy on all patients with abnormal pepsinogen.
Gastrin-17
Gastrin, produced primarily by the endocrine (G) cells in the gastric antrum, stimulates parietal cells in the corpus to secrete hydrochloric acid. Circulating gastrin has been proposed as a biomarker of gastric atrophy that could assist in the diagnosis of atrophic gastritis and gastric cancer. However, the relationship between gastrin and gastric pathology is complicated. First, gastrin is low when atrophy is restricted to the antrum, where it is produced, but it is high in the setting of isolated (usually autoimmune) corpus atrophy because the normal inhibitory feedback of acid on G cells is lost. However, atrophic gastritis of the corpus is most often a result of H. pylori infection, which causes a pan-gastritis, so low gastrin is commonly associated with atrophy of the corpus and the antrum.39 Second, G cells secrete six bioactive gastrin peptides of different length, though the 17 and 34 residue forms are predominant in plasma. For historical and technical reasons, gastrin is usually detected in serum or plasma by immunoassay of gastrin-17. However, accurate detection of gastrin-17 is difficult because it has a very short half-life, and antibodies differ in the extent to which they detect sulfated gastrin and peptides of different length. A recent comparison of commercial kits to a validated reference radioimmunoassay found that fewer than half were accurate.44 Despite these limitations, low gastrin shows some utility as a biomarker of atrophic gastritis.45,46 Since atrophy is a precursor, one might expect that gastrin would also be low in the setting of gastric cancer, but in fact it appears to be elevated, though it does not distinguish early stage from advanced disease.45
H. pylori serology
Because H. pylori is by far the most common cause of atrophic gastritis, serologic evidence of H. pylori infection has been studied in combination with pepsinogen as a biomarker for gastric cancer.34,47,48 If H. pylori antibody is absent and pepsinogen is normal, the risk of gastric cancer is extremely low, if not zero. The risk increases with H. pylori seropositivity alone, but is substantially higher if PGI/PGII is also low, indicating atrophic gastritis. Interestingly, the greatest risk for gastric cancer is sometimes found in those patients with low PGI/PGII but who are seronegative for H. pylori,34,47,48 though there are exceptions.49 While these patients could in principle have atrophic gastritis from some cause other than H. pylori infection, such as autoimmune gastritis, they more often represent the most severe, late stages of intestinal metaplasia, in which the stomach environment is inhospitable for H. pylori growth and serology reverts to negative.50 Serologic evidence of immune response to specific H. pylori virulence factors is another strategy that has been explored to identify gastric cancer biomarkers. Seropositivity to CagA and VacA have been studied most often, though the results have been mixed.51-54 Multiplex assays that detect serologic responses to a panel of H. pylori proteins may improve the performance of individual assays.55-58
Current guidelines
European and Asia-Pacific guidelines have been published regarding gastric cancer prevention,59,60 though consensus is often lacking. Screening with pepsinogen and H. pylori serology is recommended by some experts to identify patients at risk of gastric cancer, who can then be targeted for serial endoscopic evaluation. National screening programs using photofluorography or endoscopy are in place in Japan and Korea, where gastric cancer can account for as many as 20% of newly diagnosed cancers.61
Discovery of Novel Biomarkers
Current approaches to biomarkers for gastric cancer are inadequate. Here we selectively explore some approaches to discovery of novel biomarkers.
Micro RNA
MicroRNAs (miRNAs) are noncoding RNAs of approximately 22 nucleotides that regulate gene expression through post-transcriptional silencing of target genes. miRNAs act either by binding partially complementary transcripts and preventing translation of the target mRNA, or by binding perfectly complementary transcripts, resulting in cleavage of the target mRNA.62 Since they regulate expression of 30–60% of human genes,63 it is not surprising that miRNAs play important roles in processes like cell proliferation, differentiation and apoptosis, and may also function as oncogenes or tumor suppressor genes.62 Although miRNAs are relatively new contenders, they potentially have wide application as biomarkers for gastric cancer susceptibility, diagnosis, prognosis, and even prediction of response to therapy.
Individuals with a genetic predisposition to gastric cancer may be identified by single nucleotide polymorphisms (SNPs) that occur in miRNA precursors and mRNA target binding sites. Although the data are sometimes contradictory, the most studied miRNA-SNP related to gastric cancer is rs2910164 G/C of the pre-miR-146a gene.64-67 Additional miRNA-SNPs have been reported in relation to gastric cancer, including polymorphisms of the miR-196a-2 and miR-27a genes.68,69
The miRNA pattern observed in gastric cancer tissue differs from that of nonmalignant tissue of the same patient.70 Tumor-associated miRNAs can also be detected in a remarkably stable form in circulating blood or in circulating tumor cells71 and thus have potential use as non-invasive diagnostic markers for gastric cancer. Recent studies have demonstrated that several miRNAs, including miR-370, miR-146a, miR-155, miR-378 and miR-21, are involved in H. pylori infection and gastric cancer,72-76 but the reported miRNA profiles of gastric cancer have not been consistent across different studies, perhaps due to technical inconsistencies62 or because studies have analyzed different subtypes of gastric cancer (intestinal and diffuse-type cancers), which may display different miRNA expression patterns.77
Prognostic applications of miRNAs have also been reported, as levels of miRNAs expressed by gastric tumors have been associated with clinical outcomes. Several studies have correlated miRNA expression with survival time and tumor recurrence.65,78-80 miRNAs have been correlated with disease stage77,81,82 and metastasis to the lymph nodes.65,78,81,83 Levels of miRNAs expressed by gastric tumors may also affect resistance to chemotherapy.67,84,85 A more thorough discussion is available in recent reviews.62,86
DNA methylation
DNA methylation of promoter CpG islands is an epigenetic mechanism of transcriptional regulation that, when gone awry, can lead to inactivation of tumor suppressor genes and activation of oncogenes. Aberrant DNA methylation has been linked to precancerous gastric epithelial lesions and progression of gastric cancer.87 H. pylori infection, or the inflammation associated with it,88 has been implicated in the induction of aberrant DNA methylation at several loci in gastric epithelial cells, including CDH1, LOX, HAND1, THBD, HRASLS, FLNC, ARC, CDKN2A, RUNX3 and TWIST1 genes12,89-92. However, a recent meta-analysis showed that aberrant DNA methylation of more than 100 genes has been associated with gastric cancer, so the situation is complex and more work is needed.87
Changes in DNA methylation patterns occur early93 and can accumulate over the course of gastric cancer progression,91 so they show potential for use in clinical diagnostics of gastric cancer risk and prognosis. Cancer-specific methylated DNA can be found in biological fluids at levels concordant with those in cancerous tissues, suggesting it could be useful for non-invasive diagnostics.91,94 A number of genes have been repeatedly shown to be hypermethylated in gastric cancer subjects,87,95 and hypermethylation of these genes is more common in normal gastric tissue from gastric cancer patients than in non-cancer patients.87,96 DNA methylation has been correlated with survival of gastric cancer patients87,91 and also may serve as a predictor of response to chemotherapy,87,97 though only a few small studies have been performed to date.
Though promising as a future biomarker for gastric cancer, no clinical applications of DNA methylation are currently in use. Several methodological inconsistencies exist across studies, and many results still require independent validation. Perhaps of greater concern is that several genes that are differentially methylated in gastric cancer have also been identified as risk markers of other cancer types, so they are not specific markers of gastric cancer.87 Moreover, DNA methylation is not specific to cancer, as it has been associated with age, gender, smoking, intestinal metaplasia, host genetics, H. pylori, and Epstein Barr virus infection.87
Host genetics
Intestinal-type gastric cancer is considered an inflammation-induced malignancy.98 It is increasingly clear that H. pylori infection most often produces an immunotolerant phenotype, which is not associated with pathology; disease results from an aggressive Th1 and Th17-biased inflammatory response.99,100 Still unanswered, however, is the question of why most individuals develop a tolerant phenotype with no disease, while others progress to peptic ulcer or gastric cancer. While environmental and bacterial factors are important, including the gut microbiota, development of disease is no doubt influenced also by host genetics. The nature and magnitude of the chronic inflammatory response elicited by H. pylori may vary depending on the genetic background of the host.101 In general, host genetic polymorphisms that may alter susceptibility to gastric cancer are related either to recognition and signaling receptors of H. pylori or to modulation of the host pro-inflammatory response.
Polymorphisms in innate immune response genes involved in recognition and signaling of H. pylori infection may play a key role in determining the magnitude and course of the host response.102 TLR-4 is a pattern recognition receptor for LPS and ultimately signals NF-κB. A number of polymorphisms in the TLR-4 gene have been identified that lead to an amplified immune response and development of gastric cancer.98,102 For example, the TLR4 D299G mutation leads to an exaggerated immune response to H. pylori, resulting in an 8-fold increased risk for pre-malignant change and a 2-fold increased risk for malignancy.103 TLR4 T399I has also been associated with increased risk for intestinal-type gastric cancer.104 The risk for gastric mucosal atrophy is also increased substantially by the G796A mutation in NOD1, a cytosolic pattern recognition receptor that responds to peptidoglycan delivered by the H. pylori cag PAI.105
Polymorphisms in cytokine genes that drive the inflammatory response to H. pylori infection may also influence susceptibility to gastric cancer. The pro-inflammatory cytokine IL-1β has been recognized as an important candidate in the development of gastric cancer due to its roles in amplification of the host inflammatory response to H. pylori infection and strong suppression of gastric acid.106,107 A recent meta-analysis of polymorphisms in the IL-1 gene cluster in susceptibility to gastric cancer showed that the IL-1β -511 T allele and the IL-1 RN*2 VNTR are significantly associated with increased risk, especially of the intestinal-type gastric cancer, in Caucasian but not Asian or Hispanic populations.108 TNF-α is another pro-inflammatory cytokine with an effect on gastric acid suppression that has been implicated in gastric cancer susceptibility. The TNF-α-308AA genotype was associated with a 2-fold increase in risk of gastric cancer,107 which has been confirmed in a recent meta-analysis.109 Genotypes TNF-β-252G/G and HSP70–1C/G were also significantly associated with gastric cancer in a Latin American population, probably because of their association with an intense and sustained inflammatory response.110 IL-8 is a potent pro-inflammatory cytokine involved in recruitment of neutrophils and macrophages. The IL-8–251 AA genotype has been associated with increased IL-8 expression in H. pylori infected individuals,111 but there have been conflicting reports regarding its contribution to gastric cancer risk. Recently, a meta-analysis has supported the association of the IL-8–251 AA genotype with gastric cancer risk in Asians, especially intestinal-type gastric cancer.111 IL-10 downregulates the inflammatory response by inhibition of pro-inflammatory cytokines. Three polymorphisms in this gene have been connected with altered risk of gastric cancer development. Meta-analyses have revealed that the IL-10–592 AA112 and IL-10–819 TT113 genotypes are associated with reduced risk of gastric cancer in Asian populations, whereas the IL-10–1082 GG-plus-GA genotypes are associated with increased risk (especially intestinal-type gastric cancer) in Asian populations.114
Though many genetic polymorphisms have been linked to altered gastric cancer susceptibility, no genetic tests exist in clinical practice. Many of the markers are very common and lack adequate specificity for a gastric cancer screening test.98 In addition, several of the potential markers are controversial, with many of the associations specific to ethnicity.115 A thorough review and selection of the stronger candidates is needed to design validation studies in communities with different genetic backgrounds.
Glycomics
Protein glycosylation is a co- or post-translational modification in which oligosaccharide structures are enzymatically added to protein. Protein glycoslylation is sensitive to disruption, and altered glycosylation patterns are associated with various states of health and disease, including cancer.116-119 The majority of plasma proteins are glycosylated, and several studies have analyzed glycoproteins or enzymatically released glycans in plasma or serum of gastric cancer patients and controls.120,121 Although the study of protein glycosylation is a newly emerging field for biomarker discovery, it has potential application for diagnosis and prognosis of gastric cancer. For example, preliminary studies suggest that non-sulfated chondroitin/dermatan sulfate concentration is increased in gastric biopsies from cancer patients compared with normal controls.122
One target of biomarker research is the glycosylation pattern of the most abundant glycoprotein in serum, immunoglobulin G (IgG). The N-glycans on the IgG Fc region have an important effect on the structure of the IgG molecule and its binding properties to the Fcγ receptor.123 Several studies have identified increased levels of agalactosylated IgG oligosaccharides in serum from gastric cancer patients compared with controls, and the levels of these agalactosylated glycans increase with tumor progression.124,125 Interestingly, higher levels of agalactosylated IgG oligosaccharides, which increase with tumor progression, have also been reported for patients with cancer of the prostate126 and ovary.127
Proteomics
Many platforms have been applied to analyze proteins in various clinical specimens for gastric cancer biomarker discovery. Strategies include the proteomic analysis of blood, gastric fluid, tissues, and cell lines to identify aberrantly expressed or modified proteins. Though blood is a relatively non-invasive specimen to obtain, biomarker discovery from serum is challenging because the majority of the serum proteome is comprised of a few highly abundant proteins, so a large dynamic range is required to detect the low abundance proteins.128 Potential biomarkers identified through proteomic analysis of blood include complement component C9129 and apolipoproteins C-I and C-III.130 Levels of soluble E-cadherin, hepatocyte growth factor, tissue inhibitor of metalloproteinase-1 (TIMP-1) and IL-2R have also been found to have prognostic significance in gastric cancer patients.131-134 Though more invasive to obtain, gastric fluid may contain higher concentrations of tumor-secreted proteins.128 For example, α-1-antitrypsin, S100A9 and gastric intrinsic factor have been proposed as biomarkers in gastric fluid.135 Tissue-based proteomic studies have identified numerous candidate proteins, but have faced issues with little overlap and even conflicting data across studies.128 A comprehensive list of proteomic technologies and candidate proteins for gastric cancer biomarkers has been recently summarized.136 The list is steadily increasing, although to date few have been validated in large patient cohorts.137 Currently, no protein biomarkers have sufficiently high sensitivity or specificity for clinical detection of gastric cancer. However, since gastric cancers can have diverse etiologies, it is unlikely that aberrant expression of a single protein will be observed in every patient. Rather, a combination of proteins in a panel may improve overall sensitivity and specificity that individual markers lack.137
Conclusions
H. pylori-associated gastric cancer is a major worldwide healthcare burden. Although the incidence is declining in developed countries, over the coming decades the incidence of gastric cancer in developing countries will actually increase, largely because of aging of the population.138 Thus, it is in developing countries that early detection is most needed. Since resources are limited, biomarker tests must be non-invasive, simple, and cheap, which makes the task of biomarker discovery and development even more difficult. To be most efficient and economical, biomarkers will also have to be utilized in the right context. For example, it will be important to validate SNPs or other markers in different ethnic groups, and to use markers of unregulated inflammatory response, such as altered miRNA, DNA methylation, or altered glycomics and proteomics, only in older adults (probably > 40 y) where precancerous lesions are more likely. Gastric cancer is a multifactorial disease, and a proper combination of biomarkers, together with age, gender, family history, and perhaps even blood group,139 may improve their utility to identify patients at risk. Finally, since the neoplastic response to H. pylori infection is delayed in germ free mice,140 other members of the gastric microbial community might also be informative. Early detection with a combination of biomarkers, together with more intensive screening of high-risk individuals, offers the most realistic hope to bend the gastric cancer curve.
Acknowledgments
Work in the laboratory of J.V.S. on biomarkers of H. pylori-associated gastric cancer is supported by a Public Health Service Award from the NIH (CA R01136647).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/25720
References
- 1.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 2.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2005;54:209–41. doi: 10.1016/j.critrevonc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohta H, Noguchi Y, Takagi K, Nishi M, Kajitani T, Kato Y. Early gastric carcinoma with special reference to macroscopic classification. Cancer. 1987;60:1099–106. doi: 10.1002/1097-0142(19870901)60:5<1099::AID-CNCR2820600530>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, et al. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24:249–56. doi: 10.1007/s10552-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 8.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Carneiro F. Hereditary gastric cancer. Pathologe. 2012;33(Suppl 2):231–4. doi: 10.1007/s00292-012-1677-6. [DOI] [PubMed] [Google Scholar]
- 10.Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42:261–84. doi: 10.1016/j.gtc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Chan AO, Huang C, Hui WM, Cho CH, Yuen MF, Lam SK, et al. Stability of E-cadherin methylation status in gastric mucosa associated with histology changes. Aliment Pharmacol Ther. 2006;24:831–6. doi: 10.1111/j.1365-2036.2006.03032.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan AO, Lam SK, Wong BC, Wong WM, Yuen MF, Yeung YH, et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003;52:502–6. doi: 10.1136/gut.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 16.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 17.Areia M, Carvalho R, Cadime AT, Rocha Gonçalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013 doi: 10.1111/hel.12050. In press. [DOI] [PubMed] [Google Scholar]
- 18.Yeh JM, Hur C, Kuntz KM, Ezzati M, Goldie SJ. Cost-effectiveness of treatment and endoscopic surveillance of precancerous lesions to prevent gastric cancer. Cancer. 2010;116:2941–53. doi: 10.1002/cncr.25030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh JM, Kuntz KM, Ezzati M, Goldie SJ. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer. 2009;124:157–66. doi: 10.1002/ijc.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan DR, Torres J, Sexton R, Herrero R, Salazar-Martínez E, Greenberg ER, et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA. 2013;309:578–86. doi: 10.1001/jama.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamandis EP. The failure of protein cancer biomarkers to reach the clinic: why, and what can be done to address the problem? BMC Med. 2012;10:87. doi: 10.1186/1741-7015-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlou MP, Diamandis EP, Blasutig IM. The long journey of cancer biomarkers from the bench to the clinic. Clin Chem. 2013;59:147–57. doi: 10.1373/clinchem.2012.184614. [DOI] [PubMed] [Google Scholar]
- 23.Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–8. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM., Jr. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–36. doi: 10.1038/labinvest.3700719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. China Gastric Cancer Study Group Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 26.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 27.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 28.Katz MH. Multivariable analysis: a primer for readers of medical research. Ann Intern Med. 2003;138:644–50. doi: 10.7326/0003-4819-138-8-200304150-00012. [DOI] [PubMed] [Google Scholar]
- 29.Ransohoff DF. Rules of evidence for cancer molecular-marker discovery and validation. Nat Rev Cancer. 2004;4:309–14. doi: 10.1038/nrc1322. [DOI] [PubMed] [Google Scholar]
- 30.Ransohoff DF. Bias as a threat to the validity of cancer molecular-marker research. Nat Rev Cancer. 2005;5:142–9. doi: 10.1038/nrc1550. [DOI] [PubMed] [Google Scholar]
- 31.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agréus L, Kuipers EJ, Kupcinskas L, Malfertheiner P, Di Mario F, Leja M, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol. 2012;47:136–47. doi: 10.3109/00365521.2011.645501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–40. [PubMed] [Google Scholar]
- 34.Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–43. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 35.Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173–7. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 36.Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307–19. doi: 10.5009/gnl.2010.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2–10. doi: 10.1080/00365520600863720. [DOI] [PubMed] [Google Scholar]
- 38.Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, Talley NJ, et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43:1448–55. doi: 10.1080/00365520802273025. [DOI] [PubMed] [Google Scholar]
- 39.Väänänen H, Vauhkonen M, Helske T, Kääriäinen I, Rasmussen M, Tunturi-Hihnala H, et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003;15:885–91. doi: 10.1097/00042737-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Ley C, Mohar A, Guarner J, Herrera-Goepfert R, Figueroa LS, Halperin D, et al. Screening markers for chronic atrophic gastritis in Chiapas, Mexico. Cancer Epidemiol Biomarkers Prev. 2001;10:107–12. [PubMed] [Google Scholar]
- 41.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–53. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 42.Miki K, Fujishiro M, Kodashima S, Yahagi N. Long-term results of gastric cancer screening using the serum pepsinogen test method among an asymptomatic middle-aged Japanese population. Dig Endosc. 2009;21:78–81. doi: 10.1111/j.1443-1661.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- 43.Varis K, Sipponen P, Laxén F, Samloff IM, Huttunen JK, Taylor PR, et al. Helsinki Gastritis Study Group Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Scand J Gastroenterol. 2000;35:950–6. doi: 10.1080/003655200750023011. [DOI] [PubMed] [Google Scholar]
- 44.Rehfeld JF, Bardram L, Hilsted L, Poitras P, Goetze JP. Pitfalls in diagnostic gastrin measurements. Clin Chem. 2012;58:831–6. doi: 10.1373/clinchem.2011.179929. [DOI] [PubMed] [Google Scholar]
- 45.Cao Q, Ran ZH, Xiao SD. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis. 2007;8:15–22. doi: 10.1111/j.1443-9573.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 46.Shiotani A, Iishi H, Uedo N, Kumamoto M, Nakae Y, Ishiguro S, et al. Histologic and serum risk markers for noncardia early gastric cancer. Int J Cancer. 2005;115:463–9. doi: 10.1002/ijc.20852. [DOI] [PubMed] [Google Scholar]
- 47.Inoue K, Fujisawa T, Haruma K. Assessment of degree of health of the stomach by concomitant measurement of serum pepsinogen and serum Helicobacter pylori antibodies. Int J Biol Markers. 2010;25:207–12. [PubMed] [Google Scholar]
- 48.Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–8. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Xue L, Xing L, Wang J, Cui J, Mi J, et al. Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer. 2012;130:1614–9. doi: 10.1002/ijc.26172. [DOI] [PubMed] [Google Scholar]
- 50.Camorlinga-Ponce M, Flores-Luna L, Lazcano-Ponce E, Herrero R, Bernal-Sahagún F, Abdo-Francis JM, et al. Age and severity of mucosal lesions influence the performance of serologic markers in Helicobacter pylori-associated gastroduodenal pathologies. Cancer Epidemiol Biomarkers Prev. 2008;17:2498–504. doi: 10.1158/1055-9965.EPI-08-0289. [DOI] [PubMed] [Google Scholar]
- 51.Janulaityte-Günther D, Kupcinskas L, Pavilonis A, Valuckas K, Wadström T, Andersen LP. Combined serum IgG response to Helicobacter pylori VacA and CagA predicts gastric cancer. FEMS Immunol Med Microbiol. 2007;50:220–5. doi: 10.1111/j.1574-695X.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- 52.Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010;5:1885–93. doi: 10.2217/fmb.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki G, Cullings H, Fujiwara S, Hattori N, Matsuura S, Hakoda M, et al. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol Biomarkers Prev. 2007;16:1224–8. doi: 10.1158/1055-9965.EPI-06-1048. [DOI] [PubMed] [Google Scholar]
- 54.Yamaoka Y, Kodama T, Kashima K, Graham DY. Antibody against Helicobacter pylori CagA and VacA and the risk for gastric cancer. J Clin Pathol. 1999;52:215–8. doi: 10.1136/jcp.52.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flores-Luna L, Camorlinga-Ponce M, Hernandez-Suarez G, Kasamatsu E, Martínez ME, Murillo R, et al. The utility of serologic tests as biomarkers for Helicobacter pylori-associated precancerous lesions and gastric cancer varies between Latin American countries. Cancer Causes Control. 2013;24:241–8. doi: 10.1007/s10552-012-0106-8. [DOI] [PubMed] [Google Scholar]
- 56.Epplein M, Zheng W, Xiang YB, Peek RM, Jr., Li H, Correa P, et al. Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men. Cancer Epidemiol Biomarkers Prev. 2012;21:2185–92. doi: 10.1158/1055-9965.EPI-12-0792-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao L, Michel A, Weck MN, Arndt V, Pawlita M, Brenner H. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H. pylori proteins determined by novel multiplex serology. Cancer Res. 2009;69:6164–70. doi: 10.1158/0008-5472.CAN-09-0596. [DOI] [PubMed] [Google Scholar]
- 58.Gao L, Weck MN, Michel A, Pawlita M, Brenner H. Association between chronic atrophic gastritis and serum antibodies to 15 Helicobacter pylori proteins measured by multiplex serology. Cancer Res. 2009;69:2973–80. doi: 10.1158/0008-5472.CAN-08-3477. [DOI] [PubMed] [Google Scholar]
- 59.Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, et al. European Society of Gastrointestinal Endoscopy. European Helicobacter Study Group. European Society of Pathology. Sociedade Portuguesa de Endoscopia Digestiva Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia-Pacific Gastric Cancer Consensus Conference Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–65. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 61.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, et al. Asia Pacific Working Group on Gastric Cancer Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–87. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 62.Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143:35–47, e2. doi: 10.1053/j.gastro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hishida A, Matsuo K, Goto Y, Naito M, Wakai K, Tajima K, et al. Combined effect of miR-146a rs2910164 G/C polymorphism and Toll-like receptor 4 +3725 G/C polymorphism on the risk of severe gastric atrophy in Japanese. Dig Dis Sci. 2011;56:1131–7. doi: 10.1007/s10620-010-1376-1. [DOI] [PubMed] [Google Scholar]
- 65.Kogo R, Mimori K, Tanaka F, Komune S, Mori M. Clinical significance of miR-146a in gastric cancer cases. Clin Cancer Res. 2011;17:4277–84. doi: 10.1158/1078-0432.CCR-10-2866. [DOI] [PubMed] [Google Scholar]
- 66.Okubo M, Tahara T, Shibata T, Yamashita H, Nakamura M, Yoshioka D, et al. Association between common genetic variants in pre-microRNAs and gastric cancer risk in Japanese population. Helicobacter. 2010;15:524–31. doi: 10.1111/j.1523-5378.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhou F, Zhu H, Luo D, Wang M, Dong X, Hong Y, et al. A functional polymorphism in Pre-miR-146a is associated with susceptibility to gastric cancer in a Chinese population. DNA Cell Biol. 2012;31:1290–5. doi: 10.1089/dna.2011.1596. [DOI] [PubMed] [Google Scholar]
- 68.Peng S, Kuang Z, Sheng C, Zhang Y, Xu H, Cheng Q. Association of microRNA-196a-2 gene polymorphism with gastric cancer risk in a Chinese population. Dig Dis Sci. 2010;55:2288–93. doi: 10.1007/s10620-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 69.Sun Q, Gu H, Zeng Y, Xia Y, Wang Y, Jing Y, et al. Hsa-mir-27a genetic variant contributes to gastric cancer susceptibility through affecting miR-27a and target gene expression. Cancer Sci. 2010;101:2241–7. doi: 10.1111/j.1349-7006.2010.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, et al. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652–7. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 71.Mostert B, Sieuwerts AM, Martens JW, Sleijfer S. Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn. 2011;11:259–75. doi: 10.1586/erm.11.11. [DOI] [PubMed] [Google Scholar]
- 72.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, et al. Fork head box M1 is overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by hsa-miR-370. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 73.Liu H, Zhu L, Liu B, Yang L, Meng X, Zhang W, et al. Genome-wide microRNA profiles identify miR-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196–203. doi: 10.1016/j.canlet.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 74.Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Müller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J Immunol. 2011;187:3578–86. doi: 10.4049/jimmunol.1101772. [DOI] [PubMed] [Google Scholar]
- 75.Paranjape T, Slack FJ, Weidhaas JB. MicroRNAs: tools for cancer diagnostics. Gut. 2009;58:1546–54. doi: 10.1136/gut.2009.179531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao B, Zhu ED, Li N, Lu DS, Li W, Li BS, et al. Increased miR-146a in gastric cancer directly targets SMAD4 and is involved in modulating cell proliferation and apoptosis. Oncol Rep. 2012;27:559–66. doi: 10.3892/or.2011.1514. [DOI] [PubMed] [Google Scholar]
- 77.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katada T, Ishiguro H, Kuwabara Y, Kimura M, Mitui A, Mori Y, et al. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–42. [PubMed] [Google Scholar]
- 79.Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui J, et al. Combination of hsa-miR-375 and hsa-miR-142-5p as a predictor for recurrence risk in gastric cancer patients following surgical resection. Ann Oncol. 2011;22:2257–66. doi: 10.1093/annonc/mdq758. [DOI] [PubMed] [Google Scholar]
- 80.Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F, et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–33. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 81.Feng R, Chen X, Yu Y, Su L, Yu B, Li J, et al. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. doi: 10.1016/j.canlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6:e1000879. doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Zhu W, Zhang J, Huo S, Zhou L, Gu Z, et al. MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem Biophys Res Commun. 2010;395:275–80. doi: 10.1016/j.bbrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 85.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, et al. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–31. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 86.Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ. MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark. 2012;11:259–67. doi: 10.3233/CBM-2012-00284. [DOI] [PubMed] [Google Scholar]
- 87.Sapari NS, Loh M, Vaithilingam A, Soong R. Clinical potential of DNA methylation in gastric cancer: a meta-analysis. PLoS One. 2012;7:e36275. doi: 10.1371/journal.pone.0036275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, et al. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–40. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 89.Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–95. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 90.Tahara T, Arisawa T, Shibata T, Wang FY, Nakamura M, Sakata M, et al. Risk prediction of gastric cancer by analysis of aberrant DNA methylation in non-neoplastic gastric epithelium. Digestion. 2007;75:54–61. doi: 10.1159/000101775. [DOI] [PubMed] [Google Scholar]
- 91.Lu XX, Yu JL, Ying LS, Han J, Wang S, Yu QM, et al. Stepwise cumulation of RUNX3 methylation mediated by Helicobacter pylori infection contributes to gastric carcinoma progression. Cancer. 2012;118:5507–17. doi: 10.1002/cncr.27604. [DOI] [PubMed] [Google Scholar]
- 92.Shin CM, Kim N, Jung Y, Park JH, Kang GH, Kim JS, et al. Role of Helicobacter pylori infection in aberrant DNA methylation along multistep gastric carcinogenesis. Cancer Sci. 2010;101:1337–46. doi: 10.1111/j.1349-7006.2010.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paluszczak J, Baer-Dubowska W. Epigenetic diagnostics of cancer--the application of DNA methylation markers. J Appl Genet. 2006;47:365–75. doi: 10.1007/BF03194647. [DOI] [PubMed] [Google Scholar]
- 94.Shivapurkar N, Gazdar AF. DNA methylation based biomarkers in non-invasive cancer screening. Curr Mol Med. 2010;10:123–32. doi: 10.2174/156652410790963303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamashita K, Sakuramoto S, Watanabe M. Genomic and epigenetic profiles of gastric cancer: potential diagnostic and therapeutic applications. Surg Today. 2011;41:24–38. doi: 10.1007/s00595-010-4370-5. [DOI] [PubMed] [Google Scholar]
- 96.Kaise M, Miwa J, Fujimoto A, Tashiro J, Tagami D, Sano H, et al. Influence of Helicobacter pylori status and eradication on the serum levels of trefoil factors and pepsinogen test: serum trefoil factor 3 is a stable biomarker. Gastric Cancer. 2012 doi: 10.1007/s10120-012-0185-y. In press. [DOI] [PubMed] [Google Scholar]
- 97.Kato K, Iida S, Uetake H, Takagi Y, Yamashita T, Inokuchi M, et al. Methylated TMS1 and DAPK genes predict prognosis and response to chemotherapy in gastric cancer. Int J Cancer. 2008;122:603–8. doi: 10.1002/ijc.23143. [DOI] [PubMed] [Google Scholar]
- 98.McLean MH, El-Omar EM. Genetics of inflammation in the gastrointestinal tract and how it can cause cancer. Recent Results Cancer Res. 2011;185:173–83. doi: 10.1007/978-3-642-03503-6_11. [DOI] [PubMed] [Google Scholar]
- 99.Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Müller A, Solnick JV. Inflammation, immunity, and vaccine development for Helicobacter pylori. Helicobacter. 2011;16(Suppl 1):26–32. doi: 10.1111/j.1523-5378.2011.00877.x. [DOI] [PubMed] [Google Scholar]
- 101.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–70. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Castaño-Rodríguez N, Kaakoush NO, Goh KL, Fock KM, Mitchell HM. The role of TLR2, TLR4 and CD14 genetic polymorphisms in gastric carcinogenesis: a case-control study and meta-analysis. PLoS One. 2013;8:e60327. doi: 10.1371/journal.pone.0060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–12. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 104.Santini D, Angeletti S, Ruzzo A, Dicuonzo G, Galluzzo S, Vincenzi B, et al. Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypes. Clin Exp Immunol. 2008;154:360–4. doi: 10.1111/j.1365-2249.2008.03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kara B, Akkiz H, Doran F, Bayram S, Erken E, Gumurdullu Y, et al. The significance of E266K polymorphism in the NOD1 gene on Helicobacter pylori infection: an effective force on pathogenesis? Clin Exp Med. 2010;10:107–12. doi: 10.1007/s10238-009-0077-6. [DOI] [PubMed] [Google Scholar]
- 106.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 107.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. doi: 10.1016/S0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 108.Xue H, Lin B, Ni P, Xu H, Huang G. Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1604–17. doi: 10.1111/j.1440-1746.2010.06428.x. [DOI] [PubMed] [Google Scholar]
- 109.Gorouhi F, Islami F, Bahrami H, Kamangar F. Tumour-necrosis factor-A polymorphisms and gastric cancer risk: a meta-analysis. Br J Cancer. 2008;98:1443–51. doi: 10.1038/sj.bjc.6604277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Partida-Rodríguez O, Torres J, Flores-Luna L, Camorlinga M, Nieves-Ramírez M, Lazcano E, et al. Polymorphisms in TNF and HSP-70 show a significant association with gastric cancer and duodenal ulcer. Int J Cancer. 2010;126:1861–8. doi: 10.1002/ijc.24773. [DOI] [PubMed] [Google Scholar]
- 111.Xue H, Liu J, Lin B, Wang Z, Sun J, Huang G. A meta-analysis of interleukin-8 -251 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7:e28083. doi: 10.1371/journal.pone.0028083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xue H, Wang YC, Lin B, An J, Chen L, Chen J, et al. A meta-analysis of interleukin-10 -592 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7:e39868. doi: 10.1371/journal.pone.0039868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xue H, Lin B, An J, Zhu Y, Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. doi: 10.1186/1471-2407-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ni P, Xu H, Xue H, Lin B, Lu Y. A meta-analysis of interleukin-10-1082 promoter polymorphism associated with gastric cancer risk. DNA Cell Biol. 2012;31:582–91. doi: 10.1089/dna.2011.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leja M, Wex T, Malfertheiner P. Markers for gastric cancer premalignant lesions: where do we go? Dig Dis. 2012;30:268–76. doi: 10.1159/000336990. [DOI] [PubMed] [Google Scholar]
- 116.Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE, et al. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–64. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 117.Kirmiz C, Li BS, An HJ, Clowers BH, Chew HK, Lam KS, et al. A serum glycomics approach to breast cancer biomarkers. Mol Cell Proteomics. 2007;6:43–55. doi: 10.1074/mcp.M600171-MCP200. [DOI] [PubMed] [Google Scholar]
- 118.Ruhaak LR, Miyamoto S, Lebrilla CB. Developments in the identification of glycan biomarkers for the detection of cancer. Mol Cell Proteomics. 2013;12:846–55. doi: 10.1074/mcp.R112.026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saldova R, Fan Y, Fitzpatrick JM, Watson RW, Rudd PM. Core fucosylation and alpha2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 2011;21:195–205. doi: 10.1093/glycob/cwq147. [DOI] [PubMed] [Google Scholar]
- 120.Uen YH, Lin KY, Sun DP, Liao CC, Hsieh MS, Huang YK, et al. Comparative proteomics, network analysis and post-translational modification identification reveal differential profiles of plasma Con A-bound glycoprotein biomarkers in gastric cancer. J Proteomics. 2013;83:197–213. doi: 10.1016/j.jprot.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Gomes C, Almeida A, Ferreira JA, Silva L, Santos-Sousa H, Pinto-de-Sousa J, et al. Glycoproteomic analysis of serum from patients with gastric precancerous lesions. J Proteome Res. 2013;12:1454–66. doi: 10.1021/pr301112x. [DOI] [PubMed] [Google Scholar]
- 122.Weyers A, Yang B, Park JH, Kim YS, Kim SM, Lee SE, et al. Microanalysis of stomach cancer glycosaminoglycans. Glycoconj J. 2013 doi: 10.1007/s10719-013-9476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walker MR, Lund J, Thompson KM, Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989;259:347–53. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bones J, Byrne JC, O’Donoghue N, McManus C, Scaife C, Boissin H, et al. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res. 2011;10:1246–65. doi: 10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- 125.Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29:57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 126.Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Egawa S, et al. Changes in serum IgG oligosaccharide chains with prostate cancer progression. Anticancer Res. 2004;24(5B):3135–9. [PubMed] [Google Scholar]
- 127.Gerçel-Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81:71–6. doi: 10.1006/gyno.2000.6102. [DOI] [PubMed] [Google Scholar]
- 128.Wu W, Chung MC. The gastric fluid proteome as a potential source of gastric cancer biomarkers. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.04.035. In press. [DOI] [PubMed] [Google Scholar]
- 129.Chong PK, Lee H, Loh MC, Choong LY, Lin Q, So JB, et al. Upregulation of plasma C9 protein in gastric cancer patients. Proteomics. 2010;10:3210–21. doi: 10.1002/pmic.201000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cohen M, Yossef R, Erez T, Kugel A, Welt M, Karpasas MM, et al. Serum apolipoproteins C-I and C-III are reduced in stomach cancer patients: results from MALDI-based peptidome and immuno-based clinical assays. PLoS One. 2011;6:e14540. doi: 10.1371/journal.pone.0014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chan AO, Lam SK, Chu KM, Lam CM, Kwok E, Leung SY, et al. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48:808–11. doi: 10.1136/gut.48.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saito H, Tsujitani S, Ikeguchi M, Maeta M, Kaibara N. Serum level of a soluble receptor for interleukin-2 as a prognostic factor in patients with gastric cancer. Oncology. 1999;56:253–8. doi: 10.1159/000011973. [DOI] [PubMed] [Google Scholar]
- 133.Tanaka K, Miki C, Wakuda R, Kobayashi M, Tonouchi H, Kusunoki M. Circulating level of hepatocyte growth factor as a useful tumor marker in patients with early-stage gastric carcinoma. Scand J Gastroenterol. 2004;39:754–60. doi: 10.1080/00365520410005973. [DOI] [PubMed] [Google Scholar]
- 134.Yoshikawa T, Cho H, Tsuburaya A, Kobayashi O. Impact of plasma tissue inhibitor of metalloproteinase-1 on long-term survival in patients with gastric cancer. Gastric Cancer. 2009;12:31–6. doi: 10.1007/s10120-008-0494-3. [DOI] [PubMed] [Google Scholar]
- 135.Wu W, Juan WC, Liang CR, Yeoh KG, So J, Chung MC. S100A9, GIF and AAT as potential combinatorial biomarkers in gastric cancer diagnosis and prognosis. Proteomics Clin Appl. 2012;6:152–62. doi: 10.1002/prca.201100050. [DOI] [PubMed] [Google Scholar]
- 136.Lin LL, Huang HC, Juan HF. Discovery of biomarkers for gastric cancer: a proteomics approach. J Proteomics. 2012;75:3081–97. doi: 10.1016/j.jprot.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 137.Polanski M, Anderson NL. A list of candidate cancer biomarkers for targeted proteomics. Biomark Insights. 2007;1:1–48. [PMC free article] [PubMed] [Google Scholar]
- 138.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rizzato C, Kato I, Plummer M, Muñoz N, Stein A, Jan van Doorn L, et al. Risk of advanced gastric precancerous lesions in Helicobacter pylori infected subjects is influenced by ABO blood group and cagA status. Int J Cancer. 2013;133:315–22. doi: 10.1002/ijc.28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–20. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]