Abstract
Helicobacter pylori (H. pylori) and hepatitis C virus (HCV) infect millions of people and can induce cancer. We investigated if H. pylori infection promoted HCV-associated liver cancer. Helicobacter-free C3B6F1 wild-type (WT) and C3B6F1-Tg(Alb1-HCVN)35Sml (HT) male and female mice were orally inoculated with H. pylori SS1 or sterile media. Mice were euthanized at ~12 mo postinoculation and samples were collected for analyses. There were no significant differences in hepatocellular tumor promotion between WT and HT mice; however, HT female mice developed significantly larger livers with more hepatic steatosis than WT female mice. H. pylori did not colonize the liver nor promote hepatocellular tumors in WT or HT mice. In the stomach, H. pylori induced more corpus lesions in WT and HT female mice than in WT and HT male mice, respectively. The increased corpus pathology in WT and HT female mice was associated with decreased gastric H. pylori colonization, increased gastric and hepatic interferon gamma expression, and increased serum Th1 immune responses against H. pylori. HT male mice appeared to be protected from H. pylori-induced corpus lesions. Furthermore, during gastric H. pylori infection, HT male mice were protected from gastric antral lesions and hepatic steatosis relative to WT male mice and these effects were associated with increased serum TNF-α. Our findings indicate that H. pylori is a gastric pathogen that does not promote hepatocellular cancer and suggest that the HCV transgene is associated with amelioration of specific liver and gastric lesions observed during concurrent H. pylori infection in mice.
Keywords: Hepatitis, helicobacter, cancer, infection, virus, bacteria, mice, transgenic
Introduction
Helicobacter pylori (H. pylori) and hepatitis C virus (HCV) infect approximately 50% and 2% of the world’s population, respectively.1,2 The World Health Organization’s International Agency for Research on Cancer considers H. pylori and HCV carcinogens.3H. pylori infection may induce gastritis leading to gastric adenocarcinoma whereas HCV can induce hepatitis and cirrhosis leading to hepatocellular carcinoma (HCC).2,4 Gastric and liver cancers are ranked in the top ten most common cancers in both men and women worldwide and are two of the most common causes of cancer death.5
During HCV infection, environmental and/or host-associated factors are important and influence the progression to cirrhosis.6 Some of these factors are age, gender, alcohol ingestion, obesity, diabetes, and co-infection with hepatitis B virus or human immunodeficiency virus.6 Hepatic steatosis is also considered a risk factor for HCC in humans with chronic HCV infection and may affect a patient’s response to treatment.7,8
Studies suggest that H. pylori may impact HCV disease based on detection of H. pylori or “H. pylori-like” DNA in liver tissues from HCV patients.9-14 Helicobacter spp. have been detected in bile and gallbladder tissues of chronic cholecystitis patients and the need for further research investigating a potential causal role of helicobacters in human liver disease has been proposed.15,16 H. pylori was isolated from the liver of a human with Wilson’s disease-associated liver cirrhosis.17 In vitro studies using a human hepatoma cell line (Huh7) have also documented that H. pylori induces cell arrest and apoptosis of infected hepatocytes and also induces hepatocyte malfunction associated with formation of podosomes.18,19 In addition, an enterohepatic helicobacter, H. bilis, can affect the modulation of various proteins including those involved with tumorigenesis in Huh7-derived cells transfected with HCV.20 H. hepaticus, an enterohepatic helicobacter of mice, can induce and promote HCC in A/JCr and HCV transgenic mice, respectively.21,22
The use of FL-N/35 (C57BL/6 background) mice has advanced the study of HCV pathogenesis. These mice are transgenic for FL-N which encodes the complete polyprotein of a genotype 1b strain of HCV. In humans, infection with the HCV genotype 1b strain appears to increase the risk of HCC development.23 FL-N/35 mice usually develop hepatic steatosis and HCC after 10 and 13 mo of age, respectively, and these hepatic lesions appear to be more common in male than female mice.24,25 These studies highlighted the role of structural and nonstructural viral proteins in HCV pathogenesis. Further studies using FL-N/35 mice have documented an attenuation of Fas-mediated apoptosis in transgenic hepatocytes, an increased risk of HCC by iron overload, and development of hepatocellular steatosis with decreased plasma triglycerides.26-28 In the current study, we used a F1 hybrid of transgenic FL-N/35 and C3H/HeNTac mice as a mouse model of HCV to investigate the impact of H. pylori infection on liver cancer. The use of C3B6F1 mice in the current study was based on findings by the National Toxicology Program that B6C3F1 mice were susceptible to H. hepaticus-associated hepatitis and liver cancer.29 Therefore, the F1 model was useful to investigate whether another species of Helicobacter (H. pylori) could influence the pathogenesis of liver cancer in mice.
Results
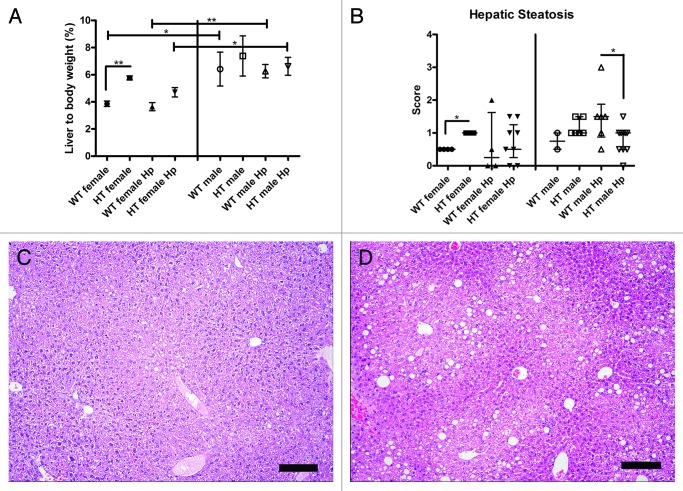
HCV transgene increases liver weight in female mice
The liver to body weight ratio (%) was significantly higher in WT male mice relative to WT female mice (p < 0.05) and in WT male Hp mice relative to WT female Hp mice (p < 0.01). In addition, the liver to body weight ratio (%) was significantly higher in HT female mice relative to WT female mice (p < 0.01) but not in HT male mice relative to WT male mice (Fig. 1A). Similar results were obtained when analyzing the absolute liver weight data. These findings suggest that male mice have a proportionately heavier liver than female mice and that the HCV transgene increases the liver weight in female mice. There were no significant differences in liver to body weight ratio (%) between HT male mice and HT male Hp mice, between HT female mice and HT female Hp mice, between WT male mice and WT male Hp mice, or between WT female mice and WT female Hp mice. Therefore, H. pylori infection did not increase liver weight in HT or WT mice.
Figure 1. (A) Liver to body weight ratio (%) in the different groups of mice. No liver or body weight was available for three mice in different groups including WT female Hp, HT female, and HT female Hp (*, p < 0.05; **, p < 0.01). (B) Hepatic steatosis scores in the different groups of mice (*, p < 0.05). (C) Normal liver in a hepatitis C virus transgenic male mouse infected with H. pylori (HT male Hp). (D) Mild fatty degeneration in the cytoplasm of centrilobular hepatocytes in a wild-type male mouse infected with H. pylori (WT male Hp). (C and D) bar size 100 µm.
HCV transgene increases hepatic steatosis in female mice
The hepatic steatosis score was significantly higher in HT female mice relative to WT female mice (p < 0.05) and in WT male Hp mice relative to HT male Hp mice (p = 0.05) (Fig. 1B, 1C, 1D). Histopathologic features of steatosis comprised hepatocellular cytoplasmic vacuolar change consistent with the formation of fat vacuoles. These findings suggest that the HCV transgene increases hepatic steatosis in HT female mice and decreases hepatic steatosis in HT male Hp mice. There were no significant differences in hepatic steatosis between HT male mice and HT male Hp mice, between HT female mice and HT female Hp mice, or between WT female mice and WT female Hp mice. The difference between WT male mice and WT male Hp mice could not be calculated due to the small sample size in the WT male group. Therefore, H. pylori did not modulate hepatic steatosis in HT mice or WT female mice.
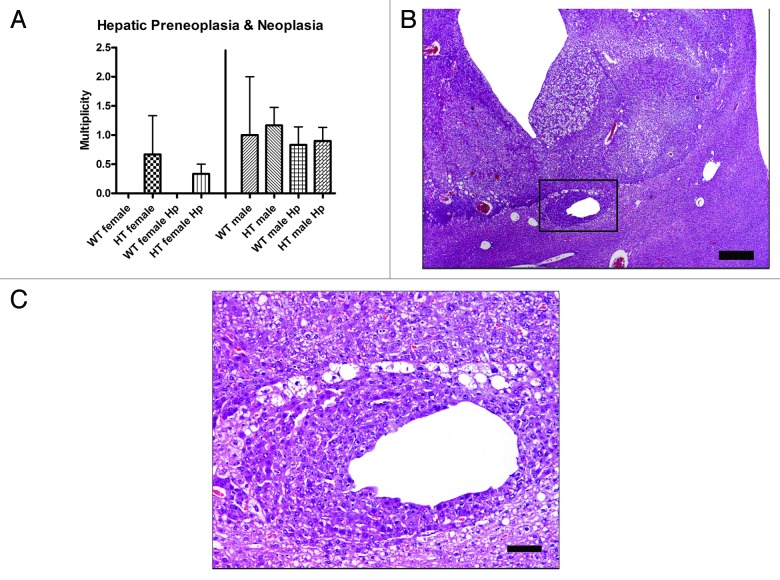
H. pylori infection does not promote hepatocellular tumors in HCV transgenic mice
The percentage of mice in each group with microscopic preneoplastic [altered hepatocellular foci (AHF) and large foci of cellular alteration (LFCA)] and/or neoplastic [hepatocellular adenoma (HCA) and hepatocellular carcinoma (HCC)] liver lesions is detailed in Table 1. Multiplicity values for microscopic preneoplastic and neoplastic liver lesions were recorded in H. pylori-infected and uninfected HT and WT mice; however, there were no significant differences in multiplicity of microscopic preneoplastic and neoplastic liver lesions between HT male mice and HT male Hp mice (p = 0.50) and between HT female mice and HT female Hp mice (p = 0.80) (Fig. 2A). There were no significant differences in the number of liver lobes with dysplasia and neoplasia between HT male mice and HT male Hp mice (p = 0.80) and between HT female mice and HT female Hp mice (p = 0.50) (data not shown). These results indicate that H. pylori infection does not promote liver tumors in C3B6F1-Tg(Alb1-HCVN)35Sml mice. HCCs were detected in livers of H. pylori-infected and uninfected HT and WT mice (Fig. 2B), and were characterized by poorly delineated proliferations of moderately-well differentiated, vacuolated, neoplastic hepatocytes, often organized in a trabecular pattern with minimal intervening stroma (Fig. 2C). The multiplicity of preneoplasia and/or neoplasia and the number of mice with microscopic neoplastic liver lesions did not differ significantly between groups. None of the groups of mice developed a median hepatitis index score ≥ 4 and no significant differences in hepatitis index scores were observed in HT male Hp mice and HT female Hp mice relative to HT male mice and HT female mice, respectively (Supplementary Material).
Table 1. Percentage of mice with microscopic preneoplastic and/or neoplastic liver lesions in each group*.
| Group (number of mice) | Mice with neoplasia | Total mice with hepatic preneoplasia (AHF and LFCA) and neoplasia | ||
|---|---|---|---|---|
| Mice with HCA only | Mice with HCC only | Total mice with HCA and HCC | ||
| WT female (n = 4) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HT female (n = 3) | 1 (33.33%) | 0 (0%) | 1 (33.33%) | 1 (33.33%) |
| WT female Hp (n = 4) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HT female Hp (n = 9) | 0 (0%) | 2 (22.22%) | 2 (22.22%) | 3 (33.33%) |
| WT male (n = 2) | 0 (0%) | 1 (50%) | 1 (50%) | 1 (50%) |
| HT male (n = 6) | 2 (33.33%) | 0 (0%) | 2 (33.33%) | 5 (83.33%) |
| WT male Hp (n = 6) | 2 (33.33%) | 1 (16.66%) | 3 (50%) | 4 (66.66%) |
| HT male Hp (n = 10) | 0 (0%) | 4 (40%) | 4 (40%) | 7 (70%) |
No statistically significant differences were observed between the groups analyzed within each column. WT, wild-type; HT, hepatitis C virus transgenic. Preneoplasia includes altered hepatocellular foci (AHF) and large foci of cellular alteration (LFCA); Neoplasia includes hepatocellular adenoma (HCA) and hepatocellular carcinoma (HCC).
Figure 2. (A) Multiplicity of hepatic preneoplasia and neoplasia in the different groups of mice. (B) Hepatocellular carcinoma in a hepatitis C virus transgenic male mouse infected with H. pylori (HT male Hp). The tumor is poorly demarcated from the normal liver tissue and there is vacuolation of neoplastic cells and dilation of sinusoids. (C) Higher magnification (box in B) view of the junction between the poorly demarcated neoplasm and adjacent normal parenchyma. Neoplastic cells have cytoplasm with a deeper basophilic staining intensity and are often vacuolated. A focus of neoplastic cells also surrounds a distended hepatic sinusoid. (B) bar size 500 µm. (C) bar size 100 µm.
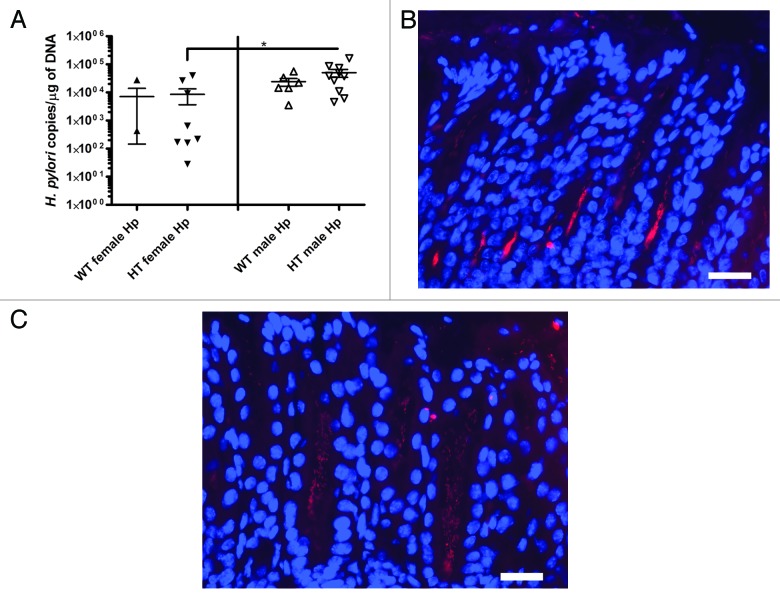
H. pylori colonizes the stomach but not the liver
No H. pylori were detected in livers by quantitative PCR (qPCR). In the stomach, qPCR detected copies of H. pylori in all but three of the H. pylori-inoculated mice analyzed in this study (Table 2). In colonized mice, the values ranged from 28 to 165862 H. pylori copies per µg of mouse DNA. qPCR did not detect H. pylori in gastric tissues from 2 WT female Hp and 1 HT female Hp mice. No significant differences in H. pylori colonization were detected by qPCR between WT female Hp mice and WT male Hp mice; however, significantly fewer H. pylori were detected in the stomachs of HT female Hp mice than in the stomachs of HT male Hp mice (p < 0.05) (Fig. 3A). This finding was consistent with the increased gastric corpus pathology observed in HT female Hp mice relative to HT male Hp mice. There were no significant differences in H. pylori colonization levels between WT female Hp mice and HT female Hp mice and between WT male Hp mice and HT male Hp mice.
Table 2. Mouse strains, groups, and numbers in this study*.
| Sham | H. pylori (Hp)-infected | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| C3B6F1† | 4 (4) | 2 (4) | 4 (5) | 6 (7) |
| C3B6F1-Tg(Alb1-HCVN)35Sml‡ | 3 (4) | 6 (6) | 9 (10) | 10 (12) |
Number in parentheses indicates number of mice at the initiation of the study. One WT male mouse was euthanized at ~6 mo postinoculation and another mouse in this group was diagnosed with osteosarcoma at the end of the study. One WT female Hp mouse was diagnosed with histiocytic sarcoma at the end of the study. One WT male Hp mouse was found dead at ~12 mo postinoculation. Two HT male Hp mice were euthanized at ~3 to 4 mo and ~4 mo postinoculation, respectively. The three mice euthanized before the end of the study did not exhibit preneoplastic and/or neoplastic liver lesions. One HT female and one HT female Hp mouse were diagnosed with lymphoma at the end of the study. The mice with other tumors (osteosarcoma, histiocytic sarcoma, and lymphoma) and the mice that were euthanized or died before the end of the study were not included in the final analyses. † Wild-type (WT); ‡, HCV transgenic (HT).
Figure 3. (A) Colonization of H. pylori in the stomach analyzed with quantitative PCR (*, p < 0.05). Values are expressed as H. pylori copies per µg of mouse DNA. Values for three mice without H. pylori colonization are not shown in the graph. (B) Fluorescent in situ hybridization (FISH) of H. pylori in the gastric antrum of an infected hepatitis C virus transgenic male mouse (HT male Hp). (C) FISH of H. pylori in the gastric antrum of an infected hepatitis C virus transgenic female mouse (HT female Hp). (B and C) bar size ~20 µm.
H. pylori were detected by fluorescent in situ hybridization (FISH) in the gastric corpus and antrum of selected gastric sections from infected mice. In gastric sections with high numbers of H. pylori, the antrum appeared to be colonized at a higher level than the corpus. H. pylori were detected on the apical surface of epithelial cells at the luminal edge and within crypts (Fig. 3B and 3C).
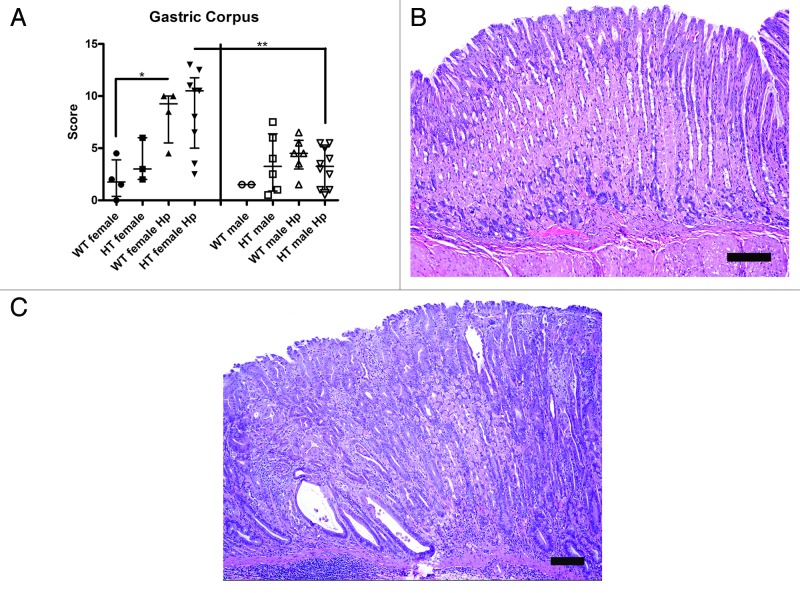
H. pylori infection causes the most severe gastric lesions in the corpus of WT and HT female mice
In the corpus, an increased gastric histologic activity index (GHAI) score was observed in WT female Hp mice relative to WT female (p < 0.05) and WT male Hp mice (p = 0.07) and in HT female Hp mice relative to HT female mice (p = 0.06) and HT male Hp mice (p < 0.01) (Fig. 4A-C). There were no significant differences in GHAI score between WT female Hp mice and HT female Hp mice and between WT male Hp mice and HT male Hp mice. The most common mucosal lesions were comprised of mild foveolar hyperplasia, oxyntic atrophy with mucous metaplasia, pseudopyloric intestinal metaplasia, epithelial dysplasia, and scattered inflammatory cell infiltrates; the latter of which also extended to involve the submucosa. The gastric GHAI scores were comparable between HT male and HT male Hp (p = 0.83). The difference between WT male mice and WT male Hp mice could not be calculated due to the small sample size in the WT male group.
Figure 4. (A) Gastric histologic activity index (GHAI) score of the corpus in the different groups of mice (*, p < 0.05; **, p < 0.01). (B) Mild foveolar hyperplasia in the gastric corpus of a hepatitis C virus transgenic male mouse infected with H. pylori (HT male Hp). (C) Mucosal lesions in the gastric corpus of a hepatitis C virus transgenic female mouse infected with H. pylori (HT female Hp). Lesions consisted of pseudopyloric intestinal metaplasia, epithelial dysplasia, and multifocal inflammation in the mucosa and submucosa. (B and C) bar size 100 µm.
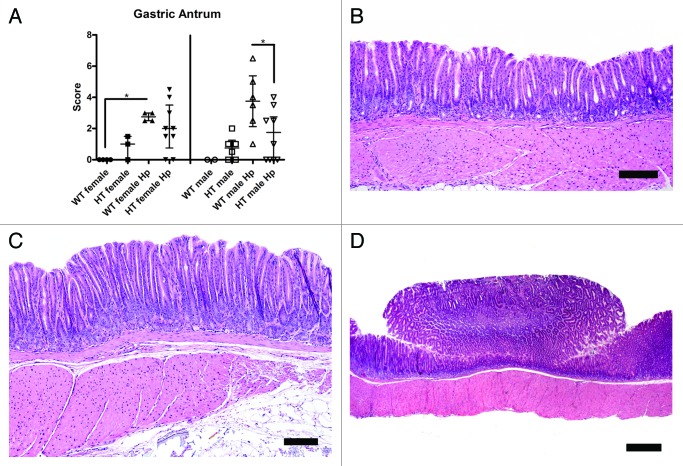
Increased gastric lesions in the antrum, characterized by mucosal hyperplasia, were observed in WT male Hp mice relative to WT male mice (no P value due to small sample size in the WT male group) and in WT female Hp mice relative to WT female mice (p < 0.05). Gastric antral lesions were significantly greater in WT male Hp mice compared with HT male Hp mice (p < 0.05) (Fig. 5A-C). The antral histopathology index scores were comparable between HT male mice and HT male Hp mice (p = 0.40) and between HT female mice and HT female Hp mice (p = 0.19).
Figure 5. (A) Total histopathology index score of the antrum in the different groups of mice (*, p < 0.05). (B) Relatively normal gastric antrum of a hepatitis C virus transgenic male mouse infected with H. pylori (HT male Hp). (C) Mucosal hyperplasia with mild dysplasia in the gastric antrum of a wild-type male mouse infected with H. pylori (WT male Hp). (D) Polypoid lesion in the gastric antrum of a wild-type male mouse infected with H. pylori (WT male Hp). (B and C) bar size 100 µm. (D) bar size 500 µm.
In the antrum, exophytic, proliferative lesions were observed grossly in 2 of 6 (33%) WT male Hp mice. These exophytic antral lesions in WT male Hp mice were epithelial polyps, characterized by a proliferation of cells with antral epithelial differentiation (Fig. 5D). These lesions did not seem to be specific to WT male Hp mice as a microscopically similar antral polypoid lesion was diagnosed in a HT male Hp mouse that was euthanized at ~4 mo postinoculation.
Female gender in combination with H. pylori infection increase the gastric and hepatic expression of interferon gamma
In the stomach, significant upregulation of Ifng was observed in HT male Hp mice relative to HT male mice and in HT female Hp mice relative to HT female mice (Fig. 6A). In addition, significant upregulation of Ifng was observed in WT female Hp mice relative to WT male Hp mice and in HT female Hp mice relative to HT male Hp mice (Fig. 6A). In the liver, significant differences in Ifng expression were observed between WT female Hp mice and WT male Hp mice and between HT female Hp mice and HT male Hp mice (Fig. 6B). There were no significant differences in hepatic Ifng expression between HT male mice and HT male Hp mice and between HT female mice and HT female Hp mice. Gastric Tnf and Il10 and hepatic Il10 were significantly upregulated in H. pylori-infected female mice relative to H. pylori-infected male mice (Supplementary Material). Hepatic Ifnb1 was significantly upregulated in HT female Hp mice relative to WT female Hp mice (Supplementary Material). The expression of gastric Tnf and Il10 and hepatic Il10 and Ifnb1 did not differ significantly between HT male mice and HT male Hp mice and between HT female mice and HT female Hp mice.
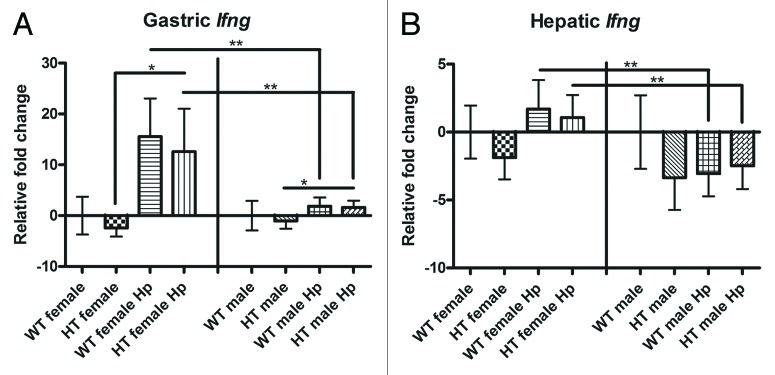
Figure 6. Gastric and hepatic gene expression in the different groups of mice: (A) Gastric interferon gamma (Ifng). (B) Hepatic Ifng. *, p < 0.05; **, p < 0.01.
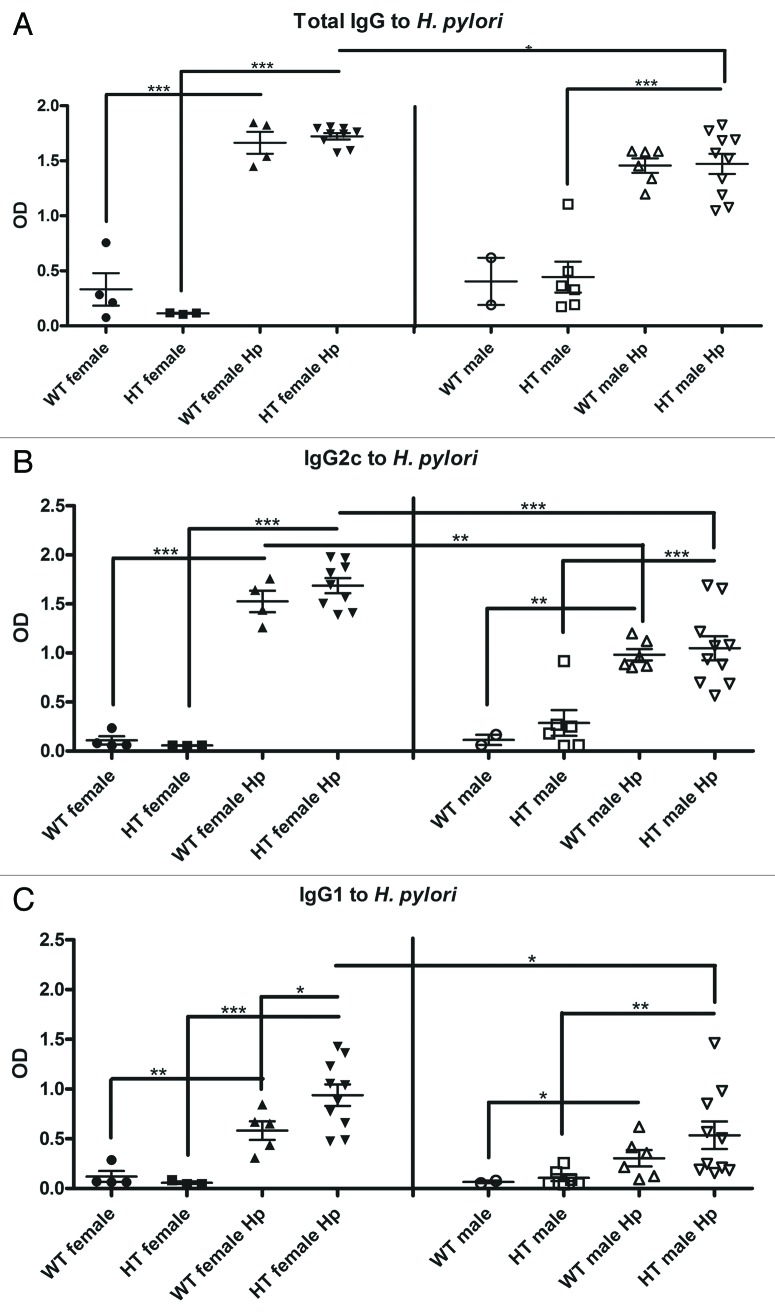
H. pylori increases serum Th1 and Th2 immune responses in female mice
Compared with uninfected controls, all mice infected with H. pylori developed similar, robust serum IgG titers to H. pylori (Fig. 7A-C). A significantly greater Th1-associated IgG2c antibody response was measured in WT female Hp mice relative to WT male Hp mice and in HT female Hp mice relative to HT male Hp mice. Th2-associated IgG1 responses were also significantly higher in HT female Hp mice compared with HT male Hp mice.
Figure 7. Serological immune response against H. pylori in the different groups of mice: (A) Total IgG. (B) Th1 (IgG2c). (C) Th2 (IgG1). OD, optical density. *, p ≤ 0.05; **, p ≤ 0.01; ***; p ≤ 0.001.
HCV transgene increases serum TNF-α during H. pylori infection in male mice
The serum concentration of TNF-α was significantly higher in HT male Hp mice relative to WT male Hp mice (185.5 ± 11.6 vs. 138.6 ± 17.1, p < 0.05) (Fig. 8). There were no significant differences in the serum concentration of TNF-α between HT male mice and HT male Hp mice and between HT female mice and HT female Hp mice. The serum concentration of IL1-β was significantly lower in HT female Hp mice relative to HT male Hp mice and the serum concentration of MCP-1 was significantly lower in WT male Hp mice relative to WT male mice. The serum IL1-β and MCP-1 findings were not consistent with the increased gastric corpus and antral pathology observed in HT female Hp and WT male Hp mice, respectively (Supplementary Material). No significant differences were observed in the serum concentration of IL-10 and IFN-ɣ when comparing the different groups of interest; however, the serum concentration of IL-17A was significantly higher in HT male mice relative to HT female mice (p < 0.01; data not shown) and the serum concentration of IL-6 was increased in WT female Hp mice relative to WT male Hp mice (p = 0.06; data not shown). There were no significant differences in the serum concentration of IL-1β, MCP-1, IL-10, IFN-ɣ, IL-17A, and IL-6 between HT male and HT male Hp mice and between HT female and HT female Hp mice.
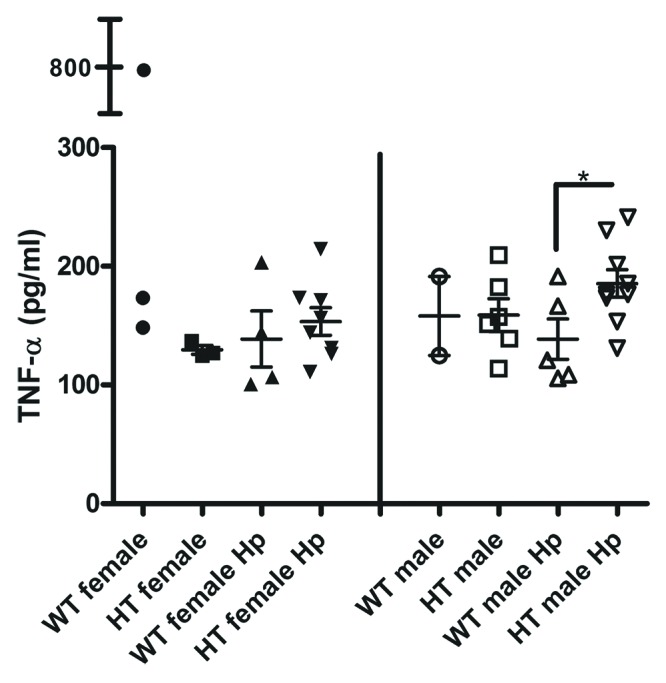
Figure 8. Serum concentration of tumor necrosis factor α (TNF-α) in the different groups of mice. *, p < 0.05.
Discussion
Previous studies in humans infected with HCV suggested that H. pylori may play a role in the pathogenesis and progression of HCC. Direct and indirect mechanisms of helicobacter-induced liver damage involving toxin(s) and induction of pro-inflammatory cytokines, respectively, have been suggested.16 The present study, utilizing C3B6F1-Tg(Alb1-HCVN)35Sml mice, characterized by hepatic steatosis in transgenic female mice, demonstrated that H. pylori colonized the stomach, but not the liver, and provides indirect evidence that H. pylori does not promote HCC during HCV infection in humans. However, we cannot exclude the possibility that H. pylori or "H. pylori-like" bacteria colonize the human liver during severe liver disease or cirrhosis as suggested by others.12,13,17,30-32 Hepatic H. pylori colonization and pathology may also depend on the immune response of the human host and/or H. pylori virulence factors such as CagA or VacA.10,33,34 Since H. pylori SS1, used in the current study, appears to have a non-functional cag pathogenicity island, additional studies are needed in order to investigate the role of the H. pylori cag pathogenicity island in liver cancer.35 It is important to note that enterohepatic Helicobacter spp. (EHS) may be involved in hepatobiliary diseases of humans.15,36-39 Although H. hepaticus was not detected in the stools of HCV or hepatitis B virus patients with HCC, a previous study from our laboratory indicated that H. hepaticus promoted liver cancer in B6C3F1-Tg(Alb1-HCVN)35Sml mice and also promoted aflatoxin-induced liver cancer in C3H/HeNCr mice with persistent intestinal colonization of H. hepaticus, despite the lack of the organism in the liver.22,40 The occurrence of HCCs in H. pylori-infected and uninfected HT and WT mice in our study is consistent with the natural susceptibility of aged B6C3F1 male mice to liver tumors.41
Hepatic steatosis is frequently observed in humans with chronic HCV infection and may increase the risk of HCC through increased oxidative stress or by promoting fibrosis.42 The C3B6F1-Tg(Alb1-HCVN)35Sml mouse model used in the current study was characterized by increased hepatic steatosis in female mice relative to WT female mice and no significant development of liver tumors. In contrast, its parental transgenic strain, the C57BL/6-Tg(Alb1-HCVN)35Sml (FL-N/35) mouse model, was characterized by hepatic steatosis and liver cancer in male mice.24,25 This difference may be due in part to the mixed genetic background in our model since another HCV transgenic mouse model on B6C3F1 and B6C3F2 genetic backgrounds was characterized by more females than males developing hepatic steatosis.43 In addition, subsequent generations of FL-N/35 mice no longer developed spontaneous liver tumors.27 The tumor promoting effects of the HCV transgene depend on the host genetic background.44 In our study, however, the HCV transgene protected from hepatic steatosis in H. pylori-infected male mice and this decrease in steatosis correlated with a significant increase in serum levels of TNF-α. Our findings are in contrast with human data in which chronic HCV patients with hepatic steatosis (and unknown H. pylori infection status) have increased serum levels of TNF-α.45 However, TNF-α/β deficient mice (C57BL/6J background) develop hepatomegaly and hepatic steatosis suggesting a role of TNF in control of lipid homeostasis.46 The role of H. pylori in hepatic steatosis associated with HCV in humans has been reported.82
We observed that H. pylori infection induced increased gastric corpus pathology in C3B6F1 (WT and HT) female mice relative to male mice. This is consistent with previous studies utilizing C57BL/6J and C57BL/6 gpt delta mouse models which have documented that female mice develop more severe gastric lesions from H. felis and H. pylori infection, respectively.47,48 H. pylori-infected female 129/Sv mice also showed increased gastric lesions compared with H. pylori-infected male 129/Sv mice.49 Furthermore, HT male mice were protected from H. pylori-induced corpus lesions. In the gastric antrum, H. pylori induced gastric lesions in WT male and female mice. However, HT male and female mice were protected from H. pylori-induced gastric antral lesions and more antral lesions were observed in WT male Hp than in HT male Hp mice. These findings suggest that the HCV transgene protected the mice against gastric corpus and antral lesions during H. pylori infection. However, studies in humans with HCV-associated chronic hepatitis did not find a correlation between liver inflammation and gastric mucosal lesions.50
Dysplasia in the gastric antrum has been observed in trefoil factor family 2 knockout male and female mice (on a mixed B6129Sv background) infected with H. pylori and this finding correlated with upregulation of gastric Ifng.51 During H. pylori infection the expression of Ifng was significantly higher in the stomach and liver of female mice relative to male mice. Similarly, in Mongolian gerbils infected with H. pylori SS1, the gastric Ifng expression was higher in females than in males at 36 weeks post-infection and females developed more gastric antral and corpus pathology than males at 36 weeks post-infection.52 In our study, the upregulation of Ifng in the stomach was associated with a significant increase in Th1 immune respose againt H. pylori, an increase in gastric corpus pathology, and a decrease in H. pylori colonization levels. These findings are consistent with the absence of H. pylori in the stomach of three female mice originally inoculated with H. pylori. Interferon gamma mediates inflammation during H. pylori infection with a subsequent reduction in H. pylori colonization.53 Increased gastric pathology in female C57BL/6 gpt delta mice at 6 mo postinfection has been associated with decreased H. pylori colonization levels.48 H. pylori also modulates the hepatic expression of innate immunity-, inflammation-, and fibrogenesis-associated genes in female C57BL/6 mice.54 Increased hepatic expression of Ifng in female mice in our study may contribute to the activation of NF-κB with a resulting pro-inflammatory response.54,55
A potential beneficial effect of H. pylori infection has been documented in humans with HCV. Increased levels of iron in the body may contribute to HCC development.56 H. pylori-positive HCV patients have lower levels of hepatic iron deposits than H. pylori-negative HCV patients.57 This finding may be consistent with the association of H. pylori infection and iron deficiency anemia.58 Interestingly, an excess-iron diet increased the risk of HCC in FL-N/35 mice whereas an iron-depleted diet increased the risk of gastric cancer in a gerbil model of H. pylori infection.27,59 In addition, chronic H. felis infection induced iron deficiency in hypergastrinemic INS-GAS mice.60 Whether H. pylori infection can decrease the risk of HCC in FL-N/35 mice fed a high iron diet requires additional studies.
H. pylori infection may prolong viral infection by decreasing the cytotoxic T cell response.61 Recent studies also suggest that the DNA of H. pylori includes a high ratio of immunoregulatory to immunostimulatory sequences that are associated with decreased type I interferon (IFN-α) and amelioration of colitis.62,63 However, in the context of HCV infection, decreased IFN-α signaling could promote chronic infection by decreasing the host immune response against the virus.64,65Ifnb1, the type I interferon gene encoding IFN-β, induces degradation of HCV proteins through autophagy.66 In addition, through nucleotide-binding oligomerization domain 1, Ifnb1 also partially protects the infected host by decreasing H. pylori gastric colonization.67 We observed significant upregulation of hepatic Ifnb1 in HT female Hp mice relative to WT female Hp mice suggesting that the HCV transgene contributed to a type I interferon response during H. pylori infection. Since the transgenic HCV mouse model used in the current study does not involve viral infection, it is not possible to determine the effect of H. pylori infection on HCV replication. The high prevalence of H. pylori in humans justifies the need for future studies designed to investigate if H. pylori and/or its DNA affect HCV replication in animal models. Mouse models with “humanized” livers and HCV infection may provide novel opportunities for delineating the effect of H. pylori on liver disease and cancer in humans.68
In summary, utilizing a transgenic mouse model of HCV pathogenesis and hepatic steatosis, we ascertained that H. pylori was not able to colonize the liver nor did the organism promote liver cancer. In contrast, previous studies using B6AF1 and AB6F1, B6C3F1-Tg(Alb1-HCVN)35Sml, and C3;B6 mice infected with the prototype EHS, H. hepaticus, demonstrated that this bacterium induced persistent hepatitis and/or liver tumors.22,69,70 Our results therefore would argue that EHS rather than H. pylori, are more likely to be associated with human hepatobiliary diseases including cancer. Our experimental evidence is supported by studies in which EHS have been identified in humans with inflammatory and neoplastic conditions including cholecystitis, bile duct and gallbladder cancer as well as by studies suggesting the presence of non-H. pylori helicobacters in patients with hepatobiliary diseases including primary biliary cirrhosis and primary sclerosing cholangitis.15,36-39 Future studies in humans with HCV and other hepatobiliary diseases should attempt to culture helicobacters not only in liver, but also the gastrointestinal tract, and compare isolates by detailed molecular techniques to ascertain their taxonomic classifications.15,17
Materials and Methods
Mice and experimental design
FL-N/35 mice [C57BL/6-Tg(Alb1-HCVN)35Sml; Mouse Genome Informatics ID: MGI:3513779] were obtained from Stanley M. Lemon (University of North Carolina School of Medicine). Male C57BL/6-Tg(Alb1-HCVN)35Sml mice were bred to C3H/HeNTac female mice and the C3B6F1-Tg(Alb1-HCVN)35Sml [HCV transgenic (HT)] and C3B6F1 wild-type (WT) progeny were used for the current study. C3B6F1 mice were genotyped for the transgene using primers HCV-F (5′-CAACCCTACGTACAGCTG-3′) and HCV-R (5′-GGTAGTCAACCATGCACC-3′) and the following polymerase chain reaction (PCR) conditions: 1 cycle of 94˚C for 5 min, 35 cycles of 94˚C for 75 s, 52˚C for 75 s and 72˚C for 75 s, and a final cycle of 72˚C for 10 min. Prior to experimental inoculation, C3B6F1 mice were determined to be free of Helicobacter spp. by PCR of their fecal DNA using primers that detect Helicobacter spp.15,71 Specific pathogen-free WT and HT mice were assigned to groups based on genotype and gender and were orally-inoculated every day for three days with 0.2 mL of media only (sham) or media with H. pylori SS1 (~2 × 108 organisms per inoculum) at ~2 mo of age. The sham inoculated groups are designated: WT female, HT female, WT male, and HT male. The H. pylori-inoculated groups are designated: WT female Hp, HT female Hp, WT male Hp, and HT male Hp (Table 2). Mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facilities and fed Prolab® RMH 3000 (LabDiet®, 5P00) and provided reverse osmosis water ad libitum. Mice were euthanized at ~12 mo postinoculation. This study was approved by the Massachusetts Institute of Technology Committee on Animal Care.
Histopathological evaluation
Immediately following euthanasia by CO2 inhalation, mouse body weight was recorded and blood was collected by cardiac puncture. A complete necropsy was performed and liver weight was measured. The stomach was collected and incised along the line of the greater gastric curvature, luminal contents were removed, and the mucosa was rinsed with sterile PBS. For histopathologic evaluation, linear strips extending from the gastric squamocolumnar junction to the proximal duodenum were taken along the lesser curvature. The liver was separated into left, right, median, and caudate lobes, and a section from each was collected. Stomach and liver sections were fixed in 10% neutral-buffered formalin and were routinely processed for histology. Sections of stomach and liver were also frozen in liquid nitrogen and stored at ~−80˚C for RNA extraction and at ≤ −20˚C for DNA extraction. Sera were also stored at ≤ −20˚C.
Five-micrometer-thick sections were stained with hematoxylin and eosin (H&E) for histopathological evaluation by a board-certified veterinary pathologist (N.M.A.P.) blinded to treatment groups. H&E-stained stomach sections were scored for corpus and antral pathology. Lesions in the corpus were scored according to a previously described scoring system, on an ascending scale of 0 to 4 for inflammation, epithelial defects, hyperplasia, dysplasia, atrophy and intestinal metaplasia; a GHAI was calculated as the sum of the scores for all parameters listed.72 Lesions in the antrum were scored based on a modification of the system used for lower intestinal lesions. The degree and frequency of epithelial defects, inflammation, and hyperplasia were scored on a scale of 0 to 4 with ascending severity (0, none; 1, minimal; 2, mild; 3, moderate; and 4, severe); epithelial dysplasia was also graded using a scale of 0 to 4 (0, normal; 1, mild dysplastic changes; 2, adenoma or low-grade dysplasia; 3, high grade dysplasia or carcinoma in situ; and 4, invasive carcinoma).51,73 A total histopathology index for the antrum was generated by adding the scores for inflammation, epithelial defects, hyperplasia, and dysplasia. For the liver, a hepatitis index was calculated by combining individual scores for lobular, portal and interface hepatitis, as well as the number of lobes (out of a total of 4) that contained 5 or more inflammatory lesions.74 Hepatitis was defined by a hepatitis index equal to, or greater than, 4.74 Liver sections were also scored for hepatic steatosis, preneoplastic (AHF and LFCA) and neoplastic (HCA and HCC) lesions.75,76 Images were obtained using an Olympus DP25 (Olympus Corporation, Tokyo, Japan) digital camera. Adobe® Photoshop® CS4 and CS6 were used for image processing. Automatic contrast and automatic color correction were performed for each image. Additionally, the eraser tool was used to remove extraneous, artifactually sloughed material in the intestinal lumen.
FISH for H. pylori
Formalin-fixed paraffin-embedded stomach sections were deparaffinized and rehydrated. After drying, pre-heated (74.5˚C for 10 min) hybridization buffer consisting of 0.9 M NaCl, 20 mM Tris-HCL (pH 7.2), 0.1% SDS, 30% formamide, and including 10 ng/µl of the Hpy-1-Cy3 probe77 was applied on the tissue sections and these were then covered with parafilm. The tissue sections were incubated in a chamber overnight at 48˚C. The next day, sections were rinsed with double-distilled water and then washed 15 min (covered to prevent light exposure) with a pre-heated washing buffer consisting of 0.9 M NaCl, 20 mM Tris-HCL (ph 7.2), and 0.01% SDS. A second 15 min (covered) wash was then performed using another pre-heated washing buffer consisting of 0.9 M NaCl and 20 mM Tris-HCL (pH 7.2). Finally, the sections were rinsed with double-distilled water and dried. Vectashield® Mounting Medium with DAPI (Vector Laboratories, H-1500) was then applied to the sections followed by placement of coverslips. Sections were examined with a Zeiss Axioskop 2 Plus microscope with filters for DAPI, rhodamine, and FITC and photos were obtained with a QIClick™ digital CCD camera (QImaging, Surrey, BC Canada). Two slides per H. pylori-infected group and one slide from an uninfected sham group (WT female) were analyzed for fluorescent organisms in a blinded fashion by a board-certified veterinary pathologist (N.M.A.P.).
H. pylori colonization
DNA was extracted from a longitudinal section of stomach incorporating antrum and corpus and from a section of the left liver lobe using High Pure PCR Template Preparation Kit (Roche). qPCR for H. pylori was utilized to assess H. pylori colonization as previously described.78 The following primers were used: 5′-CAAAATCGCTGGCATTGGT-3′ (forward) and 5′-CTTCACCGGCTAAGGCTTCA-3′ (reverse).78 The internal probe was: 5′-AACAAAGACATGCAAGATGGCGTTAAAAACA-3′.78
Gene expression analyses
RNA was extracted from a longitudinal section of stomach incorporating antrum and corpus and from the left liver lobe using TRIzol® Reagent (Invitrogen-Life Technologies). Five micrograms of RNA were converted to cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gastric and hepatic gene expression of Tumor necrosis factor (Tnf) (Assay ID: Mm99999068_m1), Interferon gamma (Ifng) (Assay ID: Mm01168134_m1), Interferon β 1, fibroblast (Ifnb1)66 (Assay ID: Mm00439546_s1), Interleukin 10 (Il10) (Assay ID: Mm01288386_m1) were measured using commercial TaqMan® primer-probe sets (Applied Biosystems-Life Technologies). The volume of each reaction consisted of 5 µl of sample cDNA, 4 µl of UltraPure™ Distilled Water (Gibco-Life Technologies, 10977), 1 µl of primer-probe, and 10 µl of TaqMan® Fast Universal PCR Master Mix (2 ×) (Applied Biosystems, 4352042). Duplicate reactions were performed for each sample. Gene expression was determined using the comparative CT method relative to Glyceraldehyde-3-phosphate dehydrogensase (Gapdh) (Applied Biosystems, 4352661), per PE Applied Biosystems’ User Bulletin # 2 ABI PRISM 7700 Sequence Detection System and using uninfected WT male and WT female mice as controls for male and female mice, respectively.
Enzyme-linked immunosorbent assay (ELISA) for serum antibody to H. pylori antigens
Serum IgG, Th1-associated IgG2c, and Th2-associated IgG1 responses to outer membrane antigens of H. pylori were measured by ELISA as previously described.79,80 Antigen was coated on Immulon II plates (Thermo Fisher Scientific, 3455) at a concentration of 1 µg/ml (IgG) or 10 µg/ml (isotypes) with sera diluted 1:100. Biotinylated secondary antibodies included goat anti-mouse IgG (Southern Biotech, 1030–03) and monoclonal anti-mouse antibodies produced by clones A85–1 and 5.7 (BD Biosciences, 553441 and 553504, respectively) for detecting IgG1 and IgG2c, respectively. Incubation with ExtrAvidin®-Peroxidase (Sigma, E2886) was followed by 2, 2’-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS®) substrate (Kirkegaard and Perry Laboratories, 50–65–02 and 50–64–02) for color development. Absorbance (optical density) development at 405/590 nm was recorded by an ELISA plate reader (Dynatech MR7000, Dynatech Laboratories, Inc., Chantilly, VA).
Serum cytokine assay (xMAP® Technology-Luminex)
Serum samples were analyzed using the Bio-Plex Pro™ Mouse Cytokine Th17 Panel A 6-Plex Group I (BIO-RAD, M60–00007NY) which included IL-1β, IL-10, IL-6, IL-17A, IFN-ɣ, and TNF-α. In addition, MCP-1 (MCAF; BIO-RAD, 171-G5019M) was added to the assay following the manufacturer’s recommendations. Most samples were analyzed in duplicate. Four serum samples were not analyzed (samples were hemolyzed).
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 5.03 for Windows (GraphPad Software). The combined multiplicity of hepatic preneoplasia and neoplasia was defined as the number of preneoplastic (AHF and LFCA) and neoplastic (HCA and HCC) microscopic liver lesions in each group divided by the total number of mice in the group.76,81 The Mann Whitney test (2 tailed) was used for analyses of corpus’ GHAI and antrum’s total histopathology index, hepatic steatosis, multiplicity of microscopic preneoplastic and neoplastic liver lesions, and hepatitis index scores. The Fisher’s exact test was used to compare the multiplicity of microscopic preneoplastic and/or neoplastic liver lesions and the numbers of mice with microscopic preneoplastic and/or neoplastic liver lesions between the groups of mice. The unpaired T test (2 tailed) was used for analyses of liver weight, liver to body weight ratio (%), number of liver lobes with dysplasia and neoplasia, multiplicity of microscopic preneoplastic and neoplastic liver lesions, H. pylori colonization, and gene expression. The unpaired T test (2 tailed) with Welch correction was used to analyze the serological immune response against H. pylori. Results are shown as median with interquartile range (for hepatic steatosis, corpus’ GHAI, antrum’s total histopathology index, and hepatitis index), mean ± standard error of the mean [for liver to body weight ratio (%), multiplicity, colonization, serological immune response against H. pylori, and serum cytokine concentrations], or as mean ± standard deviation (for gene expression). Results were considered significant if p ≤ 0.05.
Supplementary Material
Acknowledgments
The following National Institutes of Health grants provided support for this study: R01CA093405, P01CA026731, P01CA028842, P30ES002109. We would like to thank Elaine Robbins for assistance with the preparation of the figures for publication.
Glossary
Abbreviations:
- hepatitis C virus
HCV
- Helicobacter pylori
H. pylori
- wild-type
WT
- HCV transgenic
HT
- gastric histologic activity index
GHAI
- altered hepatocellular foci
AHF
- large foci of cellular alteration
LFCA
- hepatocellular adenoma
HCA
- hepatocellular carcinoma
HCC
- polymerase chain reaction
PCR
- quantitative polymerase chain reaction
qPCR
- hematoxylin and eosin
H&E
- fluorescent in situ hybridization
FISH
- enzyme-linked immunosorbent assay
ELISA
- enterohepatic helicobacter species
EHS
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Supplemental Materials
All supplemental materials may be found here: www.landesbioscience.com/journals/gutmicrobes/article/26042.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/26042
References
- 1.Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol. 2007;5:441–52. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73, e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 4.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 7.Antúnez I, Aponte N, Fernández-Carbia A, Rodríguez-Perez F, Toro DH. Steatosis as a predictive factor for treatment response in patients with chronic hepatitis C. P R Health Sci J. 2004;23(Suppl):57–60. [PubMed] [Google Scholar]
- 8.Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036–43. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 9.Ponzetto A, Pellicano R, Leone N, Cutufia MA, Turrini F, Grigioni WF, D’Errico A, Mortimer P, Rizzetto M, Silengo L. Helicobacter infection and cirrhosis in hepatitis C virus carriage: is it an innocent bystander or a troublemaker? Med Hypotheses. 2000;54:275–7. doi: 10.1054/mehy.1999.0987. [DOI] [PubMed] [Google Scholar]
- 10.Dore MP, Realdi G, Mura D, Graham DY, Sepulveda AR. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638–43. doi: 10.1023/A:1015848009444. [DOI] [PubMed] [Google Scholar]
- 11.Pellicano R, Mazzaferro V, Grigioni WF, Cutufia MA, Fagoonee S, Silengo L, Rizzetto M, Ponzetto A. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J Gastroenterol. 2004;10:598–601. doi: 10.3748/wjg.v10.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha M, Avenaud P, Ménard A, Le Bail B, Balabaud C, Bioulac-Sage P, de Magalhães Queiroz DM, Mégraud F. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396–401. doi: 10.1136/gut.2004.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castéra L, Pedeboscq A, Rocha M, Le Bail B, Asencio C, de Lédinghen V, Bernard PH, Laurent C, Lafon ME, Capdepont M, et al. Relationship between the severity of hepatitis C virus-related liver disease and the presence of Helicobacter species in the liver: a prospective study. World J Gastroenterol. 2006;12:7278–84. doi: 10.3748/wjg.v12.i45.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cindoruk M, Cirak MY, Unal S, Karakan T, Erkan G, Engin D, Dumlu S, Turet S. Identification of Helicobacter species by 16S rDNA PCR and sequence analysis in human liver samples from patients with various etiologies of benign liver diseases. Eur J Gastroenterol Hepatol. 2008;20:33–6. doi: 10.1097/MEG.0b013e3282efa4f2. [DOI] [PubMed] [Google Scholar]
- 15.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, Ericson RL, Lau CN, Correa P, Araya JC, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–63. doi: 10.1016/S0016-5085(98)70589-X. [DOI] [PubMed] [Google Scholar]
- 16.Pellicano R, Ménard A, Rizzetto M, Mégraud F. Helicobacter species and liver diseases: association or causation? Lancet Infect Dis. 2008;8:254–60. doi: 10.1016/S1473-3099(08)70066-5. [DOI] [PubMed] [Google Scholar]
- 17.de Magalhães Queiroz DM, Santos A. Isolation of a Helicobacter strain from the human liver. Gastroenterology. 2001;121:1023–4. doi: 10.1053/gast.2001.28574. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Yamaoka Y, Yoffe B, Graham DY. Disturbance of apoptosis and DNA synthesis by Helicobacter pylori infection of hepatocytes. Dig Dis Sci. 2008;53:2532–40. doi: 10.1007/s10620-007-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Roux-Goglin E, Varon C, Spuul P, Asencio C, Mégraud F, Génot E. Helicobacter infection induces podosome assembly in primary hepatocytes in vitro. Eur J Cell Biol. 2012;91:161–70. doi: 10.1016/j.ejcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Okoli AS, Raftery MJ, Mendz GL. Comparison of Helicobacter bilis-Associated Protein Expression in Huh7 Cells Harbouring HCV Replicon and in Replicon-Cured Cells. Int J Hepatol. 2012;2012:501671. doi: 10.1155/2012/501671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox JG, Li X, Yan L, Cahill RJ, Hurley R, Lewis R, Murphy JC. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–58. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox JG, Feng Y, Theve EJ, Raczynski AR, Fiala JL, Doernte AL, Williams M, McFaline JL, Essigmann JM, Schauer DB, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2010;59:88–97. doi: 10.1136/gut.2009.183749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142–54. doi: 10.1016/j.jhep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Lemon SM, Lerat H, Weinman SA, Honda M. A transgenic mouse model of steatosis and hepatocellular carcinoma associated with chronic hepatitis C virus infection in humans. Trans Am Clin Climatol Assoc. 2000;111:146–56, discussion 156-7. [PMC free article] [PubMed] [Google Scholar]
- 25.Lerat H, Honda M, Beard MR, Loesch K, Sun J, Yang Y, Okuda M, Gosert R, Xiao SY, Weinman SA, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–65. doi: 10.1053/gast.2002.31001. [DOI] [PubMed] [Google Scholar]
- 26.Disson O, Haouzi D, Desagher S, Loesch K, Hahne M, Kremer EJ, Jacquet C, Lemon SM, Hibner U, Lerat H. Impaired clearance of virus-infected hepatocytes in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2004;126:859–72. doi: 10.1053/j.gastro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, Korenaga M, Xiao SY, Weinman SA, Lemon SM, et al. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087–98. doi: 10.1053/j.gastro.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 28.Lerat H, Kammoun HL, Hainault I, Mérour E, Higgs MR, Callens C, Lemon SM, Foufelle F, Pawlotsky JM. Hepatitis C virus proteins induce lipogenesis and defective triglyceride secretion in transgenic mice. J Biol Chem. 2009;284:33466–74. doi: 10.1074/jbc.M109.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hailey JR, Haseman JK, Bucher JR, Radovsky AE, Malarkey DE, Miller RT, Nyska A, Maronpot RR. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol Pathol. 1998;26:602–11. doi: 10.1177/019262339802600503. [DOI] [PubMed] [Google Scholar]
- 30.Queiroz DM, Rocha AM, Rocha GA, Cinque SM, Oliveira AG, Godoy A, Tanno H. Association between Helicobacter pylori infection and cirrhosis in patients with chronic hepatitis C virus. Dig Dis Sci. 2006;51:370–3. doi: 10.1007/s10620-006-3150-y. [DOI] [PubMed] [Google Scholar]
- 31.El-Masry S, El-Shahat M, Badra G, Aboel-Nour MF, Lotfy M. Helicobacter pylori and Hepatitis C Virus Coinfection in Egyptian Patients. J Glob Infect Dis. 2010;2:4–9. doi: 10.4103/0974-777X.59244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmat G, El-Bendary M, Zakarya S, Ela MA, Zalata K. Role of Helicobacter pylori in patients with HCV-related chronic hepatitis and cirrhosis with or without hepatocellular carcinoma: possible association with disease progression. J Viral Hepat. 2012;19:473–9. doi: 10.1111/j.1365-2893.2011.01567.x. [DOI] [PubMed] [Google Scholar]
- 33.Silva LD, Rocha AM, Rocha GA, de Moura SB, Rocha MM, Dani R, de Melo FF, Guerra JB, de Castro LP, Mendes GS, et al. The presence of Helicobacter pylori in the liver depends on the Th1, Th17 and Treg cytokine profile of the patient. Mem Inst Oswaldo Cruz. 2011;106:748–54. doi: 10.1590/s0074-02762011000600016. [DOI] [PubMed] [Google Scholar]
- 34.Boonyanugomol W, Chomvarin C, Sripa B, Bhudhisawasdi V, Khuntikeo N, Hahnvajanawong C, Chamsuwan A. Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB (Oxford) 2012;14:177–84. doi: 10.1111/j.1477-2574.2011.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crabtree JE, Ferrero RL, Kusters JG. The mouse colonizing Helicobacter pylori strain SS1 may lack a functional cag pathogenicity island. Helicobacter. 2002;7:139–40, author reply 140-1. doi: 10.1046/j.1083-4389.2002.00071.x. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadström T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38:1072–6. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsukura N, Yokomuro S, Yamada S, Tajiri T, Sundo T, Hadama T, Kamiya S, Naito Z, Fox JG. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn J Cancer Res. 2002;93:842–7. doi: 10.1111/j.1349-7006.2002.tb01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiwari SK, Khan AA, Ibrahim M, Habeeb MA, Habibullah CM. Helicobacter pylori and other Helicobacter species DNA in human bile samples from patients with various hepato-biliary diseases. World J Gastroenterol. 2006;12:2181–6. doi: 10.3748/wjg.v12.i14.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamada T, Yokota K, Ayada K, Hirai K, Kamada T, Haruma K, Chayama K, Oguma K. Detection of Helicobacter hepaticus in human bile samples of patients with biliary disease. Helicobacter. 2009;14:545–51. doi: 10.1111/j.1523-5378.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krüttgen A, Horz HP, Weber-Heynemann J, Vucur M, Trautwein C, Haase G, Luedde T, Roderburg C. Study on the association of Helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes. 2012;3:228–33. doi: 10.4161/gmic.19922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Innes JR, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Hart ER, Pallotta AJ, Bates RR, Falk HL, Gart JJ, et al. Bioassay of pesticides and industrial chemicals for tumorigenicity in mice: a preliminary note. J Natl Cancer Inst. 1969;42:1101–14. [PubMed] [Google Scholar]
- 42.McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969–83. doi: 10.1038/onc.2010.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naas T, Ghorbani M, Alvarez-Maya I, Lapner M, Kothary R, De Repentigny Y, Gomes S, Babiuk L, Giulivi A, Soare C, et al. Characterization of liver histopathology in a transgenic mouse model expressing genotype 1a hepatitis C virus core and envelope proteins 1 and 2. J Gen Virol. 2005;86:2185–96. doi: 10.1099/vir.0.80969-0. [DOI] [PubMed] [Google Scholar]
- 44.Klopstock N, Katzenellenbogen M, Pappo O, Sklair-Levy M, Olam D, Mizrahi L, Potikha T, Galun E, Goldenberg D. HCV tumor promoting effect is dependent on host genetic background. PLoS One. 2009;4:e5025. doi: 10.1371/journal.pone.0005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349–57. doi: 10.1111/j.1365-2036.2006.03114.x. [DOI] [PubMed] [Google Scholar]
- 46.Schnyder-Candrian S, Czarniecki J, Lerondel S, Corpataux J, Ryffel B, Schnyder B. Hepatic steatosis in the absence of tumor necrosis factor in mice. Cytokine. 2005;32:287–95. doi: 10.1016/j.cyto.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Court M, Robinson PA, Dixon MF, Jeremy AH, Crabtree JE. The effect of gender on Helicobacter felis-mediated gastritis, epithelial cell proliferation, and apoptosis in the mouse model. J Pathol. 2003;201:303–11. doi: 10.1002/path.1422. [DOI] [PubMed] [Google Scholar]
- 48.Sheh A, Lee CW, Masumura K, Rickman BH, Nohmi T, Wogan GN, Fox JG, Schauer DB. Mutagenic potency of Helicobacter pylori in the gastric mucosa of mice is determined by sex and duration of infection. Proc Natl Acad Sci U S A. 2010;107:15217–22. doi: 10.1073/pnas.1009017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Every AL, Ng GZ, Skene CD, Harbour SN, Walduck AK, McGuckin MA, Sutton P. Localized suppression of inflammation at sites of Helicobacter pylori colonization. Infect Immun. 2011;79:4186–92. doi: 10.1128/IAI.05602-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orłowski M, Stalke P, Michalska Z, Sikorska K, Witczak-Malinowska K, Eilert-Zygadłowska J, Stolarczyk J. Assessment of correlation between histopathologic changes of gastric mucosa according to Whitehead’s classification and extent of liver damage according to Knodell’s scale in patients with chronic hepatopathy. Med Sci Monit. 2003;9(Suppl 3):60–3. [PubMed] [Google Scholar]
- 51.Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani M, Jones EK, Wang TC. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 -/- C57BL6 x Sv129 Helicobacter pylori-infected mice. Am J Pathol. 2007;171:1520–8. doi: 10.2353/ajpath.2007.070249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197–207. doi: 10.1002/path.1498. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Kita M, Ohno T, Iwakura Y, Sekikawa K, Imanishi J. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol Immunol. 2004;48:647–54. doi: 10.1111/j.1348-0421.2004.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 54.Ki MR, Goo MJ, Park JK, Hong IH, Ji AR, Han SY, You SY, Lee EM, Kim AY, Park SJ, et al. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β1-induced inflammatory signaling. Lab Invest. 2010;90:1507–16. doi: 10.1038/labinvest.2010.109. [DOI] [PubMed] [Google Scholar]
- 55.Cheshire JL, Baldwin AS., Jr. Synergistic activation of NF-kappaB by tumor necrosis factor alpha and gamma interferon via enhanced I kappaB alpha degradation and de novo I kappaBbeta degradation. Mol Cell Biol. 1997;17:6746–54. doi: 10.1128/mcb.17.11.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 57.Sumida Y, Kanemasa K, Yamaoka Y, Imamura S, Katoh N, Nakashima T, Tachibana S, Mitsuyoshi H, Itoh Y, Okanoue T. Influence of Helicobacter pylori infection on iron accumulation in hepatitis C. Liver Int. 2006;26:827–33. doi: 10.1111/j.1478-3231.2006.01305.x. [DOI] [PubMed] [Google Scholar]
- 58.Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter. 2008;13:323–40. doi: 10.1111/j.1523-5378.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 59.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–92. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomson MJ, Pritchard DM, Boxall SA, Abuderman AA, Williams JM, Varro A, Crabtree JE. Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PLoS One. 2012;7:e50194. doi: 10.1371/journal.pone.0050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shirai M, Arichi T, Nakazawa T, Berzofsky JA. Persistent infection by Helicobacter pylori down-modulates virus-specific CD8+ cytotoxic T cell response and prolongs viral infection. J Infect Dis. 1998;177:72–80. doi: 10.1086/513827. [DOI] [PubMed] [Google Scholar]
- 62.Luther J, Owyang SY, Takeuchi T, Cole TS, Zhang M, Liu M, Erb-Downward J, Rubenstein JH, Chen CC, Pierzchala AV, et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut. 2011;60:1479–86. doi: 10.1136/gut.2010.220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owyang SY, Luther J, Owyang CC, Zhang M, Kao JY. Helicobacter pylori DNA’s anti-inflammatory effect on experimental colitis. Gut Microbes. 2012;3:168–71. doi: 10.4161/gmic.19181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blindenbacher A, Duong FH, Hunziker L, Stutvoet ST, Wang X, Terracciano L, Moradpour D, Blum HE, Alonzi T, Tripodi M, et al. Expression of hepatitis c virus proteins inhibits interferon alpha signaling in the liver of transgenic mice. Gastroenterology. 2003;124:1465–75. doi: 10.1016/S0016-5085(03)00290-7. [DOI] [PubMed] [Google Scholar]
- 65.Duong FH, Filipowicz M, Tripodi M, La Monica N, Heim MH. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology. 2004;126:263–77. doi: 10.1053/j.gastro.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 66.Desai MM, Gong B, Chan T, Davey RA, Soong L, Kolokoltsov AA, Sun J. Differential, type I interferon-mediated autophagic trafficking of hepatitis C virus proteins in mouse liver. Gastroenterology. 2011;141:674–85, e1-6. doi: 10.1053/j.gastro.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645–62. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–33. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 69.García A, Ihrig MM, Fry RC, Feng Y, Xu S, Boutin SR, Rogers AB, Muthupalani S, Samson LD, Fox JG. Genetic susceptibility to chronic hepatitis is inherited codominantly in Helicobacter hepaticus-infected AB6F1 and B6AF1 hybrid male mice, and progression to hepatocellular carcinoma is linked to hepatic expression of lipogenic genes and immune function-associated networks. Infect Immun. 2008;76:1866–76. doi: 10.1128/IAI.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Roux-Goglin E, Dubus P, Asencio C, Jutand MA, Rosenbaum J, Mégraud F. Hepatic lesions observed in hepatitis C virus transgenic mice infected by Helicobacter hepaticus. Helicobacter. 2013;18:33–40. doi: 10.1111/j.1523-5378.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- 71.Lofgren JL, Esmail M, Mobley M, McCabe A, Taylor NS, Shen Z, Erdman S, Hewes C, Whary MT, Fox JG. Prevalence of murine Helicobacter spp. Infection is reduced by restocking research colonies with Helicobacter-free mice. J Am Assoc Lab Anim Sci. 2012;51:436–42. [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 73.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers AB, Theve EJ, Feng Y, Fry RC, Taghizadeh K, Clapp KM, Boussahmain C, Cormier KS, Fox JG. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67:11536–46. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- 75.Gilat T, Leikin-Frenkel A, Goldiner I, Juhel C, Lafont H, Gobbi D, Konikoff FM. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC) Hepatology. 2003;38:436–42. doi: 10.1053/jhep.2003.50348. [DOI] [PubMed] [Google Scholar]
- 76.García A, Zeng Y, Muthupalani S, Ge Z, Potter A, Mobley MW, Boussahmain C, Feng Y, Wishnok JS, Fox JG. Helicobacter hepaticus--induced liver tumor promotion is associated with increased serum bile acid and a persistent microbial-induced immune response. Cancer Res. 2011;71:2529–40. doi: 10.1158/0008-5472.CAN-10-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trebesius K, Panthel K, Strobel S, Vogt K, Faller G, Kirchner T, Kist M, Heesemann J, Haas R. Rapid and specific detection of Helicobacter pylori macrolide resistance in gastric tissue by fluorescent in situ hybridisation. Gut. 2000;46:608–14. doi: 10.1136/gut.46.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maurer KJ, Rogers AB, Ge Z, Wiese AJ, Carey MC, Fox JG. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am J Physiol Gastrointest Liver Physiol. 2006;290:G175–82. doi: 10.1152/ajpgi.00272.2005. [DOI] [PubMed] [Google Scholar]
- 79.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–92. doi: 10.1016/S0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 80.Whary MT, Morgan TJ, Dangler CA, Gaudes KJ, Taylor NS, Fox JG. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–8. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi M, Dinse GE, Foley JF, Hardisty JF, Maronpot RR. Comparative prevalence, multiplicity, and progression of spontaneous and vinyl carbamate-induced liver lesions in five strains of male mice. Toxicol Pathol. 2002;30:599–605. doi: 10.1080/01926230290105776. [DOI] [PubMed] [Google Scholar]
- 82.Sakr SA, Badrah GA, Sheir RA. Histological and histochemical alterations in liver of chronic hepatitis C patients with Helicobacter pylori infection. Biomed Pharmacother. 2013;67:367–74. doi: 10.1016/j.biopha.2013.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.