Abstract
Precisely regulated patterns of gene expression are dependent on the binding of transcription factors and chromatin-associated determinants referred to as co-activators and co-repressors. These regulatory components function with the core transcriptional machinery to serve in critical activities to alter chromatin modification and regulate gene expression. While we are beginning to understand that cell-type specific patterns of gene expression are necessary to achieve selective cardiovascular developmental programs, we still do not know the molecular machineries that localize these determinants in the heart. With clear implications for the epigenetic control of gene expression signatures, the ENCODE (Encyclopedia of DNA Elements) Project Consortium determined that about 90% of the human genome is transcribed while only 1-2% of transcripts encode proteins. Emerging evidence suggests that non-coding RNA (ncRNA) serves as a signal for decoding chromatin modifications and provides a potential molecular basis for cell type-specific and promoter-specific patterns of gene expression. The discovery of the histone methyltransferase enzyme EZH2 in the regulation of gene expression patterns implicated in cardiac hypertrophy suggests a novel role for chromatin-associated ncRNAs and is the focus of this article.
Keywords: cardiac hypertrophy, gene regulation, long non-coding RNA, chromatin, histone modifications
Introduction
The mammalian heart is the first organ to form in the vertebrate embryo. During development, heart chambers undergo structural changes mediated by specific cellular and extracellular cues such as hormone stimulation. Heart development involves stage-specific changes that are precisely regulated by spatial and temporal events on chromatin to regulate specific gene expression patterns.1 For example, genes expressed at later stages in cardiac development, such as cardiomyocyte maturation and terminal differentiation, show mono-methylation of histone H3 lysine 4 (H3K4me1) at early stages of development, whereas activation at later stages are often specified by H3K4me3 modification.1 During lineage commitment there are stage-specific acetylation (H3K27ac) and methylation (H3K4me1, H3K4me3 and H3K27me3) of lysine residues on histone H3 regulating gene expression and cardiac differentiation. For example, both methylation and acetylation of histone proteins at distinct lysine positions determine specific histone modification signatures that predict gene expression patterns that serve as transcription-factor binding sites as well as the exchange of co-regulatory complexes on promoters.2 Gene activation in pluripotent stem cells is associated with H3K4me1 patterns at gene promoters, which are also activated at later stages in the cardiac lineage, which is in striking contrast to H3K27me3 patterns and genes destined for suppression. Genes that code for the adult isoform cardiac contractile protein such as α-myosin heavy chain (α-MHC) and the transcription factor NKX2.5, are activated specifically at later stages of cardiac differentiation. These genes show high levels of H3K27me3 deposition at pluripotent stage, which are gradually erased and replaced by H3K4me3 modification.3
Cardiomyocyte cells respond using adaptive mechanisms to changing environmental stimuli such as increased workload. Such physiological changes are marked by an increase in cardiomyocyte size and ventricular mass, which is referred to as cardiac hypertrophy. Chronic exercise training or pregnancy can increase heart muscle mass and contractile ability, often referred to as physiological hypertrophy.4 However, there is a fine balance between physiological and pathological hypertrophy which are distinguished by cardiac failure. Pathophysiological surroundings such as acute and chronic myocardial stress including hypertension, valvular disease, and myocardial infarction, can dramatically increase the size of the ventricular chamber.4,5 This is referred to as pathological-cardiac hypertrophy and, like physiological hypertrophy, stimulates a phase of neurohumoral and biomechanical signals within the myocardium. While it is considered that physiological hypertrophy is generally advantageous as well as reversible, pathological hypertrophy causes irreversible remodeling leading to deformation of the ventricles and reduced heart contractility.6
The discovery of specific activator and repressor complexes important in cardiac development has revealed several mechanistic insights into myocardial function, cardiac development as well as heart disease. Ventricular hypertrophy is associated with re-activation of fetal genes that include ANP, BNP, and β-MHC as well as the suppression of SERCA2a and α-MHC genes in the adult heart.6 The recruitment of ATPase-dependent chromatin remodeling complexes that belong to the SWI/SNF family7 have been shown to contextually associate with either histone acetyltransferases (HATs) or histone deacetylases (HDACs) to regulate cardiac gene expression.8 Indeed, the recruitment and binding of p300 HAT enzyme on gene promoters is closely associated with chamber-specific gene expression patterns conferred by histone acetylation under physiological states.9 In addition, recent studies have expanded the complexity of regulatory determinants that participate in cardiac gene function, for example histone modifying proteins such as EZH2 and ASXL2 specify MHC gene expression in postnatal cardiac homeostasis.10,11 Human homologs of Drosophila genes (Enhancer of zeste homolog 2 and Additional sex combs-like protein 2) EZH2 and ASXL2 are members of the Polycomb group (PcG) protein family and implicated in maintaining gene repressive states by chromatin modification during later stages of heart development.
Mechanisms that regulate gene expression are under the direct control of specific classes of transcription factors and core machinery that serve to alter chromatin structure and function. However the precise actions of transcription factors and chromatin remodeling determinants, including histone and non-histone modifying enzymes in gene transcription are poorly characterized in the heart. Moreover, the diversity of transcription factors and chromatin modifying enzymes specifying gene expression patterns presents a major conceptual problem when attempting to predict specific interactions with target genes. In this article we explore the basis of cell type-specific and gene-specific patterns of gene regulation that integrate chromatin-interacting ncRNAs with histone modifying enzymes that functionally serve to alter gene structure and expression. Recent experimental observations show that chromatin remodeling and histone modification confer important transcriptional programs as a result of development and cardiac disease.12-15 The diverse interplay of histone modifying enzymes interacting with long non-coding RNAs (lncRNA) that serve to localize DNA-binding proteins as well as direct specific post-translational modifications to regulate gene expression has been described and is the focus of our discussion.16-18
Physiological Roles of lncRNAs in the Heart
Recent advances in nucleic acid sequencing technologies has revealed that nearly 90% of the genome is transcribed in one tissue type or another, with estimates that between 70-98% constitute ncRNAs.19-21 These transcripts are broadly classified in two groups according to nucleotide length: short ncRNAs (<200 nt), such as microRNA (miRNA) and long ncRNAs (>200 nt), such as the natural antisense transcripts (NATs) (Table 1). Interestingly, ncRNAs have been thought for some time to interact with DNA to regulate important nuclear functions. Indeed, Jacob and Monod explored this concept of base complementarity between RNA and DNA sequences22 which later was experimentally examined in triplex-forming sequences derived from human c-MYC.23 Direct evidence of interacting ncRNA mediating gene silencing-epigenetic changes exposed recruitment of important regulatory components in RNA-dependent DNA methylation.24
Table 1. Classification of functional ncRNAs. Transcriptional gene silencing functions of short (grey background) and long ncRNAs by chromatin interaction.
| ncRNA class | Chromatin interaction |
|---|---|
| MicroRNA (miRNA) | Yes 114 |
| Small interfering RNA (siRNA) | Yes 115 |
| Piwi-interacting RNA (piRNA) | Yes 116 |
| Small nuclearRNA (snRNA) | Yes 92 |
| Small nucleolarRNA (snoRNA) | Yes 117 |
| Natural antisense transcript (NAT) | Yes80 |
| Large intergenic ncRNA (lincRNA) | Yes35 |
| Promoter associated RNA (paRNA) | Yes118 |
| Circular RNA (circRNA) | Yes78 |
| Enhancer RNA (eRNA) | Yes119 |
| Pseudogene RNA (trans-NAT) | Yes120,121 |
| Transcribed ultraconserved regions (T-UCRs) | Yes122 |
| Short-lived RNA transcripts (SLiTs) | Yes32 |
| Telomeric repeat-containing RNA (TERRA) | Yes123 |
| Transfer RNA (tRNA) | Not reported |
| Ribosomal RNA (rRNA) | Not reported |
When, in 1993 two studies published back-to-back in Cell described a putative role for short ncRNAs in C. elegans development, the importance of these critical findings was probably underappreciated in transcription biology.25,26 How ncRNAs recognize and interact with target sequences to regulate gene expression still remains poorly characterized. Although short ncRNAs are strongly conserved but of unknown function, the seminal discoveries by the groups led by Ambros and Ruvkun have revealed a regulatory complexity mediated by ncRNAs. The field has expanded tremendously with a better understanding of the significance in biology and disease. Recent studies now show that during development, ncRNAs are expressed in a dynamic fashion and regulated by specific cellular and environmental cues.16,17
The importance of short ncRNAs in heart development was elegantly demonstrated by cardiac-specific deletion of miRNA-processing enzyme, DICER.27 Abundantly expressed in the heart, miR-1 and miR-133 are associated with cardiovascular development and myeloid differentiation.28-30 Recently, functional paradigms for several lncRNAs have also been described such as the participation in embryonic differentiation and cell-lineage development as well as transcriptional control.16,17,31,32 While lncRNAs can serve as spliceosome and ribosome components in eukaryotic RNA metabolism, recent experimental observations indicate a role in organizing chromatin conformation and shaping the genome. For example, chromatin interacting lncRNAs were recently identified as key determinants of gene imprinting (such as XIST and KCNQ1OT1 as well as AIR), whereas the recruitment of PRC2 components are implicated in gene suppression events that involve HOTAIR and TUG1.33-35 Recently, knockdown of lncRNAs expressed in embryonic stem cells has revealed more than one hundred functional lncRNAs associated with the maintenance of pluripotency.36 In addition, several lncRNAs have been implicated in normal heart physiology. For example, in the mouse, Braveheart (Bvht) and Fendrr are thought to have critical roles in cardiac lineage specification during embryonic development.16,17 The silencing of Bvht in mES cells results in the loss of cardiomyocyte beating in embryoid bodies (EB) at day 11 of differentiation.16 Whereas the expression of tissue-specific Fendrr is a regulator of heart and body wall development.17 While these results are not fully understood, it is hypothesized that Bvht and Fendrr control gene expression by interacting with the regulatory cofactors, PRC2 and TrxG/MLL complexes. These studies highlight the importance of lncRNA transcripts defining chromatin structure and gene expression necessary for heart development. Recent studies have also identified putative roles for over expressed lncRNAs in cancer (MALAT1 and HOTAIR) and Alzheimer’s (BACE1-AS), as well as reduced expression of lncRNAs in anemia (LincRNA-EPS) and Huntington’s disease (HTT-AS).37-40 In addition to the general involvement of DNA-binding motifs that function in the recruitment of transcription factors, new roles for lncRNAs in mediating chromatin-protein interactions have recently been described.20,41 Several lncRNAs have putative sequence motifs and structural domains implicated in protein association and interacting with specific gene targets. Indeed, several chromatin-interacting proteins have recently been described to have ncRNA-binding domains such as the polycomb-group (PcG) proteins, which are involved in the suppression of gene expression mediated by chromatin modification.42,43
Non-Coding RNAs Connect EZH2 with Chromatin
The expression of lncRNAs and natural antisense transcripts have recently been shown to regulate gene transcription and protein translation in the heart.14,15 The antisense (AS) transcripts to NPPA (AS-NPPA) and β-MHC (AS-β-MHC) are examples of regulatory lncRNAs in the myocardium. These transcripts are thought to associate with chromatin and regulate the expression of sense counterparts, NPPA and β-MHC whose expressions are regulated by EZH2 in the heart. The EZH2 lysine methyltransferase has a binding domain that is thought to mediate interaction with lncRNAs.42 For instance, phosphorylation of threonine (T365) of EZH2 interacts with HOTAIR and XIST.33 Although well characterized in cancer, the specific interactions of ncRNAs with histone modifying determinants such as EZH2, remain poorly described in the heart.44 Several lysine methyltransferase proteins have a conserved SET-domain region, which is thought to be critical to chromatin association as well as enzymatic activity. A number of methyl-writing SET-domain family members such as G9A, SET7, SMYD3, SET2, SET1, and EZH2, can bind to single-stranded DNA and RNA.45-48 In addition, several MLL family proteins that contain the SET-domain are known to interact with ncRNA either directly or indirectly.49,50 The methyl-erasing enzyme, LSD1, is thought to bind directly to the 3’ end of the HOTAIR lncRNA to regulate HOXD gene expression.51
Recent data published by several groups suggest putative roles for antisense transcripts in mediating EZH2 interactions with chromatin (Table 2).10,52 The expression of genes encoding contractile proteins and transcription factors implicated in heart disease are altered in EZH2-knockout mouse models.10 Deep sequencing of chromatin immunoprecipitated from the mouse heart using antibodies that recognize EZH2 show direct interaction with genes implicated in cardiac disease (Table 2).52 Interestingly, EZH2 appears to bind novel bi-directional promoter (bdP) sequence to regulate sense and antisense RNA expression. For example, the heart displays altered expression of tumor suppressor related genes CDKN2B, CDKN2A, and ARF encoding the INK4/ARF locus at chromosome 9p21 in EZH2-null mice.10,52 The ANRIL antisense is thought to regulate these genes by PcG-dependent silencing.53 But, whether ANRIL directly regulates EZH2 chromatin interaction at the 9p21 region in cardiomyocyte cells remains to be determined. In favor of a role in cardiac homeostasis, individuals homozygous for the SNP allele at the 9p21 region show altered ANRIL expression and increased susceptibility to atherogenic plaque development and coronary heart disease (CHD) as well as diabetes.54,55 While CDKN2A expression levels were reduced in 9p21 knockout hearts, there was no evidence for cardiac hypertrophy or cardiovascular pathology.56 Other studies also report ANRIL interactions with PcG proteins such as CBX7 and SUZ12 to regulate CDKN2B and CDKN2A gene expression.57,58 Overexpression of ANRIL in cultured cells significantly altered the expression of a large number of distant genes proposing ANRIL as a trans regulatory element.58 Ontology analysis has identified genes involved in the regulation of chromatin structure and function.58
Table 2. Chromatin immunoprecipitation in mouse left ventricle shows specific interaction of EZH2 at genes with bi-directional transcription.
| Ink4a, Ink4b, Ak148321/ANRIL |
| Pax6, Pax6ost1 |
| Nppa, Nppa-as1 |
| Miat, 1700028D13Rik |
| α-MHC, β-MHC, AS β-MHC |
| Foxd2, 9130206I24Rik |
| Hoxc11, Hoxc12, Hotair |
| Gata3, 4930412O13Rik |
| Dio3, Dio3os |
| Ucn, Ucn-as |
| Islr2, 1600029o15Rik |
| Dll4, Gm14207 |
| Pou3f3, 2610017I09Rik |
| 2610100L16Rik, Gm10724 |
| Hoxa4, Hoxa5, Hoxa6, Hoxa7, 2700086A05Rik |
| Irx5, 4933436c20Rik |
| Fbxo44, Fbxo2 |
| Otx2, Otx2os |
| H2-K2, AA388235 |
| Pcnxl2, Bc021891 |
| Dlx6, Dlx6as-1 |
| Tbx2, 2610027K06Rik |
| Myl4 (ALC-1), Myl4-AS |
| cTn1 (Tnnt3), cTn1-AS |
| Tgfβ3, Tgfβ3-AS |
Listed are genes as enriched by ChIP using antibodies that recognize EZH2 and H3K27me3 modification.52 Genes on sense and antisense strands are distinguished by an underline. Significant proportion of the genes enriched by EZH2-ChIP in the mouse heart show specific binding of EZH2 at key cardiac genes with antisense RNA expression. Several cardiac genes with antisense RNA expression including the cardiac regulatory lncRNA genes ANRIL, MIAT, and NPPA-AS appear to be bound by EZH2. Genes encoding non-cardiomyocyte expression programs such as the PAX6, which expresses opposite strand transcript is also repressed by direct binding of EZH2 in the heart. Increased expression of Myosin light chain (MYL4) and TGFβ-3 genes was observed in EZH2 deficient mice,10,52 both of which are known to express regulatory antisense transcripts, however, show no direct association of EZH2 at these promoters.52
Cardiac hypertrophy and heart failure are associated with changes in the expression of α- and β-MHC mRNAs and this shift in myosin-isoform distribution serves important roles in cardiac muscle fiber shortening.59 The silencing of α-MHC in the failing hearts has led renewed interest to restore expression of this gene in hypertrophic tissue.59 The MHC genes are clustered on chromosome 14 in humans and mice (chromosome 15 in rat) and the α- and β- MHC genes are separated by an intergenic sequence of ~4.5 kb in length (Fig. 1).60 The β-MHC gene is upstream of α-MHC and both transcribe mature mRNA approximately 7 kb in length.60 The complexity of MHC gene regulation presents interesting conceptual problems as well as experimental challenges, with the identification of transcripts on opposing DNA strands. This complementary sequence to the canonical mRNA represents the antisense or non-coding RNA.61 The intergenic region of MHC is thought to contain a bdP that transcribes both AS β-MHC and α-MHC in opposite directions.61 Transcription of AS β-MHC progresses in the direction of the β-MHC gene and is thought to regulate the expression of MHC genes in response to pressure overload.61,62
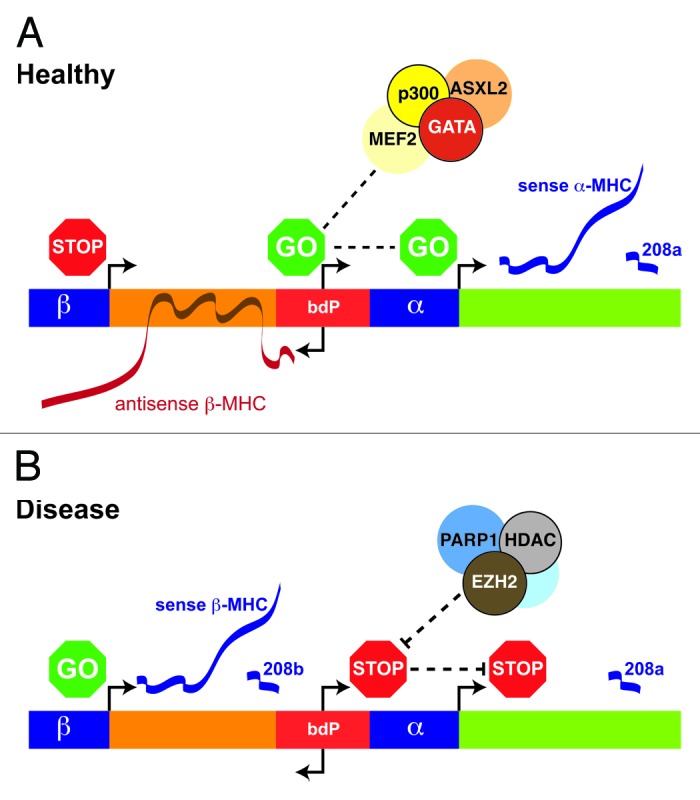
Figure 1. Interplay of chromatin modifications and non-coding RNAs regulate MHC genes in the heart. The expression of cardiac α- and β-MHC genes is regulated in (A) healthy and (B) diseased heart. The bi-directional promoter (bdP) of the α- and β-MHC intergenic region comprises binding sequences for GATA, CTF1/NF1, RAR, T3R, MEF-2 transcription factors. Both the α- and β-MHC genes encode miRNA-208a and miRNA-208b that function in heart health and disease. The bdP is known to transcribe AS β-MHC which serves to regulate β-MHC sense (mRNA) transcription by chromatin interaction. The co-regulatory chromatin determinants BRG1, histone deacetylases (HDACs), and EZH2 are involved in the suppression of AS β-MHC and α-MHC genes in disease.
The regulation of MHC isoforms involves the coordinated actions of core machinery that include DNA transcription factors, chromatin remodeling, and expression of antisense RNA transcripts. Perhaps the most interesting of recent experimental results highlights the complex regulation of the MHC genes includes both transcriptional and post-transcriptional changes. Recent experiments in EZH2 mutant mice reveal changes to MHC isoform regulation characteristic of the hypertrophic heart.10 In addition to H3K27me3 modification by EZH2 is the direct involvement of histone-modifying enzymes such as HDAC9, ASXL2, and chromatin remodeling enzymes, such as BRG1 and PARP1 which interact with the bdP (Fig. 1).11,63 DNase hypersensitive sites are also associated with MHC gene expression at various developmental stages of the heart.64 Whatever the role of EZH2, showing its involvement in chromatin dependent association with ncRNA is the first step in revealing how MHC isoform expression is regulated in heart disease.
Novel ncRNAs in the Heart
Long ncRNA expression recently described in the heart with regulatory roles involving chromatin modification and function is summarized in Table 3. RNA sequencing of the myocardium has revealed specific transcriptome profiles for coding and non-coding transcripts that distinguish the stages of the failing heart.65 Recent studies have identified more than 1300 previously unannotated exons with altered expression levels in animal models of heart failure.65 Among these, almost 682 exons displayed differential expression and the majority (81%) of unannotated RNAs expressed were non-coding RNAs. For example, the expression of H19 lncRNA was highest in heart failure tissue when compared to cardiac hypertrophy. The function of H19 in the myocardium remains poorly characterized, as for human heart explants, transcriptome profiling has shown the expression of putative ncRNAs associated with the development of cardiomyopathy.66 These studies suggest that a large number of novel transcripts are dynamically expressed in the myocardium. Serial analysis of gene expression (SAGE) of different human tissue types has identified cardiac-specific expression of NCRNA00116.67 Despite the tremendous advances in technology used to identify novel RNA species, the physiological function of these molecules remains largely uncharted.68
Table 3. Long ncRNA expression in the heart.
| Long ncRNA | Cardiac function | Disease association | Expression in disease (↑/↓) | Methods of identification | Mechanism of regulation | Splice variants | |
|---|---|---|---|---|---|---|---|
| ANRIL | Regulation of INK4/ARF locus, genes involved in nuclear and chromatin architecture56 | Cardiac hypertrophy, Atherosclerosis | ↑ | RNA-ChIP, RACE-PCR, circRNA assays | Chromatin interaction | Reported | |
| cTnI-AS | Regulation of cTnI mRNA72 | Unknown | Unknown | RACE | RNA duplex formation | None reported | |
| NPPA-AS1 | Regulation of NPPA mRNA68 | Unknown | Unknown | RACE | RNA duplex formation | Reported | |
| AS-UCN | Regulation of sense transcription/translation124 | Unknown | Unknown | RNase Protection Assay | Overlapping sense transcription | None reported | |
| MIAT or Gomafu | Splicing, Retinal cell fate specification125 | Myocardial Infarction | ↑ | Northern blot, RACE | Chromatin interaction / Nanog TF binding | Reported | |
| Fendrr | Cardiac mesoderm formation17 | Unknown | Unknown | RACE, RNA-ChIP, ISH | Chromatin interaction | None reported | |
| MHM | Cardiomyocyte Proliferation126 | Cardiac hypertrophy, arrhythmia | Unknown | Northern blot, In Situ hybridization |
Chromatin interaction | Reported | |
| H19 | Imprinting and Igf2 regulation65 | Hypertrophy & Heart failure | ↑ | RNA-ChIP, Strand-specific PCR | Chromatin interaction | Reported | |
| 91H (AS-H19) | Regulation of Igf2127 | Unknown | Unknown | Strand-specific PCR | Unknown | None reported | |
| Kcnq1ot1 | Embryonic heart formation, Regulation of Cdkn1c, KvLQT1 genes91 | Unknown | Unknown | RACE, FISH, RNA-ChIP | Chromatin interaction | Reported | |
| FMR1-AS1 or FMR4 | Cell proliferation128 | Proposed | Unknown | RACE, Northern blot | Chromatin interaction proposed | Reported | |
| Air | Embryonic heart formation, Imprinting of Igf2r in adult hearts129 | Unknown | Unknown | RNA-ChIP, FISH |
Chromatin interaction | Reported | |
| MLC-ALC-1 antisense | Regulation of MLC-1 mRNA130 | ToF, HOCM | ↑ | Strand-specific PCR | Unknown | None reported | |
| AS-TGFβ3 | Hear chamber formation131 | Unknown | Unknown | RNase protection assay | RISC-mediated silencing proposed | None reported | |
|
sONE (AS-eNOS) |
eNOS synthesis132 | Unknown | Unknown | Strand-specific PCR, In Situ hybridization | Unknown | None reported | |
| SRA | Myogenesis, SRA proteins synthesis133 | DCM | ↓ | Strand-specific PCR, Splice variant assays, RNA-ChIP | Chromatin interaction | Reported | |
| AS β-MHC | β-MHC gene transcription61 | Cardiac hypertrophy | ↓ | Strand-specific PCR | Chromatin interaction | None reported | |
| Braveheart | Cardiovascular lineage commitment16 | Unknown | Unknown | RACE, native RNA-IP | Chromatin interaction | Reported | |
ANRIL, Antisense non-coding RNA in the INK4 locus; cTnI, Cardiac troponin I; NPPA-AS1, natriuretic peptide precursor A-antisense transcript 1; AS-UCN, Urocortin antisense; MIAT, Myocardial Infarction associated transcript; MHM, Male HyperMethylated; MLC-ALC-1, myosin light chain-atrial light chain-1;AS-TGFβ3, Transforming growth factor β-3 antisense RNA; SRA, steroid receptor RNA activator; ToF, tetrology of fallot; HOCM, Hypertrophic obstructive cardiomyopathy; DCM, dilated cardiomyopathy. RACE, Rapid amplification of cDNA ends; FISH, Fluorescent In Situ Hybridization.
Analysis of ncRNA Dependent-Chromatin Interactions
Recent methodological developments in transcript analysis have seen a tremendous amount of information generated from massive parallel sequencing. While historically difficult to ascribe function to the large number of non-coding RNAs, these transcripts are readily identifiable using RNA sequencing approaches. A number of lncRNAs contain chromatin binding domains and other sequences involved in the interactions with proteins as well as regulating gene expression.41,43 In the next section, we discuss some of the methodological developments that have enabled the characterization long ncRNA dependent-chromatin interactions.
Methods Used in the Detection and Characterization of lncRNAs
Important protein-coding genes including those implicated in heart disease have antisense transcription and ncRNA expression.69,70 Conventionally, in first-strand synthesis, complementary DNA (cDNA) is generated at low temperatures (37 °C) using random/oligo-dT primers that are non-specific to gene sequences as well as lacking strand-specific (5’ to 3’ orientation) information. To distinguish sense from antisense, strand-specific oligonucleotides are used to anneal either mRNA (sense) or ncRNA (antisense) at high temperatures (50‒60 oC) followed by first-strand cDNA synthesis. For example, strand-specific primers to cardiac MHC and troponin genes have been used to quantitatively assay sense (mRNA) and antisense (ncRNA) expression in the heart.71,72 Recently, several novel procedures have been developed to quantify strand-specific expression of the transcriptome (Table 4).73,74
Table 4. Methodologies for the detection, characterization and structural analysis of lncRNA. ncRNA-chromatin interaction assays are highlighted with grey background.
| Method | Advantage |
|---|---|
| Strand-specific qRT-PCR | Sense and antisense RNA quantification71,72 |
| ASSAGE | Reveals transcript direction73 |
| RNA ligation using distinct adaptors | Reveals transcript direction74 |
| NET-Seq | Transcriptional pausing87 |
| GRO-Seq | Immediate, transient changes to transcriptome86 |
| Exon-scanning | Splice variant detection68,79 |
| RACE | Splice variant detection, Obtain full-length transcript sequence50,80 |
| RNA CaptureSeq | Detection of transcripts of low abundance, Novel splice variant detection82 |
| BRIC-Seq | Transcript stability, RNA decay32 |
| SAGE (SuperSAGE) | Novel, tissue-specific lncRNA detection67 |
| PolyA- RNA-Seq | Identification of bimorphic transcripts and circular RNAs104 |
| RNA bisulfite conversion | RNA methylation, RNA folding, footprint sequences73 |
| PTES identification | Splice variants, circular RNA prediction109 |
| FragSeq | Intra- and inter- RNA base pairing112 |
| RNaseR assay | Circular transcriptome studies104,107 |
| Native chromatin preparation | Purifies CARs, PolyA- ncRNAs 134 |
| RNA-FISH | Cellular compartmentalization of transcripts, chromatin interaction 89 , 90 |
| RNA-ChIP | Protein-dependent RNA interaction with chromatin 80 |
| Native RNA-ChIP | Protein-dependent RNA interaction with chromatin 92 |
| ChIRP | RNA-dependent chromatin interaction 95 |
| CHART | RNA-dependent chromatin interaction 96 |
| HITS-CLIP | Cross-linking of directly interacting RNA-protein complexes 97 |
| PAR-CLIP | Cross-linking of directly interacting RNA-protein complexes 98 |
ASSAGE, Asymmetric strand specific analysis of gene expression; GRO-Seq, Global Run-on sequencing; NET-Seq, Native elongating transcript sequencing; RACE, Rapid amplification of cDNA ends; BRIC-Seq, 5’-Bromo-uridine Immunoprecipitation chase-deep sequencing; SAGE, Serial analysis of gene expression; PTES, Post-transcriptional exon scrambling; CARs, Chromatin associated RNAs; FISH, Fluorescent In Situ Hybridization; ChIP, Chromatin immunoprecipitation; ChIRP, Chromatin Isolation by RNA purification; CHART, Capture hybridization analysis of RNA targets; HITS-CLIP, High-throughput sequencing of RNA isolated by crosslinking immunoprecipitation; PAR-CLIP, Photoactivatable-Ribonucleoside-Enhanced crosslinking and immunoprecipitation.
Almost 90% of the human transcriptome is alternatively spliced in terminally differentiated cardiomyocytes and neurons.75 RNA splice variants greatly increase biodiversity of proteins.76 For example, distinct alternative splicing of the cardiac steroid receptor activator (SRA) transcript can generate SRA protein-coding transcript as well as non-coding regulatory SRA transcript.77 Consistent with this idea, splice variants in the heart are known to exist for ANRIL and regulate circularization of this transcript, whereby one variant type interacts with EZH2 whilst the other is masked for the EZH2 binding domain.78 Alternative splicing of ncRNA is perhaps key to understanding ncRNA dependent-chromatin interactions. Several strategies, such as exon-scanning and rapid amplification of cDNA ends (RACE) have successfully identified splice variants to cardiac troponin I- and NPPA- antisense transcripts (Table 4).68,79 Other examples of lncRNAs identified include KCNQ1OT1 and HOTTIP.50,80
RNA sequencing (RNA-Seq) approaches generate millions of reads that often fail to accurately identify gene structure as well as result in missing detection of low-abundant transcripts and non-polyadenylated ncRNAs.81 Transcript profiling can be studied using tiling arrays or targeted RNA CaptureSeq (RNA capture sequencing).82,83 For example, Mercer et al.82 used this approach because rare transcripts are thought to be below the detection limits of conventional RNA-Seq. Surprisingly, the study reported complex ncRNA transcription and widespread expression of novel transcripts.82 The authors characterize alternative splice junctions to the HOTAIR transcript predicted to interfere with PcG binding.82 Taken together, these data suggest that post-transcriptional splicing can regulate ncRNA dependent-chromatin binding.
Protein expression may also be determined by RNA stability and recent experimental observations suggest dynamic regulation of ncRNA stability in response to specific environmental cues. Pulse labeling of RNA followed by sequencing or 5'-bromo-uridine immunoprecipitation chase–deep sequencing analysis (BRIC-Seq) has identified novel and highly stable lncRNAs.32 This technique is used to study RNA decay and has revealed that some lncRNAs infact have short half-lives (t1/2 < 4 h) such as the cardiac ANRIL transcript, HOTAIR, TUG1, and GAS5. Other intriguing observations from the study highlighted that hundreds of short-lived regulatory RNAs designated as short-lived non-coding transcripts (SLiTs) have putative roles in nuclear function.32 An alternative method of studying RNA stability is transcriptional inhibition by Actinomycin D (ActD).84 Mouse neuroblastoma cells exposed to ActD over a 32 h period identified over 800 lncRNAs and 12 000 mRNAs that were classified highly stable with a half-life > 16 h or low stability with a half-life < 2 h.85 The regulatory RNA, NEAT1 was identified as one of the least stable ncRNAs which is thought to be dynamically regulated. Similarly, global run-on sequencing (GRO-Seq) and native elongating transcript sequencing (NET-Seq) techniques have been be used to assay nascent RNA transcripts.86,87 These studies identified immediate transcriptional response to estrogen signaling demonstrating that lncRNAs are dynamically regulated.86 The most obvious conclusion is that low stability lncRNAs are non-functional, but this argument is perhaps overly simplistic, when interpreted slightly differently, long non-coding RNAs may act immediately after transcription to mediate chromatin-dependent interactions.
Long ncRNA-Chromatin Interaction Assays
Long ncRNAs that stably interact with chromatin at specific genomic sites can be detected by fluorescent in situ hybridization (FISH) of the target RNA using antisense probes.88 FISH has traditionally been the method of choice to study long ncRNA dependent-chromatin interactions.89,90 More recently, FISH was employed to assay changes in chromatin architecture for KCNQ1OT1 a lncRNA that regulates KCNQ1 expression in the developing heart.91 Alternatively, locus-specific lncRNA interactions can be examined using formaldehyde fixation and chromatin immunoprecipitation methods (RNA-ChIP) that use antibodies that recognize RNA-binding proteins such as EZH2 and G9A.80 Alternatively, native RNA-ChIP using MNase digestion have also been successfully applied to the study of chromatin associated RNAs.92 In striking contrast to formaldehyde crosslinking, immunoprecipitation of native soluble chromatin allows for direct mapping of mono-, di- and tri-nucleosomal structures.93
Long ncRNAs can interact in a locus-specific manner using homologous complementary sequences.94 The applicability of biotinylated RNA tiling probes complementary to target lncRNA was recently used to immunoprecipitate interacting DNA sequences and proteins. Examples of these methods include ChIRP (chromatin isolation by RNA purification) and CHART (chromatin hybridization analysis of RNA targets) which have identified novel genome-wide interactions for HOTAIR and ROX2.95,96 Infact, with the advent of high-throughput sequencing it has been possible to identify novel RNAs using crosslinking immunoprecipitation (HITS-CLIP) and photoactivatable ribonucleoside enhanced crosslinking and immunoprecipitation (PAR-CLIP).97,98 These methodologies were recently used to identify the interaction of EZH2 with several ncRNAs, including ANRIL which has been associated with many diseases including coronary artery disease, diabetes and cancer.99
Structural Analysis of lncRNAs
Besides sequence-based chromatin recognition, RNA folding can also influence ncRNA dependent-chromatin interactions.100 For example, genes that code for DMD, P450, MLL, and ETS-1 are circular transcripts with diverse functions.101-104 The hypertrophy responsive NCX1 gene is thought to produce circular poly(A-) transcripts in the human heart, however the biological significance of circular RNAs has remained elusive.105 Recent evidence now suggests that circular RNAs function as miRNA sponges that compete with RNA binding proteins to form a class of post-transcriptional regulators.106,107 Accordingly, circular antisense RNAs are targeted by RISC components for gene regulation.108 The mechanism of RNA circularization is a result of non-canonical post-transcriptional exon scrambling (PTES). Non-canonical PTES appears to be a predominant event in human liver as well as the heart.109 Because of their low abundance, the majority of circular transcripts are largely undetectable by conventional RNA-sequencing. To investigate the circular component of the transcriptome, protocols employ RNaseR, an enzyme that degrades linear but not circular transcripts.104 Coupled with RNaseR, next generation sequencing has identified PTES mediated circular RNA transcripts to hundreds of human genes, the majority of which were not polyadenylated.104 Infact, circular and linear forms of cardiac antisense RNA, ANRIL have been reported.78 The expression of circular ANRIL might be associated with atherosclerotic vascular disease. Thousands of human mRNA and ncRNA transcripts are extensively methylated110 and these RNA modifications are thought to alter Argonaute binding as well as transcript folding.111 Moreover, recent identification of specific ncRNA structures such as the TINCR boxes regulate the interaction of these transcripts with regulatory proteins.100 FragSeq or fragmentation sequencing is a novel method that integrates RNA structure analysis with genome-wide sequencing.112 The Nuclease P1 enzyme is used to cleave single-stranded nucleic acids thereby preserving the intra- and inter-molecular RNA interactions. The development of these methodologies has revolutionized genome-wide analysis of cellular RNAs , which will be critical in defining regulatory networks at the genomic scale.113
Conclusions and Future Considerations
Recent experimental observations show lncRNAs regulate cardiac gene expression. This is probably best exemplified at the bidirectional promoter of the MHC genes which involves the interaction of EZH2 with the antisense β-MHC transcript to regulate MHC isoform switch (Fig. 1). While always considered to be integral elements in the post-transcriptional control of gene expression it is the recent technological developments that have been critical to understand the role of ncRNAs in the heart. The advent of massive parallel sequencing has brought improved understanding of the regulatory mechanisms underlying cardiac pathology and developmental growth as well as integrating functional genomics. Although the relevance of the non-coding genome to cardiac disease has mainly been studied in the context of the widespread disruption of expression, studies now show that ncRNAs are also critical determinants of gene regulation. Taken together with their emerging role with chromatin modification, the non-coding genome should provide new strategies and specific targets to prevent, restore or reverse the effects of pathological hypertrophy in the failing heart.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge grant and fellowship support from the National Health and Medical Research Council (NHMRC) and the National Heart Foundation of Australia (NHF). Mathiyalagan P was awarded a Monash Graduate Scholarship and El-Osta A and Du X-J are Senior Research Fellows of the NHMRC. Supported in part by the Victorian Government’s Operational Infrastructure Support program.
Glossary
Abbreviations:
- EZH2
enhancer of zeste homolog 2
- ncRNA
non-coding RNA
- lncRNA
long non-coding RNA
- MHC
myosin heavy chain
- HDAC
histone deacetylase
- HAT
histone acetyltransferase
- miRNA
MicroRNA
- NAT
natural antisense transcript
- PcG
polycomb-group
- AS
antisense
- SET
Su(var)3-9 and enhancer of zeste
- bdP
bi-directional promoter
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/26405
References
- 1.Wamstad JA, Alexander JM, Truty RM, Shrikumar A, Li F, Eilertson KE, Ding H, Wylie JN, Pico AR, Capra JA, et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151:206–20. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R, Pabon L, Reinecke H, Pratt G, Keller G, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–32. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–35. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Kiriazis H, Gao XM, Du XJ, El-Osta A. Cardiac genes show contextual SWI/SNF interactions with distinguishable gene activities. Epigenetics. 2011;6:760–8. doi: 10.4161/epi.6.6.16007. [DOI] [PubMed] [Google Scholar]
- 8.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 9.Mathiyalagan P, Chang L, Du XJ, El-Osta A. Cardiac ventricular chambers are epigenetically distinguishable. Cell Cycle. 2010;9:612–7. doi: 10.4161/cc.9.3.10612. [DOI] [PubMed] [Google Scholar]
- 10.Delgado-Olguín P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG, Tarakhovsky A, Bruneau BG. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat Genet. 2012;44:343–7. doi: 10.1038/ng.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai HL, Grachoff M, McGinley AL, Khan FF, Warren CM, Chowdhury SA, Wolska BM, Solaro RJ, Geenen DL, Wang QT. Maintenance of adult cardiac function requires the chromatin factor Asxl2. J Mol Cell Cardiol. 2012;53:734–41. doi: 10.1016/j.yjmcc.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han P, Hang CT, Yang J, Chang CP. Chromatin remodeling in cardiovascular development and physiology. Circ Res. 2011;108:378–96. doi: 10.1161/CIRCRESAHA.110.224287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguín P, Holloway AK, Mori AD, Wylie JN, Munson C, Zhu Y, et al. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun. 2011;2:187. doi: 10.1038/ncomms1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonrock N, Harvey RP, Mattick JS. Long noncoding RNAs in cardiac development and pathophysiology. Circ Res. 2012;111:1349–62. doi: 10.1161/CIRCRESAHA.112.268953. [DOI] [PubMed] [Google Scholar]
- 15.Luther HP. Role of endogenous antisense RNA in cardiac gene regulation. J Mol Med (Berl) 2005;83:26–32. doi: 10.1007/s00109-004-0613-5. [DOI] [PubMed] [Google Scholar]
- 16.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–83. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–14. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattick JS. RNA as the substrate for epigenome-environment interactions: RNA guidance of epigenetic processes and the expansion of RNA editing in animals underpins development, phenotypic plasticity, learning, and cognition. Bioessays. 2010;32:548–52. doi: 10.1002/bies.201000028. [DOI] [PubMed] [Google Scholar]
- 19.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 20.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–7. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 21.Clark MB, Amaral PP, Schlesinger FJ, Dinger ME, Taft RJ, Rinn JL, Ponting CP, Stadler PF, Morris KV, Morillon A, et al. The reality of pervasive transcription. PLoS Biol. 2011;9:e1000625–, discussion e1001102. doi: 10.1371/journal.pbio.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 23.Belotserkovskii BP, De Silva E, Tornaletti S, Wang G, Vasquez KM, Hanawalt PC. A triplex-forming sequence from the human c-MYC promoter interferes with DNA transcription. J Biol Chem. 2007;282:32433–32441. doi: 10.1074/jbc.M704618200. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz KM, Mayer C, Postepska A, Grummt I. Interaction of noncoding RNA with the rDNA promoter mediates recruitment of DNMT3b and silencing of rRNA genes. Genes Dev . 2010;24:2264–2269. doi: 10.1101/gad.590910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 26.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–62. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–6. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlesinger J, Schueler M, Grunert M, Fischer JJ, Zhang Q, Krueger T, Lange M, Tönjes M, Dunkel I, Sperling SR. The cardiac transcription network modulated by Gata4, Mef2a, Nkx2.5, Srf, histone modifications, and microRNAs. PLoS Genet. 2011;7:e1001313. doi: 10.1371/journal.pgen.1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–17. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 30.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–9. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 31.Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, Gardiner BB, Askarian-Amiri ME, Ru K, Soldà G, Simons C, et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–45. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tani H, Mizutani R, Salam KA, Tano K, Ijiri K, Wakamatsu A, Isogai T, Suzuki Y, Akimitsu N. Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Res. 2012;22:947–56. doi: 10.1101/gr.130559.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko S, Li G, Son J, Xu CF, Margueron R, Neubert TA, Reinberg D. Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA. Genes Dev. 2010;24:2615–20. doi: 10.1101/gad.1983810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107:555–9. doi: 10.1002/jso.23264. [DOI] [PubMed] [Google Scholar]
- 35.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt LH, Spieker T, Koschmieder S, Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–92. doi: 10.1097/JTO.0b013e3182307eac. [RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth.] [DOI] [PubMed] [Google Scholar]
- 38.Hu W, Yuan B, Flygare J, Lodish HF. Long noncoding RNA-mediated anti-apoptotic activity in murine erythroid terminal differentiation. Genes Dev. 2011;25:2573–8. doi: 10.1101/gad.178780.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, 3rd, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–30. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung DW, Rudnicki DD, Yu L, Margolis RL. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum Mol Genet. 2011;20:3467–77. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanhere A, Jenner RG. Noncoding RNA localisation mechanisms in chromatin regulation. Silence. 2012;3:2. doi: 10.1186/1758-907X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benetatos L, Voulgaris E, Vartholomatos G, Hatzimichael E. Non-coding RNAs and EZH2 interactions in cancer: long and short tales from the transcriptome. Int J Cancer. 2013;133:267–74. doi: 10.1002/ijc.27859. [DOI] [PubMed] [Google Scholar]
- 45.Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–20. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- 46.Pagans S, Kauder SE, Kaehlcke K, Sakane N, Schroeder S, Dormeyer W, Trievel RC, Verdin E, Schnolzer M, Ott M. The Cellular lysine methyltransferase Set7/9-KMT7 binds HIV-1 TAR RNA, monomethylates the viral transactivator Tat, and enhances HIV transcription. Cell Host Microbe. 2010;7:234–44. doi: 10.1016/j.chom.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krajewski WA, Nakamura T, Mazo A, Canaani E. A motif within SET-domain proteins binds single-stranded nucleic acids and transcribed and supercoiled DNAs and can interfere with assembly of nucleosomes. Mol Cell Biol. 2005;25:1891–9. doi: 10.1128/MCB.25.5.1891-1899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu S, Wu J, Sun B, Zhong C, Ding J. Structural and biochemical studies of human lysine methyltransferase Smyd3 reveal the important functional roles of its post-SET and TPR domains and the regulation of its activity by DNA binding. Nucleic Acids Res. 2011;39:4438–49. doi: 10.1093/nar/gkr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–4. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou DC, Cahan P, et al. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res. 2012;110:406–15. doi: 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–62. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, Green F, Clarke R, Collins R, Franzosi MG, Tognoni G, et al. PROCARDIS consortium Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet. 2008;17:806–14. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 55.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–91. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, Blow MJ, Cohen JC, Rubin EM, Pennacchio LA. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–12. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–74. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato K, Nakagawa H, Tajima A, Yoshida K, Inoue I. ANRIL is implicated in the regulation of nucleus and potential transcriptional target of E2F1. Oncol Rep. 2010;24:701–7. doi: 10.3892/or_00000910. [DOI] [PubMed] [Google Scholar]
- 59.Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004;44:2390–7. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 60.Mahdavi V, Chambers AP, Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984;81:2626–30. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haddad F, Qin AX, Bodell PW, Zhang LY, Guo H, Giger JM, Baldwin KM. Regulation of antisense RNA expression during cardiac MHC gene switching in response to pressure overload. Am J Physiol Heart Circ Physiol. 2006;290:H2351–61. doi: 10.1152/ajpheart.01111.2005. [DOI] [PubMed] [Google Scholar]
- 62.Haddad F, Jiang W, Bodell PW, Qin AX, Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol. 2010;299:H1968–80. doi: 10.1152/ajpheart.00644.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, Zhou B, Chang CP. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–7. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang WY, Liew CC. A conserved GATA motif in a tissue-specific DNase I hypersensitive site of the cardiac alpha-myosin heavy chain gene. Biochem J. 1997;325:47–51. doi: 10.1042/bj3250047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JH, Gao C, Peng G, Greer C, Ren S, Wang Y, Xiao X. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ Res. 2011;109:1332–41. doi: 10.1161/CIRCRESAHA.111.249433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S, Down T, Siggens L, Vujic A, Simeoni I, Penkett C, et al. Distinct epigenomic features in end-stage failing human hearts. Circulation. 2011;124:2411–22. doi: 10.1161/CIRCULATIONAHA.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gibb EA, Vucic EA, Enfield KS, Stewart GL, Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S, Brown CJ, et al. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6:e25915. doi: 10.1371/journal.pone.0025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Annilo T, Kepp K, Laan M. Natural antisense transcript of natriuretic peptide precursor A (NPPA): structural organization and modulation of NPPA expression. BMC Mol Biol. 2009;10:81. doi: 10.1186/1471-2199-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katayama S, Tomaru Y, Kasukawa T, Waki K, Nakanishi M, Nakamura M, Nishida H, Yap CC, Suzuki M, Kawai J, et al. RIKEN Genome Exploration Research Group. Genome Science Group (Genome Network Project Core Group) FANTOM Consortium Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–6. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 70.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–8. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haddad F, Qin AX, Giger JM, Guo H, Baldwin KM. Potential pitfalls in the accuracy of analysis of natural sense-antisense RNA pairs by reverse transcription-PCR. BMC Biotechnol. 2007;7:21. doi: 10.1186/1472-6750-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voigtsberger S, Bartsch H, Baumann G, Luther HP. Cell type-specific expression of endogenous cardiac Troponin I antisense RNA in the neonatal rat heart. Mol Cell Biochem. 2009;324:1–11. doi: 10.1007/s11010-008-9974-3. [DOI] [PubMed] [Google Scholar]
- 73.He Y, Vogelstein B, Velculescu VE, Papadopoulos N, Kinzler KW. The antisense transcriptomes of human cells. Science. 2008;322:1855–7. doi: 10.1126/science.1163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levin JZ, Yassour M, Adiconis X, Nusbaum C, Thompson DA, Friedman N, Gnirke A, Regev A. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods. 2010;7:709–15. doi: 10.1038/nmeth.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–6. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mironov AA, Fickett JW, Gelfand MS. Frequent alternative splicing of human genes. Genome Res. 1999;9:1288–93. doi: 10.1101/gr.9.12.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chooniedass-Kothari S, Emberley E, Hamedani MK, Troup S, Wang X, Czosnek A, Hube F, Mutawe M, Watson PH, Leygue E. The steroid receptor RNA activator is the first functional RNA encoding a protein. FEBS Lett. 2004;566:43–7. doi: 10.1016/j.febslet.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 78.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartsch H, Voigtsberger S, Baumann G, Morano I, Luther HP. Detection of a novel sense-antisense RNA-hybrid structure by RACE experiments on endogenous troponin I antisense RNA. RNA. 2004;10:1215–24. doi: 10.1261/rna.5261204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D, Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32:232–46. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 81.van der Brug M, Nalls MA, Cookson MR. Deep sequencing of coding and non-coding RNA in the CNS. Brain Res. 2010;1338:146–54. doi: 10.1016/j.brainres.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mercer TR, Gerhardt DJ, Dinger ME, Crawford J, Trapnell C, Jeddeloh JA, Mattick JS, Rinn JL. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol. 2012;30:99–104. doi: 10.1038/nbt.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kampa D, Cheng J, Kapranov P, Yamanaka M, Brubaker S, Cawley S, Drenkow J, Piccolboni A, Bekiranov S, Helt G, et al. Novel RNAs identified from an in-depth analysis of the transcriptome of human chromosomes 21 and 22. Genome Res. 2004;14:331–42. doi: 10.1101/gr.2094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hurwitz J, Furth JJ, Anders M, Evans A. The role of deoxyribonucleic acid in ribonucleic acid synthesis. II. The influence of deoxyribonucleic acid on the reaction. J Biol Chem. 1962;237:3752–9. [PubMed] [Google Scholar]
- 85.Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, Moscato P, Dinger ME, Mattick JS. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–98. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hah N, Danko CG, Core L, Waterfall JJ, Siepel A, Lis JT, Kraus WL. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145:622–34. doi: 10.1016/j.cell.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Churchman LS, Weissman JS. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature. 2011;469:368–73. doi: 10.1038/nature09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levsky JM, Singer RH. Fluorescence in situ hybridization: past, present and future. J Cell Sci. 2003;116:2833–8. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- 89.Chureau C, Chantalat S, Romito A, Galvani A, Duret L, Avner P, Rougeulle C. Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet. 2011;20:705–18. doi: 10.1093/hmg/ddq516. [DOI] [PubMed] [Google Scholar]
- 90.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korostowski L, Sedlak N, Engel N. The Kcnq1ot1 long non-coding RNA affects chromatin conformation and expression of Kcnq1, but does not regulate its imprinting in the developing heart. PLoS Genet. 2012;8:e1002956. doi: 10.1371/journal.pgen.1002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mondal T, Rasmussen M, Pandey GK, Isaksson A, Kanduri C. Characterization of the RNA content of chromatin. Genome Res. 2010;20:899–907. doi: 10.1101/gr.103473.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gregory RI, Randall TE, Johnson CA, Khosla S, Hatada I, O’Neill LP, Turner BM, Feil R. DNA methylation is linked to deacetylation of histone H3, but not H4, on the imprinted genes Snrpn and U2af1-rs1. Mol Cell Biol. 2001;21:5426–36. doi: 10.1128/MCB.21.16.5426-5436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zappulla DC, Cech TR. RNA as a flexible scaffold for proteins: yeast telomerase and beyond. Cold Spring Harb Symp Quant Biol. 2006;71:217–24. doi: 10.1101/sqb.2006.71.011. [DOI] [PubMed] [Google Scholar]
- 95.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44:667–78. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simon MD, Wang CI, Kharchenko PV, West JA, Chapman BA, Alekseyenko AA, Borowsky ML, Kuroda MI, Kingston RE. The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A. 2011;108:20497–502. doi: 10.1073/pnas.1113536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–5. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 98.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr., Jungkamp AC, Munschauer M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–41. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guil S, Soler M, Portela A, Carrère J, Fonalleras E, Gómez A, Villanueva A, Esteller M. Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nat Struct Mol Biol. 2012;19:664–70. doi: 10.1038/nsmb.2315. [DOI] [PubMed] [Google Scholar]
- 100.Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, Lee CS, Flockhart RJ, Groff AF, Chow J, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–5. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–40. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 102.Surono A, Takeshima Y, Wibawa T, Ikezawa M, Nonaka I, Matsuo M. Circular dystrophin RNAs consisting of exons that were skipped by alternative splicing. Hum Mol Genet. 1999;8:493–500. doi: 10.1093/hmg/8.3.493. [DOI] [PubMed] [Google Scholar]
- 103.Zaphiropoulos PG. Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Mol Cell Biol. 1997;17:2985–93. doi: 10.1128/mcb.17.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li XF, Lytton J. A circularized sodium-calcium exchanger exon 2 transcript. J Biol Chem. 1999;274:8153–60. doi: 10.1074/jbc.274.12.8153. [DOI] [PubMed] [Google Scholar]
- 106.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 107.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 108.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–22. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al-Balool HH, Weber D, Liu Y, Wade M, Guleria K, Nam PL, Clayton J, Rowe W, Coxhead J, Irving J, et al. Post-transcriptional exon shuffling events in humans can be evolutionarily conserved and abundant. Genome Res. 2011;21:1788–99. doi: 10.1101/gr.116442.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–33. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Motorin Y, Helm M. RNA nucleotide methylation. Wiley Interdiscip Rev RNA. 2011;2:611–31. doi: 10.1002/wrna.79. [DOI] [PubMed] [Google Scholar]
- 112.Underwood JG, Uzilov AV, Katzman S, Onodera CS, Mainzer JE, Mathews DH, Lowe TM, Salama SR, Haussler D. FragSeq: transcriptome-wide RNA structure probing using high-throughput sequencing. Nat Methods. 2010;7:995–1001. doi: 10.1038/nmeth.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Westhof E, Romby P. The RNA structurome: high-throughput probing. Nat Methods. 2010;7:965–7. doi: 10.1038/nmeth1210-965. [DOI] [PubMed] [Google Scholar]
- 114.Benhamed M, Herbig U, Ye T, Dejean A, Bischof O. Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nat Cell Biol. 2012;14:266–75. doi: 10.1038/ncb2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–92. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 116.Huang XA, Yin H, Sweeney S, Raha D, Snyder M, Lin H. A major epigenetic programming mechanism guided by piRNAs. Dev Cell. 2013;24:502–16. doi: 10.1016/j.devcel.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schubert T, Pusch MC, Diermeier S, Benes V, Kremmer E, Imhof A, Längst G. Df31 protein and snoRNAs maintain accessible higher-order structures of chromatin. Mol Cell. 2012;48:434–44. doi: 10.1016/j.molcel.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 118.Han J, Kim D, Morris KV. Promoter-associated RNA is required for RNA-directed transcriptional gene silencing in human cells. Proc Natl Acad Sci U S A. 2007;104:12422–7. doi: 10.1073/pnas.0701635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Oude Vrielink JA, Elkon R, Melo SA, Léveillé N, Kalluri R, et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell. 2013;49:524–35. doi: 10.1016/j.molcel.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 120.Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grandér D, Morris KV. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol. 2013;20:440–6. doi: 10.1038/nsmb.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hawkins PG, Morris KV. Transcriptional regulation of Oct4 by a long non-coding RNA antisense to Oct4-pseudogene 5. Transcription. 2010;1:165–75. doi: 10.4161/trns.1.3.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bond AM, Vangompel MJ, Sametsky EA, Clark MF, Savage JC, Disterhoft JF, Kohtz JD. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12:1020–7. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Horard B, Gilson E. Telomeric RNA enters the game. Nat Cell Biol. 2008;10:113–5. doi: 10.1038/ncb0208-113. [DOI] [PubMed] [Google Scholar]
- 124.Haeger P, Cuevas R, Forray MI, Rojas R, Daza C, Rivadeneira J, Gysling K. Natural expression of immature Ucn antisense RNA in the rat brain. Evidence favoring bidirectional transcription of the Ucn gene locus. Brain Res Mol Brain Res. 2005;139:115–28. doi: 10.1016/j.molbrainres.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 125.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087–99. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 126.Roeszler KN, Itman C, Sinclair AH, Smith CA. The long non-coding RNA, MHM, plays a role in chicken embryonic development, including gonadogenesis. Dev Biol. 2012;366:317–26. doi: 10.1016/j.ydbio.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 127.Tran VG, Court F, Duputié A, Antoine E, Aptel N, Milligan L, Carbonell F, Lelay-Taha MN, Piette J, Weber M, et al. H19 antisense RNA can up-regulate Igf2 transcription by activation of a novel promoter in mouse myoblasts. PLoS One. 2012;7:e37923. doi: 10.1371/journal.pone.0037923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khalil AM, Faghihi MA, Modarresi F, Brothers SP, Wahlestedt C. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS One. 2008;3:e1486. doi: 10.1371/journal.pone.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–3. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 130.Ritter O, Luther HP, Haase H, Baltas LG, Baumann G, Schulte HD, Morano I. Expression of atrial myosin light chains but not alpha-myosin heavy chains is correlated in vivo with increased ventricular function in patients with hypertrophic obstructive cardiomyopathy. J Mol Med (Berl) 1999;77:677–85. doi: 10.1007/s001099900030. [DOI] [PubMed] [Google Scholar]
- 131.Potts JD, Vincent EB, Runyan RB, Weeks DL. Sense and antisense TGF beta 3 mRNA levels correlate with cardiac valve induction. Dev Dyn. 1992;193:340–5. doi: 10.1002/aja.1001930407. [DOI] [PubMed] [Google Scholar]
- 132.Robb GB, Carson AR, Tai SC, Fish JE, Singh S, Yamada T, Scherer SW, Nakabayashi K, Marsden PA. Post-transcriptional regulation of endothelial nitric-oxide synthase by an overlapping antisense mRNA transcript. J Biol Chem. 2004;279:37982–96. doi: 10.1074/jbc.M400271200. [DOI] [PubMed] [Google Scholar]
- 133.Friedrichs F, Zugck C, Rauch GJ, Ivandic B, Weichenhan D, Müller-Bardorff M, Meder B, El Mokhtari NE, Regitz-Zagrosek V, Hetzer R, et al. HBEGF, SRA1, and IK: Three cosegregating genes as determinants of cardiomyopathy. Genome Res. 2009;19:395–403. doi: 10.1101/gr.076653.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodríguez-Campos A, Azorín F. RNA is an integral component of chromatin that contributes to its structural organization. PLoS One. 2007;2:e1182. doi: 10.1371/journal.pone.0001182. [DOI] [PMC free article] [PubMed] [Google Scholar]


