Abstract
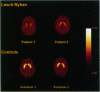
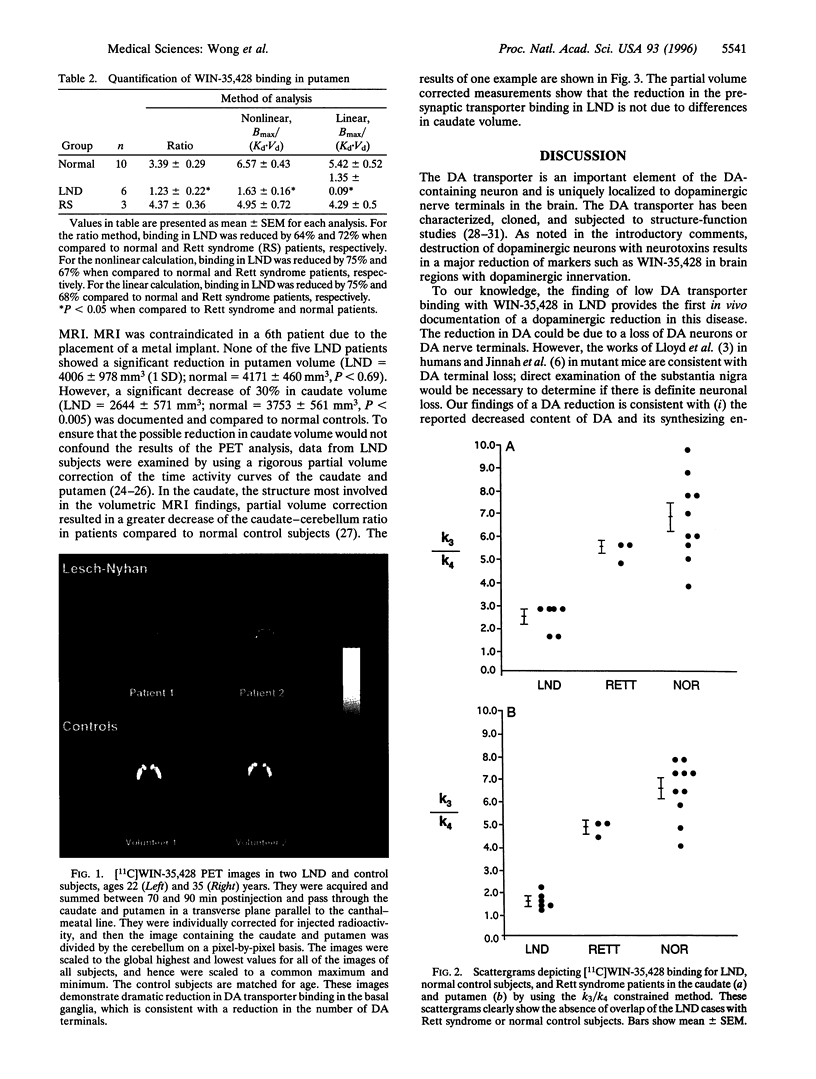
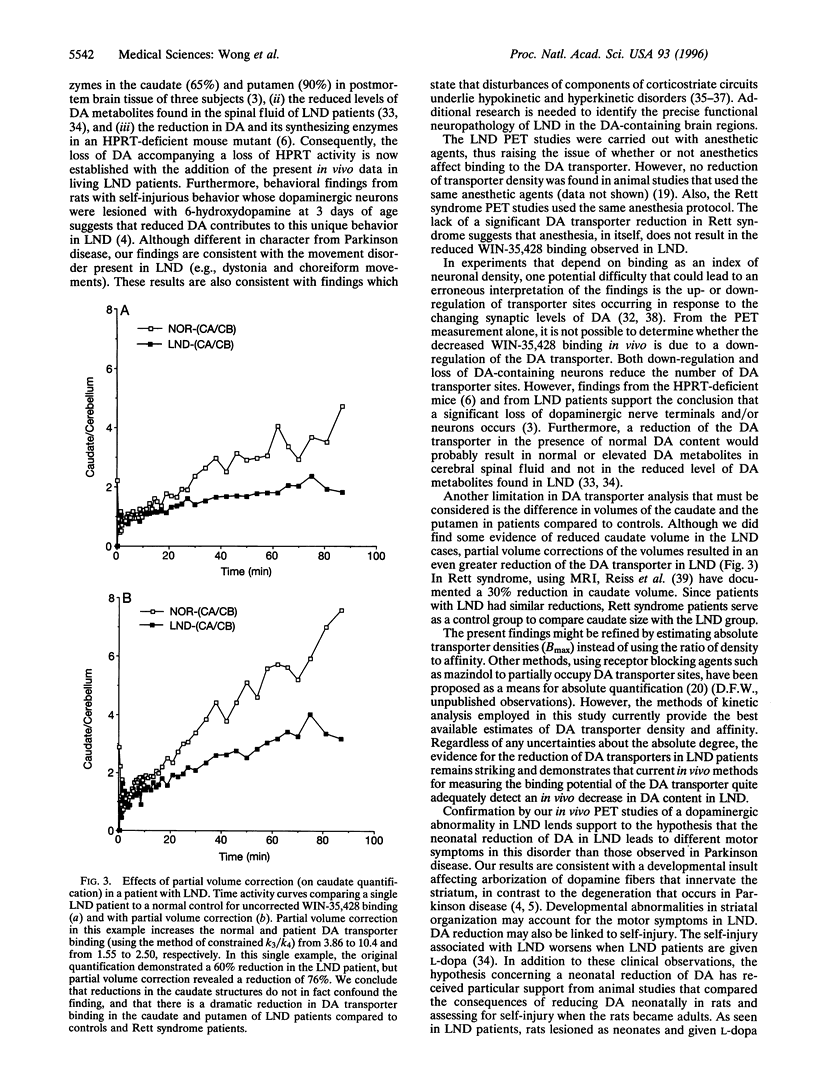
Dopamine (DA) deficiency has been implicated in Lesch-Nyhan disease (LND), a genetic disorder that is characterized by hyperuricemia, choreoathetosis, dystonia, and compulsive self-injury. To establish that DA deficiency is present in LND, the ligand WIN-35,428, which binds to DA transporters, was used to estimate the density of DA-containing neurons in the caudate and putamen of six patients with classic LND. Comparisons were made with 10 control subjects and 3 patients with Rett syndrome. Three methods were used to quantify the binding of the DA transporter so that its density could be estimated by a single dynamic positron emission tomography study. These approaches included the caudate- or putamen-to-cerebellum ratio of ligand at 80-90 min postinjection, kinetic analysis of the binding potential [Bmax/(Kd x Vd)] using the assumption of equal partition coefficients in the striatum and the cerebellum, and graphical analysis of the binding potential. Depending on the method of analysis, a 50-63% reduction of the binding to DA transporters in the caudate, and a 64-75% reduction in the putamen of the LND patients was observed compared to the normal control group. When LND patients were compared to Rett syndrome patients, similar reductions were found in the caudate (53-61%) and putamen (67-72%) in LND patients. Transporter binding in Rett syndrome patients was not significantly different from the normal controls. Finally, volumetric magnetic resonance imaging studies detected a 30% reduction in the caudate volume of LND patients. To ensure that a reduction in the caudate volume would not confound the results, a rigorous partial volume correction of the caudate time activity curve was performed. This correction resulted in an even greater decrease in the caudate-cerebellar ratio in LND patients when contrasted to controls. To our knowledge, these findings provide the first in vivo documentation of a dopaminergic reduction in LND and illustrate the role of positron emission tomography imaging in investigating neurodevelopmental disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. E., DeLong M. R., Strick P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Breese G. R., Baumeister A. A., McCown T. J., Emerick S. G., Frye G. D., Crotty K., Mueller R. A. Behavioral differences between neonatal and adult 6-hydroxydopamine-treated rats to dopamine agonists: relevance to neurological symptoms in clinical syndromes with reduced brain dopamine. J Pharmacol Exp Ther. 1984 Nov;231(2):343–354. [PMC free article] [PubMed] [Google Scholar]
- Breese G. R., Criswell H. E., Duncan G. E., Mueller R. A. A dopamine deficiency model of Lesch-Nyhan disease--the neonatal-6-OHDA-lesioned rat. Brain Res Bull. 1990 Sep;25(3):477–484. doi: 10.1016/0361-9230(90)90240-z. [DOI] [PubMed] [Google Scholar]
- Cerruti C., Pilotte N. S., Uhl G., Kuhar M. J. Reduction in dopamine transporter mRNA after cessation of repeated cocaine administration. Brain Res Mol Brain Res. 1994 Mar;22(1-4):132–138. doi: 10.1016/0169-328x(94)90040-x. [DOI] [PubMed] [Google Scholar]
- DeLong M. R., Alexander G. E., Mitchell S. J., Richardson R. T. The contribution of basal ganglia to limb control. Prog Brain Res. 1986;64:161–174. doi: 10.1016/S0079-6123(08)63411-1. [DOI] [PubMed] [Google Scholar]
- DeLong M. R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990 Jul;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Frost J. J., Rosier A. J., Reich S. G., Smith J. S., Ehlers M. D., Snyder S. H., Ravert H. T., Dannals R. F. Positron emission tomographic imaging of the dopamine transporter with 11C-WIN 35,428 reveals marked declines in mild Parkinson's disease. Ann Neurol. 1993 Sep;34(3):423–431. doi: 10.1002/ana.410340331. [DOI] [PubMed] [Google Scholar]
- Giros B., el Mestikawy S., Bertrand L., Caron M. G. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991 Dec 16;295(1-3):149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a re-examination. Brain Res. 1982 Jun;257(2):237–274. doi: 10.1016/0165-0173(82)90018-2. [DOI] [PubMed] [Google Scholar]
- Hantraye P., Brownell A. L., Elmaleh D., Spealman R. D., Wüllner U., Brownell G. L., Madras B. K., Isacson O. Dopamine fiber detection by [11C]-CFT and PET in a primate model of parkinsonism. Neuroreport. 1992 Mar;3(3):265–268. doi: 10.1097/00001756-199203000-00013. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Seibyl J. P., Scanley B. E., Laruelle M., Abi-Dargham A., Wallace E., Baldwin R. M., Zea-Ponce Y., Zoghbi S., Wang S. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11965–11969. doi: 10.1073/pnas.90.24.11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J., Caskey T. C., Stout J. T., Butler I. J. Lesch-Nyhan syndrome: a study of motor behavior and cerebrospinal fluid neurotransmitters. Ann Neurol. 1988 May;23(5):466–469. doi: 10.1002/ana.410230507. [DOI] [PubMed] [Google Scholar]
- Jinnah H. A., Wojcik B. E., Hunt M., Narang N., Lee K. Y., Goldstein M., Wamsley J. K., Langlais P. J., Friedmann T. Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. J Neurosci. 1994 Mar;14(3 Pt 1):1164–1175. doi: 10.1523/JNEUROSCI.14-03-01164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. J., Madras B. K. Distribution of cocaine recognition sites in monkey brain: II. Ex vivo autoradiography with [3H]CFT and [125I]RTI-55. Synapse. 1992 Oct;12(2):99–111. doi: 10.1002/syn.890120203. [DOI] [PubMed] [Google Scholar]
- Kilty J. E., Lorang D., Amara S. G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991 Oct 25;254(5031):578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J., Sanchez-Roa P. M., Wong D. F., Dannals R. F., Grigoriadis D. E., Lew R., Milberger M. Dopamine transporter: biochemistry, pharmacology and imaging. Eur Neurol. 1990;30 (Suppl 1):15–20. doi: 10.1159/000117169. [DOI] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- Lekman A., Witt-Engerström I., Gottfries J., Hagberg B. A., Percy A. K., Svennerholm L. Rett syndrome: biogenic amines and metabolites in postmortem brain. Pediatr Neurol. 1989 Nov-Dec;5(6):357–362. doi: 10.1016/0887-8994(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Lloyd K. G., Hornykiewicz O., Davidson L., Shannak K., Farley I., Goldstein M., Shibuya M., Kelley W. N., Fox I. H. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981 Nov 5;305(19):1106–1111. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- Logan J., Fowler J. S., Volkow N. D., Wolf A. P., Dewey S. L., Schlyer D. J., MacGregor R. R., Hitzemann R., Bendriem B., Gatley S. J. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990 Sep;10(5):740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Madras B. K. 11C-WIN 35,428 for detecting dopamine depletion in mild Parkinson's disease. Ann Neurol. 1994 Mar;35(3):376–377. doi: 10.1002/ana.410350326. [DOI] [PubMed] [Google Scholar]
- Madras B. K., Spealman R. D., Fahey M. A., Neumeyer J. L., Saha J. K., Milius R. A. Cocaine receptors labeled by [3H]2 beta-carbomethoxy-3 beta-(4-fluorophenyl)tropane. Mol Pharmacol. 1989 Oct;36(4):518–524. [PubMed] [Google Scholar]
- Meltzer P. C., Liang A. Y., Brownell A. L., Elmaleh D. R., Madras B. K. Substituted 3-phenyltropane analogs of cocaine: synthesis, inhibition of binding at cocaine recognition sites, and positron emission tomography imaging. J Med Chem. 1993 Apr 2;36(7):855–862. doi: 10.1021/jm00059a010. [DOI] [PubMed] [Google Scholar]
- Pilotte N. S., Sharpe L. G., Kuhar M. J. Withdrawal of repeated intravenous infusions of cocaine persistently reduces binding to dopamine transporters in the nucleus accumbens of Lewis rats. J Pharmacol Exp Ther. 1994 Jun;269(3):963–969. [PubMed] [Google Scholar]
- Reiss A. L., Faruque F., Naidu S., Abrams M., Beaty T., Bryan R. N., Moser H. Neuroanatomy of Rett syndrome: a volumetric imaging study. Ann Neurol. 1993 Aug;34(2):227–234. doi: 10.1002/ana.410340220. [DOI] [PubMed] [Google Scholar]
- Rousset O., Ma Y., Kamber M., Evans A. C. 3D simulations of radiotracer uptake in deep nuclei of human brain. Comput Med Imaging Graph. 1993 Jul-Oct;17(4-5):373–379. doi: 10.1016/0895-6111(93)90031-h. [DOI] [PubMed] [Google Scholar]
- Scheffel U., Boja J. W., Kuhar M. J. Cocaine receptors: in vivo labeling with 3H-(-)cocaine, 3H-WIN 35,065-2, and 3H-WIN 35,428. Synapse. 1989;4(4):390–392. doi: 10.1002/syn.890040415. [DOI] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Shaya E. K., Scheffel U., Dannals R. F., Ricaurte G. A., Carroll F. I., Wagner H. N., Jr, Kuhar M. J., Wong D. F. In vivo imaging of dopamine reuptake sites in the primate brain using single photon emission computed tomography (SPECT) and iodine-123 labeled RTI-55. Synapse. 1992 Feb;10(2):169–172. doi: 10.1002/syn.890100210. [DOI] [PubMed] [Google Scholar]
- Shimada S., Kitayama S., Lin C. L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991 Oct 25;254(5031):576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Silverstein F. S., Johnston M. V., Hutchinson R. J., Edwards N. L. Lesch-Nyhan syndrome: CSF neurotransmitter abnormalities. Neurology. 1985 Jun;35(6):907–911. doi: 10.1212/wnl.35.6.907. [DOI] [PubMed] [Google Scholar]
- Usdin T. B., Mezey E., Chen C., Brownstein M. J., Hoffman B. J. Cloning of the cocaine-sensitive bovine dopamine transporter. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11168–11171. doi: 10.1073/pnas.88.24.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk G. L., O'Leary M., Nemeroff C. B., Bissette G., Moser H., Naidu S. Neurochemical alterations in Rett syndrome. Brain Res Dev Brain Res. 1993 Jul 16;74(1):67–72. doi: 10.1016/0165-3806(93)90084-n. [DOI] [PubMed] [Google Scholar]
- Wong D. F., Yung B., Dannals R. F., Shaya E. K., Ravert H. T., Chen C. A., Chan B., Folio T., Scheffel U., Ricaurte G. A. In vivo imaging of baboon and human dopamine transporters by positron emission tomography using [11C]WIN 35,428. Synapse. 1993 Oct;15(2):130–142. doi: 10.1002/syn.890150205. [DOI] [PubMed] [Google Scholar]