Abstract
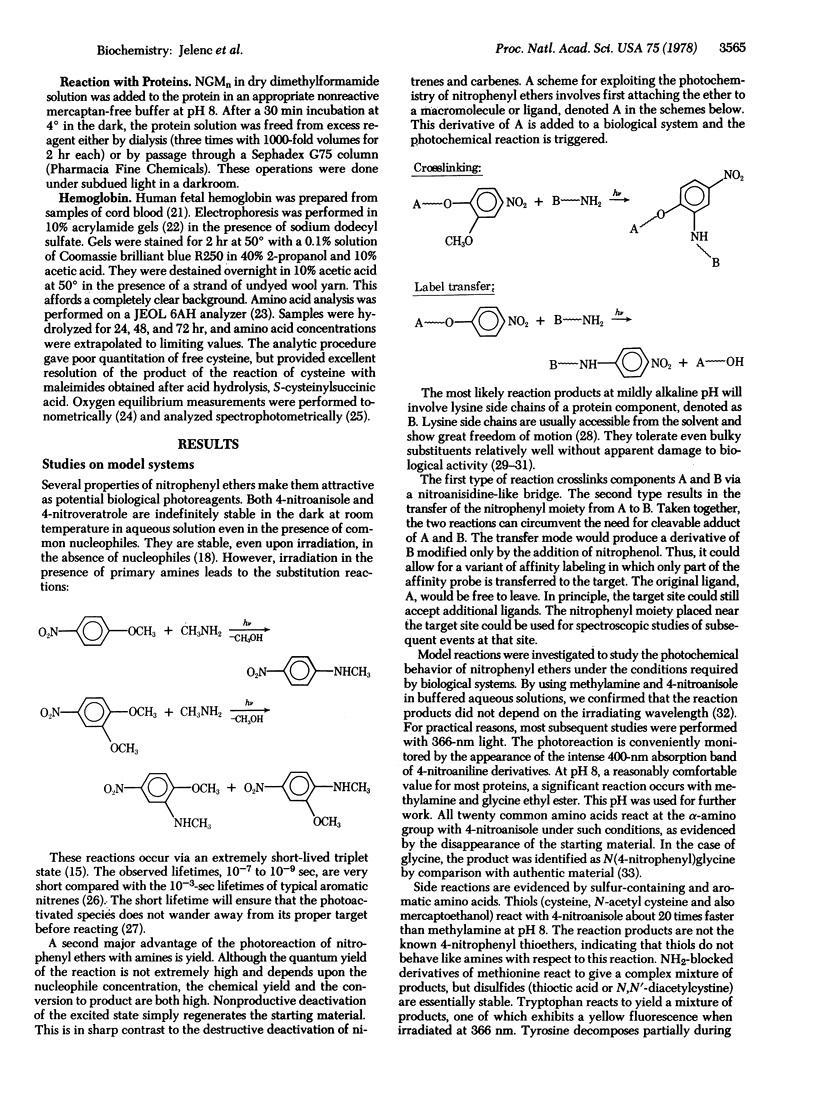
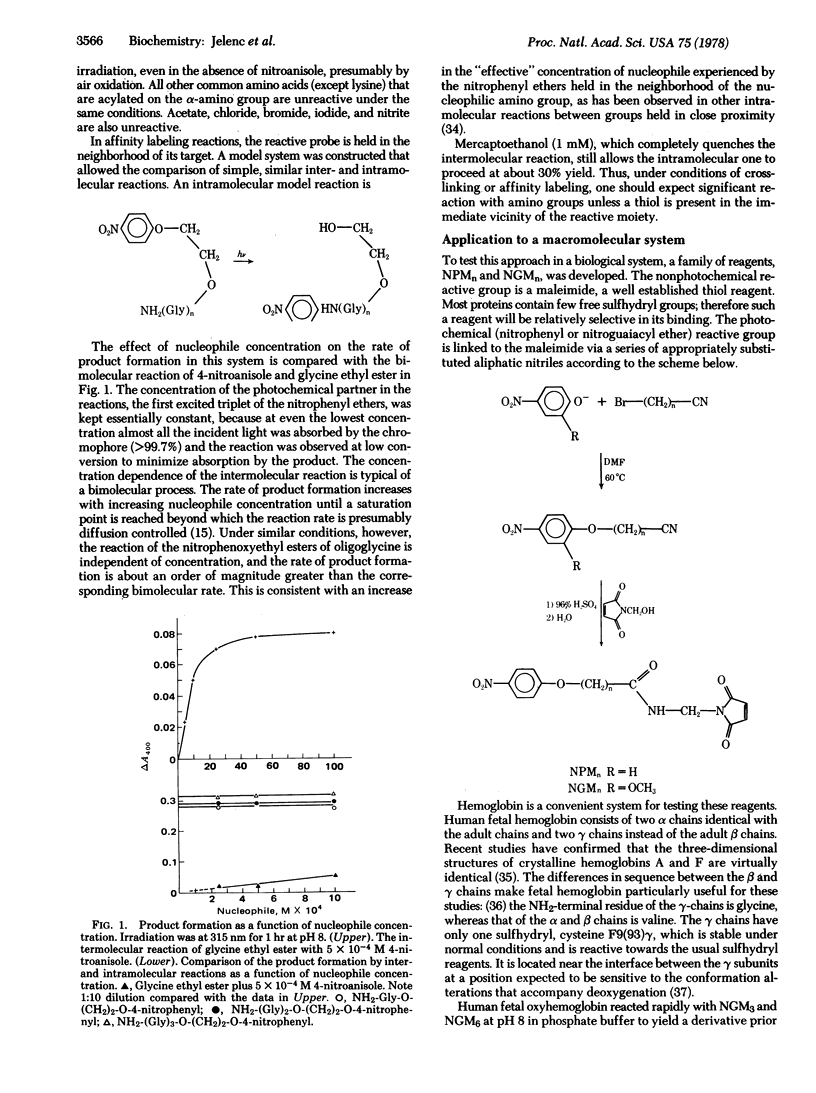
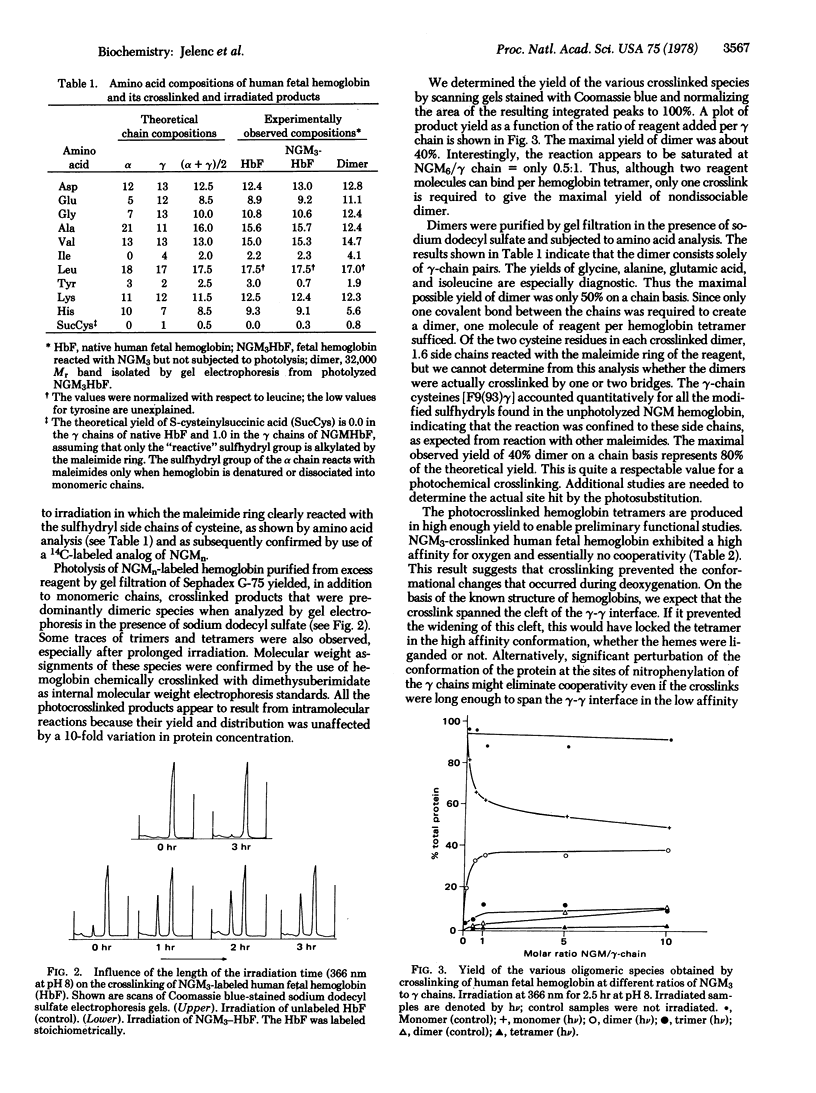
4-Nitrophenyl ethers are proposed as new high-yield photoreagents for protein crosslinking and affinity labeling. These are totally unreactive in the dark under biological conditions, but react quantitatively with amines at pH 8 upon irradiation with 366-nm light. The reaction of monoalkoxy-p-nitrobenzenes with an amine yields the corresponding free alcohol and substituted nitrophenylamine. In essence, the nitrophenyl group is transferred from the alcohol to the amine. Bifunctional affinity reagents of this type could be especially useful for placing the p-nitrophenyl chromophore adjacent to a binding site without blocking it. The corresponding 2-methoxy-4-nitrophenyl ethers react with amines by displacement of the methoxyl group. Thus, bifunctional reagents of this class could be photocrosslinkers. A maleimide-containing 2-methoxy-4-nitrophenyl ether was attached to human fetal hemoglobin at γ-cysteine F9 stoichiometrically. Subsequent ultraviolet irradiation yielded a γ-γ crosslinked hemoglobin in 80% yield. The oxygenation properties of the derivative indicate that it is locked in a high affinity conformation and that all cooperativity is lost.
Keywords: nitrophenyl ethers, phototransfer substitution reactions, maleimides, fetal hemoglobin, oxygen affinity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. Structure and function of haemoglobin. Prog Biophys Mol Biol. 1975;29(3):225–320. doi: 10.1016/0079-6107(76)90024-9. [DOI] [PubMed] [Google Scholar]
- Chowdhry V., Vaughan R., Westheimer F. H. 2-diazo-3,3,3-trifluoropropionyl chloride: reagent for photoaffinity labeling. Proc Natl Acad Sci U S A. 1976 May;73(5):1406–1408. doi: 10.1073/pnas.73.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Jamieson J. D., Printz M. P. Photoaffinity labeling of peptide hormone binding sites. J Biol Chem. 1974 Jun 10;249(11):3510–3518. [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Printz M. P. Benzophenone triplet: a new photochemical probe of biological ligand-receptor interactions. Nat New Biol. 1973 Mar 28;242(117):127–128. doi: 10.1038/newbio242127a0. [DOI] [PubMed] [Google Scholar]
- Glushko V., Lawson P. J., Gurd F. R. Conformational states of bovine pancreatic ribonuclease A observed by normal and partially relaxed carbon 13 nuclear magnetic resonance. J Biol Chem. 1972 May 25;247(10):3176–3185. [PubMed] [Google Scholar]
- Hixson S. H., Hixson S. S. P-Azidophenacyl bromide, a versatile photolabile bifunctional reagent. Reaction with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Sep 23;14(19):4251–4254. doi: 10.1021/bi00690a016. [DOI] [PubMed] [Google Scholar]
- Lutter L. C., Ortanderl F., Fasold H. The use of a new series of cleavable protein-crosslinkers on the Escherichia coli ribosome. FEBS Lett. 1974 Nov 15;48(2):288–292. doi: 10.1016/0014-5793(74)80488-6. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Konigsberg W. The characterization of modified human hemoglobin. II. Reaction with 1-fluoro-2,4-dinitrobenzene. J Biol Chem. 1968 Apr 25;243(8):1966–1970. [PubMed] [Google Scholar]
- Perham R. N., Richards F. M. Reactivity and structural role of protein amino groups in tobacco mosaic virus. J Mol Biol. 1968 May 14;33(3):795–807. doi: 10.1016/0022-2836(68)90320-3. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Moore S., Stein W. H. Activity of bovine pancreatic deoxyribonuclease A with modified amino groups. J Biol Chem. 1971 Feb 25;246(4):939–945. [PubMed] [Google Scholar]
- Ruoho A. E., Kiefer H., Roeder P. E., Singer S. J. The mechanism of photoaffinity labeling. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2567–2571. doi: 10.1073/pnas.70.9.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J., JONES R. T. THE AMINO ACID SEQUENCE OF THE GAMMA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Sep-Oct;2:992–1008. doi: 10.1021/bi00905a016. [DOI] [PubMed] [Google Scholar]
- SINGH A., THORNTON E. R., WESTHEIMER F. H. The photolysis of diazoacetylchymotrypsin. J Biol Chem. 1962 Sep;237:3006–3008. [PubMed] [Google Scholar]
- SPOEK G. L., BAKKER H., WOLVEKAMP H. P. EXPERIMENTS ON THE HAEMOCYANIN-OXYGEN EQUILIBRIUM OF THE BLOOD OF THE EDIBLE SNAIL (HELIX POMATIA L.). Comp Biochem Physiol. 1964 Jun;12:209–221. doi: 10.1016/0010-406x(64)90175-6. [DOI] [PubMed] [Google Scholar]
- Schwartz I., Ofengand J. Photo-affinity labeling of tRNA binding sites in macromolecules. I. Linking of the phenacyl-p-azide of 4-thiouridine in (Escherichia coli) valyl-tRNA to 16S RNA at the ribosomal P site. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3951–3955. doi: 10.1073/pnas.71.10.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. R., Arndt D. J., Konigsberg W. H. Structure and functional properties of chemically modified horse hemoglobin. I. Determination of the functional properties. J Mol Biol. 1971 May 28;58(1):69–77. doi: 10.1016/0022-2836(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Bollen A., Sun T. T., Hershey J. W., Sundberg J., Pierce L. R. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973 Aug 14;12(17):3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Shimizu K. Effect of organic phosphates on the difference in oxygen affinity between fetal and adult human hemoglobin. Fed Proc. 1970 May-Jun;29(3):1112–1114. [PubMed] [Google Scholar]
- WOFSY L., METZGER H., SINGER S. J. Affinity labeling-a general method for labeling the active sites of antibody and enzyme molecules. Biochemistry. 1962 Nov;1:1031–1039. doi: 10.1021/bi00912a013. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wind M., Stern A., Simon S., Law L. Relative stabilities of the two quaternary conformations of human fetal hemoglobin. Biochemistry. 1976 Nov 16;15(23):5161–5167. doi: 10.1021/bi00668a033. [DOI] [PubMed] [Google Scholar]


