Abstract
Nutritional status is known to alter immune function, a suspected risk factor for non-Hodgkin lymphoma (NHL). To investigate whether long-term over, or under, nutrition is associated with NHL self-reported anthropometric data on weight and height from over 10000 cases of NHL and 16000 controls were pooled across 18 case-control studies identified through the International Lymphoma Epidemiology Consortium. Study-specific odds ratios (OR) were estimated using logistic regression and combined using a random-effects model. Severe obesity, defined as BMI of 40 kg m−2 or more, was not associated with NHL overall (pooled OR=1.00, 95% confidence interval (CI) 0.70–1.41) or the majority of NHL subtypes. An excess was however observed for diffuse large B-cell lymphoma (pooled OR=1.80, 95% CI 1.24–2.62), although not all study-specific ORs were raised. Among the overweight (BMI 25–29.9 kg m−2) and obese (BMI 30–39.9 kg m−2), associations were elevated in some studies and decreased in others, while no association was observed among the underweight (BMI<18.5 kg m−2). There was little suggestion of increasing ORs for NHL or its subtypes with every 5 kg m−2 rise in BMI above 18.5 kg m−2. BMI components height and weight were also examined, and the tallest men, but not women, were at marginally increased risk (pooled OR=1.19, 95% CI 1.06–1.34). In summary, whilst we conclude that there is no evidence to support the hypothesis that obesity is a determinant of all types of NHL combined, the association between severe obesity and diffuse large B-cell lymphoma may warrant further investigation.
Keywords: non-Hodgkin lymphoma, lymphoma, body mass index, weight, height, epidemiology
Introduction
Non-Hodgkin lymphomas (NHL) can arise following rare inherited disorders of the immune system, long-term immunosuppressive drug therapy and viral infections such as human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV). In such instances, severe immunosuppression resulting from exposure usually leads to the development of specific NHL subtypes. For the majority of NHL however, the cause remains unknown but it is suspected that factors which affect the immune system are involved. In particular, it has been suggested that the degree of adiposity might be important since over (as well as under) nutrition can alter immune function (1;2). However, while several epidemiological studies have reported associations between excess weight and NHL (3–15) the evidence is far from conclusive (16–28). Here we present a pooled analysis of self-reported height and weight on over 10000 NHL cases and 16000 controls from 18 case-control studies identified through the International Lymphoma Epidemiology Consortium (InterLymph: http://epi.grants.cancer.gov/InterLymph/).
Materials and Methods
Through the InterLymph forum, 18 case-control studies of NHL with anthropometric data collected across 13 countries in parts of North America, Europe, and Japan between 1983 and 2004 were identified. Study designs are briefly outlined in Table 1, and more details are published elsewhere (3;8;14;18;24;29–36). Cases were identified using rapid ascertainment techniques, while controls were randomly selected from population registers (8 studies), outpatient clinics (3 studies) or inpatients (7 studies) hospitalised for a variety of non-neoplastic conditions such as circulatory, digestive or respiratory problems, or with traumatic or non-traumatic orthopaedic conditions. The appropriate ethical committees’ approval was granted for each study and informed consent was given by all participants.
Table 1.
Characteristics of case-control studies included in the pooled analysis.
| Study | Location | Year of Diagnosis | Age Range | Cases (N=10453) | Controls (N=16507) | Participation (%) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| N | Participation (%) | Source | N | ||||||
| NCI-SEER | Detroit, Michigan; Iowa; Los Angeles, California; Seattle, Washington, USA | 1998–2001 | 20–70 | 527 | 76 | <65 years RDD; 65+ years random selection from Centers for Medicare and Medicaid Services, stratified by study area, age, sex and race | 468 | 52 | (8) |
| Nebraska NHL Study | Nebraska, USA | 1999–2002 | 20–75 | 387 | 74 | RDD, frequency matched by age and sex | 535 | 78 | (14) |
| Mayo Clinic Phase 1 | Iowa, Wisconsin, Minnesota, USA | 2002–2005 | 18+ | 499 | 66 | Frequency matched by age, sex and county of residence | 499 | 70 | n/a |
| UCSF | San Francisco, USA | 1988–1995 | 21–74 | 1304 | 72 | RDD, frequency matched by age, sex, and county of residence | 2402 | 78 | (3) |
| British Columbia Study | Vancouver and Victoria, British Columbia, Canada | 2000–2004 | 20–82 | 828 | 78 | Random selection from Client Registry of the Ministry of Health, frequency matched by age, sex and region | 848 | 46 | (36) |
| UK | Yorkshire, Lancashire, South Lakeland and parts of Southwest England | 1998–2003 | 16–69 | 833 | 70 | Random selection from general practice lists, individually matched by age, sex and region of residence | 1141 | 69 | (29) |
| SCALE | Denmark and Sweden | 2000–2002 | 18–74 | 3055 | 81 | Random selection from population register, frequency matched by sex and age | 3187 | 71 | (24) |
| EpiLymph Ireland | Six hospitals on the East Coast of the Republic of Ireland | 2001–2003 | 18–80 | 135 | 90 | Hospital controls matched by age (±5 years), sex and study region | 208 | 75 | (30) |
| EpiLymph Finland | Finland | 2001–2003 | 18–80 | 87 | Hospital controls matched by age (±5 years), sex and study region | 75 | n/a | ||
| EpiLymph Germany | Ludwigshafen/Upper Palatinate, Heidelberg/Rhine-Neckar County, Würzburg/Lower Frankonia, Hamburg, Bielefeld and Munich | 1999–2002 | 18–80 | 496 | 88 | Random selection from population register, individually matched by sex, age and study region | 710 | 44 | (31) |
| EpiLymph France | Amiens, Dijon and Montpellier | 2000–2003 | 18–80 | 206 | 91 | Hospital controls matched by age (±5 years), sex and study region | 276 | 74 | (30) |
| EpiLymph Czech Republic | 1 centre in Czech Republic | 2001–2003 | 18–80 | 195 | 90 | Hospital controls individually matched by age (±5 years), sex and study region | 304 | 60 | (30) |
| EpiLymph Spain | Barcelona, Tortosa, Reus and Madrid | 1998–2002 | 18–80 | 428 | 82 | Hospital controls matched by age (±5 years), sex and study region | 631 | 96 | (32) |
| EpiLymph Italy | 2 centres in Italy | 1998–2004 | 18–80 | 222 | 93 | Random selection from population census list, matched by age (±5 years), sex and study region | 336 | 66 | (30) |
| Northern Italy | Aviano & Milan | 1983–1992 | 17–79 | 429 | >97 | Patients admitted for acute, nonneoplastic, nonimmunologic conditions in the hospitals where cases diagnosed | 1157 | >97 | (18) |
| Italy | Aviano & Naples | 1999–2002 | 18–84 | 225 | 97 | Hospital controls, frequency matched by age (in 5-year bands), sex and study centre to cases of lymphohematopoietic neoplasms including NHL and hepatocellular carcinoma | 504 | 91 | (33) |
| HERPACC1 | Aichi Cancer Centre, Nagoya, Japan | 1988–2000 | 18–79 | 416 | ≈99 | Random sample of patients not diagnosed with cancer, individually matched by age and sex | 2260 | ≈99 | (34;35) |
| HERPACC2 | Aichi Cancer Centre, Nagoya, Japan | 2001–2004 | 18–79 | 181 | ≈99 | Random sample of patients not diagnosed with cancer, individually matched by age and sex | 966 | ≈99 | (35) |
NHL diagnoses were pathologically confirmed and subsequently coded to the World Health Organisation (WHO) classification (37) (15 studies), the REAL classification (the 1999–2002 Italian study), or Working Formulation (North Italy and UCSF). Cases with HIV were excluded. Diagnostic codes from the different studies were combined as previously described (38). The analysis here considers specific B-cell subtypes of NHL (diffuse large B-cell lymphoma: ICDO3 codes 9679/3, 9680/3, 9684/3; follicular lymphoma: 9690/3, 9691/3, 9695/3, 9698/3; chronic lymphocytic leukaemia/small lymphocytic lymphoma: 9670/3, 9823/3; marginal zone lymphoma: 9689/3, 9699/3; mantle cell lymphoma: 9673/3; Burkitt lymphoma: 9687/3, 9826/3; and other unspecified B-cell lymphoma: 9671/3, 9728/3), and T-cell lymphomas as a whole (9700/3, 9701/3, 9702/3, 9705/3, 9708/3, 9709/3, 9714/3, 9716/3, 9717/3, 9718/3, 9719/3, 9729/3, 9827/3) as well as NHL in total (defined by the above ICDO3 codes and 9591/3, 9675/3, and 9727/3).
In all studies, information on anthropometrics, demographics, lifestyle, occupations and medical histories were collected by in-person or telephone interviews. For the purposes of the present analyses, anonymised data were provided and checked for inconsistencies before coding uniformly. Within each study, height in metres was categorised using sex-specific quintiles of the height distribution among controls, and data were then combined across studies to reflect the relative position, rather than the absolute value, of this variable. In the statistical analysis, the referent category for height was taken as the 3rd quintile, since this central group contains the median and has the narrowest range. Usual adult weight was requested in 10 studies (Nebraska, UCSF, SCALE and EpiLymph studies). Elsewhere different questions were used (weight at diagnosis/interview (HERPACC1, HERPACC2); one year (NCI-SEER, British Columbia, North Italy, Italy); two years (Mayo Phase 1); or five years (UK) prior to diagnosis/interview).
For the pooled analysis, body mass index (BMI) was computed by dividing weight in kilograms by the square of height in metres where each study’s weight variable was considered at the closest time point prior to diagnosis/interview, or else the usual adult weight. BMI was grouped using the World Health Organisation categories of underweight (<18.5 kg m−2), normal (18.5–24.99 kg m−2), grade 1 overweight (25–29.99 kg m−2), grade 2 obese (30–39.99 kg m−2) and grade 3 obese (40 kg m−2 or more) (39). For a person 1.7 m (5′ 7″) tall, these cut-off points relate to weights of 53 kg (118 lb), 72 kg (159 lb), 87 kg (191 lb), and 116 kg (255 lb) respectively. Socioeconomic status was defined by the level of education attained, except in British Columbia and the UK where self-reported household income and a census-based household deprivation indicator were used respectively; and no socioeconomic status information was collected in the Japanese studies (HERPACC1 and 2).
Statistical analysis followed similar methods to those employed in previous InterLymph pooling projects (40–44). Firstly, individual data were combined in an unconditional logistic regression model adjusting for study, age, sex, and race. To test for between-study heterogeneity, this model was compared using the likelihood ratio test with the model that included an additional term for interaction between the anthropometric variable and a variable indexing the studies. Heterogeneity was assumed to be present when the likelihood ratio test yielded a p-value<0.05. This flexible approach utilises all data and provides one statistic to test for heterogeneity. Where the likelihood ratio test was not statistically significant, the pooled adjusted OR and 95% CI computed from all individual data in an unconditional logistic regression model are presented.
Between-study heterogeneity was further examined among risk estimates at each category of the anthropometric variables. Study-specific odds ratios (OR) and 95% confidence intervals (CI) adjusted for sex, age, and race were computed using unconditional logistic regression (45). For each category of height or BMI, the study-specific ORs were combined using a random effects meta-analysis to produce a combined OR and corresponding 95% CI. The extent of heterogeneity for each category was indicated by Cochran’s Q-statistic which was considered statistically significant when p<0.10. The I2-statistic was also reported to describe the percentage of total variation in the study-specific ORs which was due to heterogeneity (46).
Since the ORs were diverse across studies, a variety of approaches were applied to explore heterogeneity (47). To assess relative obesity within study populations rather than the absolute value, BMI was grouped into quintiles based on the control distributions within each study before combining the relative quintile groupings across studies; these analyses are not presented here since their findings were similar to those reported. Sensitivity analyses using various stratifications and subsets of data were also conducted (48). Study-specific ORs were combined by continent, study design and time period (corresponding to the original lymphoma classification used) as well as by level of participation. Given that the study-specific associations with BMI were heterogeneous in all analyses, forest plots with ORs pooled by continent were judged to be the most informative. Pooled ORs stratified by study design are also presented.
Within studies, analyses were performed separately for men, women, Caucasian subjects and persons aged 18 to 65. The resulting study-specific ORs were combined in a random-effects meta-analysis to examine heterogeneity. Potential confounding factors, such as smoking, alcohol and socioeconomic status, were assessed by comparing study-specific regression models with and without the confounding factor using the likelihood ratio test. A factor was considered a confounder when the likelihood ratio test was significant and the adjusted OR changed by more than 10%. Continuous variables corresponding to 10 cm increases in height and 5 kg m−2 increases in BMI were created to assess trends. All analyses were conducted using Stata (49).
Results
The pooled dataset from the 18 case-control studies comprised anthropometric information from 10453 cases of NHL and 16507 controls. Most cases (85%) were diagnosed with a B-cell lymphoma, 5% with a T-cell lymphoma and for 11%, immunophenotype was not known. The three most common NHL subtypes were diffuse large B-cell lymphoma (DLBCL) (32%), follicular lymphoma (FL) (22%) and chronic lymphocytic lymphoma/small lymphocytic lymphoma (CLL/SLL) (16%). A slightly higher proportion of cases were men (57%), 90% of all cases were Caucasian and the median age was 60 years. Cases tended to be older in age, of white race and of lower socioeconomic status than controls (data not shown).
Height distributions among male and female controls varied by study; for both sexes, the median height was highest in the American studies, generally decreased from Northern to Southern Europe, and was lowest in the two Japanese studies (data not shown). Among men, compared to the third quintile the odds ratio was increased in the highest quintile (OR=1.19, 95% CI 1.06–1.34), but was close to one in the lowest two quintiles (Supplementary Table 1). When examining trend within studies, no consistent population pattern emerged; most studies showed no evidence of a trend with 10 cm increases in height, six a significant positive trend, and two a significant negative trend (data not shown). Similar patterns were observed for the majority of NHL subtypes. Little association between height and NHL, or its subtypes, was observed among women (Supplementary Table 1).
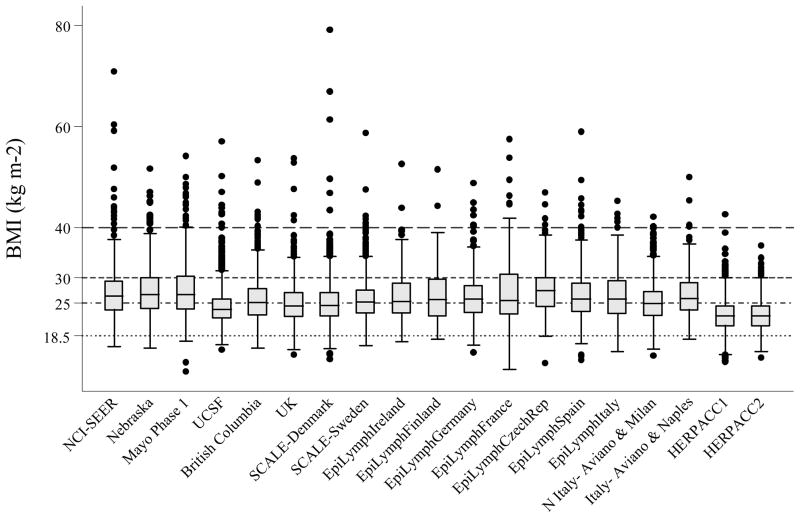
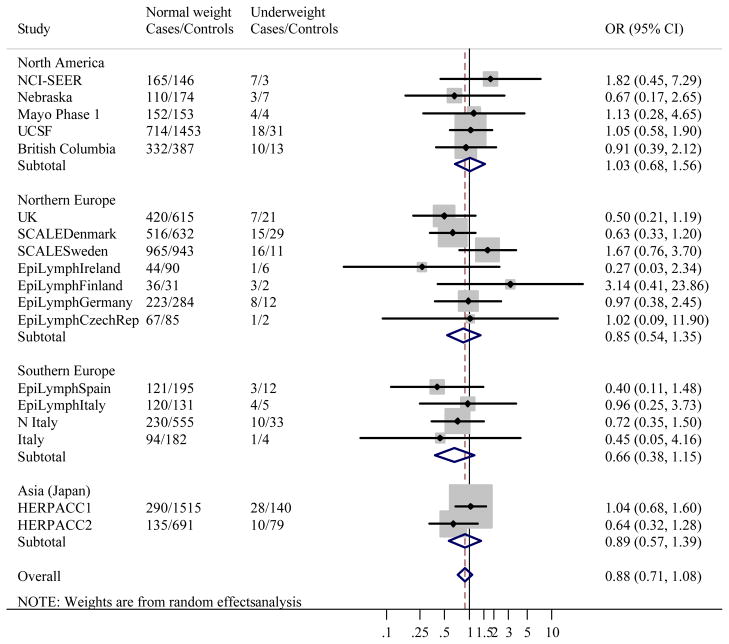
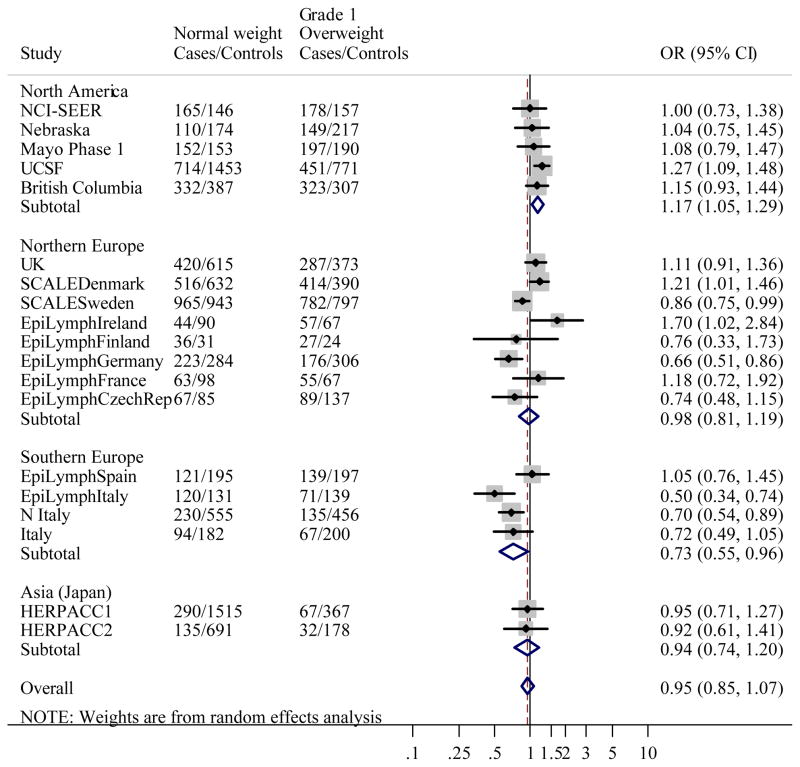
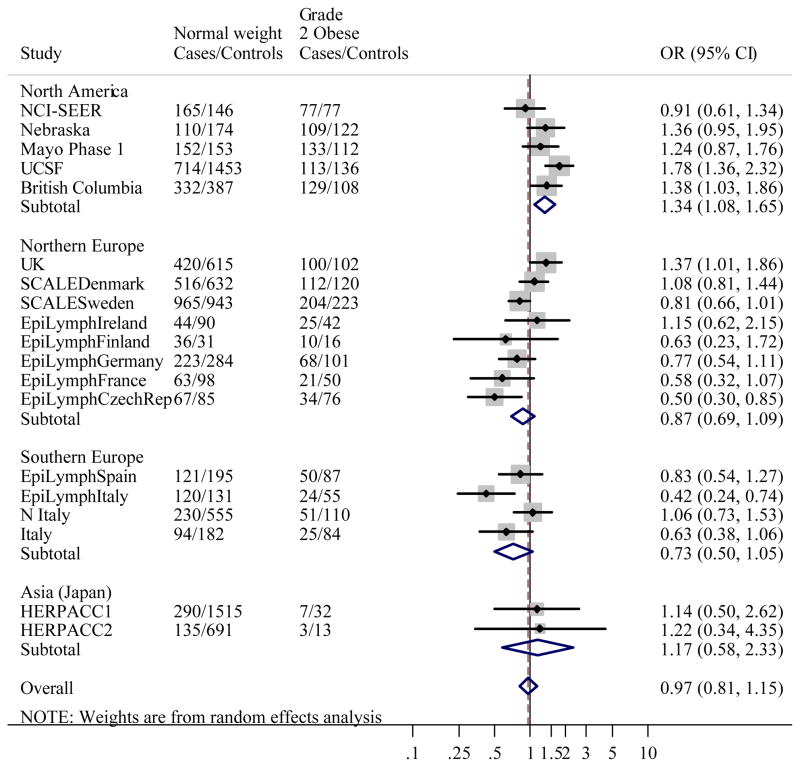
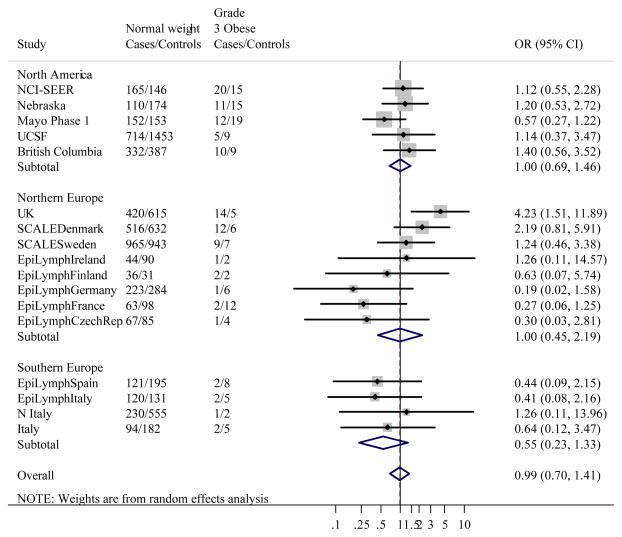
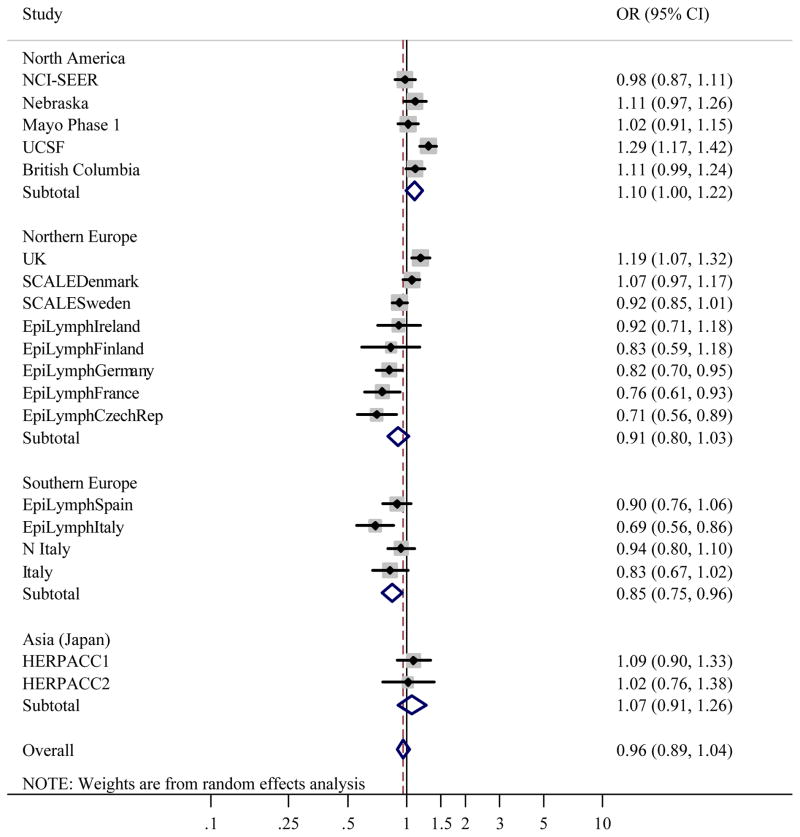
Figure 1 gives the distribution of BMI among controls by study. Like height, studies conducted in the US had the greatest median BMI, and Japan the lowest. When BMI was classified using WHO categories, associations between BMI and NHL were heterogeneous between studies (likelihood ratio test: χ2=139.1, p<0.0001). Study-specific ORs showed that the heterogeneity was most marked in Grade 1 overweight, where ORs ranged from 0.50 (95% CI 0.34–0.74) in EpiLymph Italy to 1.70 (95% CI 1.02–2.84) in EpiLymph Ireland and Grade 2 obese (ranging from OR=0.42, 95% CI 0.24–0.74 in EpiLymph Italy to OR=1.78, 95% CI 1.36–2.32 in UCSF) (Figures 2(b) and (c)). In the underweight and Grade 3 obese categories, where the numbers of subjects were small, ORs were also diverse (ranging from OR=0.27, 95% CI 0.03–2.34 in EpiLymph Ireland to OR=3.14, 95% CI 0.41–23.9 in EpiLymph Finland; and from OR=0.19, 95% CI 0.02–1.58 in EpiLymph Germany to OR=4.23, 95% CI 1.51–11.9 in UK, respectively) (Figures 2(a) and (d)). Trends with a 5 kg m−2 increase in BMI above 18.5 kg m−2 were significantly increased in two studies, significantly decreased in four studies and showed little effect in the remaining studies (Figure 3). ORs were pooled across North America, Northern Europe, Southern Europe and Japan. In North America, a homogeneous increased OR was suggested for Grade 1 overweight (Figure 2(b)) but no effect was found among Grade 3 obese (Figure 2(d)), and with the exception of the Californian study (UCSF), no significant positive trends were observed (Figure 3). Heterogeneity was still evident when the analyses were restricted to population-based studies conducted in the period 1998–2005; to those designed to code to the WHO classification; or to those where control participation rates were 70% or more. Similarly study-specific ORs were heterogeneous among men or women; subjects aged 18 to 65; or Caucasian subjects (data not shown).
Figure 1.
Box-Whisker Plot of Body Mass Index among Controls by Study.
Body mass index considered to be: Underweight if <18.5 kg m−2; Normal weight-for-height if 18.5–24.99 kg m−2; Grade 1 Overweight if 25–29.99 kg m−2; Grade 2 Obese if 30–39.99 kg m−2; and Grade 3 Obese if ≥40 kg m−2 (55).
Figure 2.
Figure 2(a). Meta-analysis of the risk of NHL associated with BMI <18.5 kg m−2 (Underweight) compared to BMI 18.5–24.99 kg m−2 (Normal weight).
Overall test for heterogeneity: Q=13.0, p=0.73; Variation in odds ratios (OR) attributable to heterogeneity: I2= 0.0%. For continents: North America: Q=1.04, p=0.90, I2=0.0%; Northern Europe: Q=7.87, p=0.25, I2=23.7%; Southern Europe: Q=1.03, p=0.80, I2=0.0%; Asia (Japan): Q=1.38, p= 0.24, I2=27.5%. Test for heterogeneity between continents: Q=1.82, p=0.61. Pooled odds ratios by study design were: Population-based studies: OR=0.91, 95% CI 0.68–1.21, Q=6.75, p=0.56, I2=0.0%; Clinic-based studies: OR=0.92, 95% CI 0.65–1.31, Q=1.47, p=0.48, I2=0.0%; Hospital-based studies: OR=0.67, 95% CI 0.39–1.17, Q=3.79, p=0.58, I2=0.0%. Test for heterogeneity between study designs: Q=1.04, p=0.59.
Figure 2(b). Meta-analysis of the risk of NHL associated with BMI 25–29.99 kg m−2 (Grade 1 overweight) compared to BMI 18.5–24.99 kg m−2 (Normal weight).
Overall test for heterogeneity: Q=60.0, p<0.001; Variation in odds ratios (OR) attributable to heterogeneity: I2=70.0%. For continents: North America: Q=2.76, p=0.60, I2=0.0%; Northern Europe: Q=25.0, p=0.001, I2=72.1%; Southern Europe: Q=8.59, p=0.04, I2=65.1%; Asia (Japan): Q=0.02, p=0.90, I2=0.0%. Test for heterogeneity between continents: Q=23.4, p<0.001. Pooled odds ratios by study design were: Population-based studies: OR=0.97, 95% CI 0.82–1.14, Q=41.6, p<0.001, I2=80.8%; Clinic-based studies: OR=0.99, 95% CI 0.82–1.20, Q=0.44, p=0.80, I2=0.0%; Hospital-based studies: OR=0.91, 95% CI 0.72–1.16, Q=14.0, p=0.03, I2=57.1%. Test for heterogeneity between study designs: Q=3.93, p=0.14.
Figure 2(c). Meta-analysis of the risk of NHL associated with BMI 30–39.99 kg m−2 (Grade 2 obese) compared to BMI 18.5–24.99 kg m−2 (Normal weight).
Overall test for heterogeneity: Q=59.7, p<0.001; Variation in odds ratios (OR) attributable to heterogeneity: I2=69.8%. For continents: North America: Q=8.18, p=0.08, I2=51.1%; Northern Europe: Q=18.1, p=0.01, I2=61.2%; Southern Europe: Q=7.88, p=0.05, I2=62.0%; Asia (Japan): Q=0.01, p=0.93, I2=0.0%. Test for heterogeneity between continents: Q=25.4, p<0.001. Pooled odds ratios by study design were: Population-based studies: OR=1.06, 95% CI 0.83–1.34, Q=41.3, p<0.001, I2=80.7%; Clinic-based studies: OR=1.22, 95% CI 0.90–1.67, Q=0.03, p=0.99, I2=0.0%; Hospital-based studies: OR=0.77, 95% CI 0.60–0.98, Q=8.51, p=0.20, I2=29.5%. Test for heterogeneity between study designs: Q=9.81, p=0.007.
Figure 2(d). Meta-analysis of the risk of NHL associated with BMI ≥40 kg m−2 (Grade 3 obese) compared to BMI 18.5–24.99 kg m−2 (Normal weight).
Overall test for heterogeneity: Q=21.9, p=0.15; Variation in odds ratios (OR) attributable to heterogeneity: I2=26.8%. For continents: North America: Q=2.89, p=0.58, I2=0.0%; Northern Europe: Q=15.3, p=0.03, I2=54.4%; Southern Europe: Q=0.69, p=0.88, I2=0.0%. Test for heterogeneity between continents: Q=2.91, p=0.23. Pooled odds ratios by study design were: Population-based studies: OR=1.33, 95% CI 0.88–2.00, Q=11.4, p=0.18, I2=29.7%; Clinic-based studies: OR=0.57, 95% CI 0.26–1.22, No test for heterogeneity as only 1 study; Hospital-based studies: OR=0.51, 95% CI 0.25–1.05, Q=2.07, p=0.91, I2=0.0%. Test for heterogeneity between study designs: Q=8.41, p=0.015.
Figure 3.
Meta-analysis of the risk of NHL associated with 5 kg m−2 increase in BMI above 18.5 kg m−2 (Normal weight and above).
Overall test for heterogeneity: Q=87.5, p<0.001; Variation in odds ratios (OR) attributable to heterogeneity: I2=79.4%. For continents: North America: Q=15.5, p=0.004, I2=74.1%; Northern Europe: Q=37.4, p<0.001, I2=81.3%; Southern Europe: Q=5.32; p=0.15; I2=43.6%; Asia (Japan): Q=0.12, p=0.73, I2=0.0%. Test for heterogeneity between continents: Q=29.0, p<0.001. Pooled odds ratios by study design were: Population-based studies: OR=1.02, 95% CI 0.92–1.13, Q=57.7, p<0.001, I2=86.1%; Clinic-based studies: OR=1.04, 95% CI 0.94–1.14, Q=0.34, p=0.84, I2=0.0%; Hospital-based studies: OR=0.85, 95% CI 0.79–0.92, Q=6.09, p=0.41, I2=1.4%. Test for heterogeneity between study designs: Q=23.4, p<0.001.
Statistically significant between-study heterogeneity was also present for the three most common NHL subtypes (likelihood ratio tests for WHO BMI and DLBCL: χ2=104.2, p=0.002; FL: χ2=82.7, p=0.003; CLL/SLL: χ2=58.7, p=0.04). For these three subtypes, as for NHL as a whole, study-specific ORs varied around one in all WHO BMI groups, with tests for heterogeneity in the two-stage random effects model being significant among Grade 1 overweight and Grade 2 obese (DLBCL: Supplementary Figures 1(a)–(d); FL: Supplementary Figures 3(a)–(d); CLL/SLL: Supplementary Figures 4(a)–(d)). In the underweight and Grade 3 obese groups, the meta-analyses generally suggested that ORs were more homogeneous and the combined risk estimates were not significantly different from one. The pooled OR for DLBCL among Grade 3 obese was increased (OR=1.80, 95% CI 1.24–2.62, Q=16.7, p=0.40, I2=4.4%), being elevated in North America and Northern Europe, but as with all analyses in this BMI group, study-specific risk estimates were diverse, based on small numbers of subjects, and with wide and overlapping confidence intervals (Supplementary Figure 1(d)). Like NHL as a whole, a 5 kg m−2 increase in BMI did not consistently increase the risk of DLBCL (Supplementary Figure 2) or the other subtypes (data not shown). ORs for the rarer B-cell lymphomas and T-cell lymphoma were mostly not significantly different between studies, probably due to the small number of cases, and there was little suggestion of associations between these NHL subtypes and BMI (Supplementary Table 2).
Pooling data from studies with the highest WHO BMI prevalences of overweight/obese controls (EpiLymph Czech Republic, Nebraska, Mayo Phase 1, EpiLymph Italy, EpiLymph Germany, Italy-Aviano and Naples, and EpiLymph Finland) gave more homogeneous ORs (likelihood ratio test: χ2=32.3, p=0.12). Within this subset of seven studies, there was still little evidence that higher than average BMI increases the risk of NHL and its subtypes (Table 2). These findings were consistent when data were stratified by sex, age, or race.
Table 2.
Number of cases and controls, pooled odds ratios and 95% confidence intervals for body mass index by all NHL subtypes and the three most common NHL subtypes in studies with the highest prevalence of overweight/obese controlsa.
| BMIb | Controls (N=2963) | NHLc (N=2108) | ORd | 95% CI | DLBCLc (N=659) | ORd | 95% CI | FLc (N=457) | ORd | 95% CI | CLL/SLLd (N=381) | ORc | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO category (kg m−2): | |||||||||||||
| <18.5 | 36 | 24 | 0.85 | 0.50–1.44 | 8 | 1.09 | 0.48–2.49 | 5 | 0.96 | 0.36–2.59 | 3 | 1.47 | 0.40–5.42 |
| 18.5–24.99 | 1040 | 802 | 1 | - | 273 | 1 | - | 182 | 1 | - | 121 | 1 | - |
| 25–29.99 | 1213 | 776 | 0.79 | 0.69–0.90 | 222 | 0.68 | 0.56–0.84 | 149 | 0.71 | 0.56–0.91 | 163 | 0.96 | 0.74–1.25 |
| 30–39.99 | 566 | 403 | 0.84 | 0.72–0.99 | 111 | 0.72 | 0.56–0.93 | 100 | 0.96 | 0.73–1.26 | 85 | 1.09 | 0.80–1.49 |
| ≥40 | 56 | 31 | 0.63 | 0.40–0.99 | 14 | 0.94 | 0.51–1.72 | 5 | 0.54 | 0.21–1.40 | 2 | 0.37 | 0.09–1.61 |
| Missing | 52 | 72 | 31 | 16 | 7 | ||||||||
| Test for heterogeneitye | χ2=32.2 | p=0.12 | χ2=25.6 | p=0.27 | χ2=24.7 | p=0.05 | χ2=17.8 | p=0.16 | |||||
Studies with highest prevalence of overweight/obese controls were EpiLymph Czech Republic, Nebraska, Mayo Phase 1, EpiLymph Italy, EpiLymph Germany, Italy-Aviano & Naples, and EpiLymph Finland.
Body mass index grouped: using WHO categories where <18.5 kg m−2 is considered Underweight; 18.5–24.99 kg m−2 Normal weight; 25–29.99 kg m−2 Grade 1 Overweight; 30–39.99 kg m−2 Grade 2 Obese; and ≥40 kg m−2 Grade 3 Obese.
NHL: non-Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; CLL/SLL: chronic lymphocytic leukaemia/ small lymphocytic lymphoma.
Odds ratios and 95% confidence intervals adjusted for study, sex, age, and race were estimated using unconditional logistic regression.
Test for heterogeneity was conducted by testing for evidence of interaction between BMI and studies using the likelihood ratio test.
Confidence interval estimated using exact methods.
Discussion
The present InterLymph analysis, which is based on 18 studies from 13 countries, found little evidence to support the hypothesis that excess weight-for-height is associated with NHL. A slightly increased OR amongst the tallest men was observed compared to those who were of mid-range height but no association was found among women. The large number of subjects included in this analysis enabled examination of risks for subtypes of NHL. While findings for most were consistent with total NHL, an increased risk for DLBCL among persons with a BMI of 40 kg m−2 or more was observed in a meta-analysis of study-specific ORs. For DLBCL, ORs were elevated with overweight/obesity in North America and amongst the most obese in Northern Europe, yet studies in either region did not show an increasing trend with a 5 kg m−2 rise in BMI. Marked heterogeneity between studies was present for all categories of BMI, which remained when studies were combined by continent, study design, time period, WHO lymphoma classification used; and when data were restricted to men or women, persons aged 18 to 65, Caucasians alone or studies with participation rates of 70% or more. ORs were less heterogeneous amongst studies with the greatest proportions of controls with a BMI of 25 kg m−2 or more. Of the seven studies in this subset, no effect of BMI on NHL risk was observed, and the lack of association with obesity was consistent across NHL subtypes, amongst men and women, and at age ≤45, 46–55, 56–65, and ≥66 years.
Six of the case-control studies included in this pooled analysis have previously published data on NHL and obesity (3;8;11;14;23;24;50) and a further 12 are included here for the first time. Apart from case-control studies, adiposity has been investigated in cohorts where height and weight were measured (9;10;12;13;25–27) or self-reported (5;15;20;21), and among persons with a hospital discharge for obesity (4;19;22). Cohort studies have the advantage of prospectively collected information, although not necessarily at a relevant time point. Positive associations with obesity have been reported for some cohorts (5;9;10;12;13;15), but not for others (4;19–22;25–27); and a further case-control investigation nested within a cohort reported a reduced risk based on measured height and weight (16). Only one additional study of case-control design- which is not part of the InterLymph consortium- has published its findings, observing an excess risk of NHL with obesity (6).
Hitherto only a few individual case-control studies and two cohort studies have considered lymphoma subtypes, proposing an association with excess adiposity for DLBCL, but less so for FL and CLL/SLL (8;11;14;15;21;22;24;50). A recent meta-analysis of published risk estimates suggested a slight increased risk of NHL, particularly DLBCL based upon data from both case-control and cohort studies (51). The pooled analysis presented here has the advantage of being less susceptible to positive publication bias since it is based on all studies within the InterLymph consortium that collected anthropometric information - around 40% of the data have not been presented before. Another advantage of pooling individual records is that it permits uniform categorization of data, as well as the assessment of the effects of potentially confounding factors. In this regard, adjustment for smoking and alcohol consumption did not greatly alter the risk estimates.
With respect to potential biases, participation rates were generally lower in controls than cases, and a particular concern is whether controls are representative of the populations from which cases were drawn. It is reassuring to note that pooling data from studies with control participation rates of 70% or more gave findings similar to those reported overall. Nonetheless, it is still possible that poor control participation could have influenced our findings since we cannot rule out the possibility that those with obesity-related health problems (e.g. type 2 diabetes, cardiovascular disease, respiratory difficulties, chronic musculoskeletal problems) may have been (more or) less likely to participate. If the latter applied, the increased risks in the highest BMI category could be an artefact of differential case-control participation.
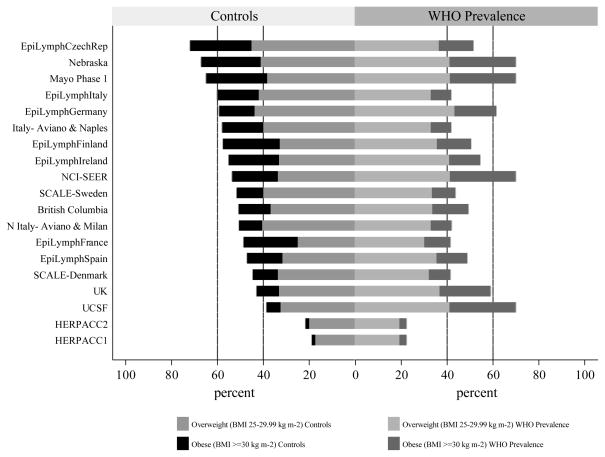
The rapidly changing prevalence of obesity is a growing public health problem, and to further investigate the issue, age-standardised data calculated from height and weight measurements were sourced from the World Health Organisation Global Database on Body Mass Index (http://www.who.int/bmi/). Interestingly, the relative order of the overweight (25–29.99 kg m−2) /obesity (≥30 kg m−2) prevalence across studies among our controls and that of the corresponding country-specific WHO BMI prevalence from around the year 2000 are not strongly correlated (Spearman’s ρ=0.41, p=0.08) (Figure 4). WHO data place the USA, Germany and the UK at the top while our self-reported information rate the Czech Republic, USA and Italy as having the highest overweight/obesity prevalence. Whilst differences between our data and WHO are likely to be related to factors such as age, sex and time period, they serve to illustrate the rapidly changing patterns and wide variations that exist around the world.
Figure 4.
Comparison of Control and WHO Overweight/Obesity Prevalences by Study.
Overweight (BMI 25–29.99 kg m−2) and Obesity (BMI ≥ 30 kg m−2) prevalence from the World Health Organisation (WHO) Global Database on Body Mass Index (http://www.who.int/bmi/). WHO prevalence was derived from the most recent published age- and sex- standardised BMI data calculated from height and weight measured in clearly defined population samples; these data were largely from around the year 2000. The relative order of control overweight/obesity prevalences across studies was not similar to that from data reported on the WHO Global database for BMI (Spearman’s ρ=0.41, p=0.08).
Self-reports of anthropometric information is known to be inexact, with height tending to be overestimated and weight underestimated (52). The nature of individual misreporting is likely to be complex, being related not only to their actual size but also to other factors such as age and sex. In a cohort of British adults, for instance, where self-reported and measured data were compared, height was overestimated most by older people, shorter men and heavier women, while the greatest underestimation of weight was amongst heavier men and women (53). This tendency for people to report BMI closer to ‘normal’ may have diluted our odds ratios. It is also possible that weight loss associated with lymphoma may have influenced the recall of cases differently to that of controls. Because of this, at interview subjects were either asked to recall their usual weight or their weight at a specified times before diagnosis/interview, and restricting the analyses to the six studies (NCI-SEER, Mayo Phase 1, British Columbia, UK, North Italy and Italy) where data were requested at 1 or 5 years prior to diagnosis yielded similar results to the findings overall.
Whilst BMI derived from height and weight acts as an easily obtained estimate of adiposity, its use as a marker of obesity has several potential weaknesses. Across different ethnic groups, for example, a given BMI may not correspond to the same proportions of body fat (54). Moreover since the index was originally devised as a means of assessing average body composition among sedentary individuals of working age it may not truly reflect the degree of adiposity across the population as a whole. For instance, among the elderly where muscle mass may have started to decline, body fat mass may be underestimated by BMI whereas amongst athletes it may be overestimated. To account for the potential variation in BMI as a marker of body fat across different populations, we grouped our data according to study-specific control distributions as well as WHO BMI categories. We also repeated the analyses restricting data to Caucasians, and to North American and Northern European studies combined. Sensitivity analyses were conducted too among persons aged 65 or less (71% of our subjects), and among those who were not regular heavy exercisers where this information was requested (NCI-SEER, British Columbia and HERPACC2). These additional investigations gave similar findings to the presented results. More specific estimates of adiposity may be derived from total body fat mass and, as a marker for abdominal fat distribution, waist-to-hip ratios, but such data were not obtained in the studies included here and have only rarely been investigated with respect to NHL elsewhere, showing little effect (15;21;26).
In conclusion, this pooled analysis of case-control studies from 13 countries, crossing 3 continents, did not find an association between NHL and increased BMI. ORs were raised in studies from some countries, namely the US, Canada and Northern European nations, but even within this group, heterogeneity was observed, questioning the validity of a combined odds ratio. The findings presented here were based on individual data from a large number of subjects enrolled in 18 studies, pooling of which were accomplished through the InterLymph consortium. Some of the advantages of this pooled analysis include information on confounders and NHL subtypes as well as data on height and weight, the constituent components of BMI. One potential confounding factor not assessed here is diet but dietary data will be examined, in conjunction with BMI, in a future InterLymph pooled analysis. Such investigations may further elucidate whether NHL or its subtypes are associated with obesity per se.
Supplementary Material
Acknowledgments
Studies that contributed data to this pooled analysis were supported by: NCI contracts PC65064, PC67008, PC67009, PC67010, PC71105 and the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI-SEER study); American Institute for Cancer Research grant 99B083 (Nebraska study); grants CA92153 (Mayo Clinic study); grant CA50850 from NCI (USCF study); the Canadian Cancer Society through the National Cancer Institute of Canada, the Canadian Institutes for Health Research and the Chan Sisters Foundation (British Columbia study); the Leukaemia Research Fund (UK study); NIH grant 5RO1 CA69269-02, Swedish Cancer Society grant 02 661, Plan Denmark grant, The Danish National Research Foundation grant (SCALE); European Commission 5th Framework Program, Quality of Life (grant No. QLK4-CT-2000-00422); the Spanish Ministry of Health (grant No. 04-0091, CIBER 06/0073); La Fondation de France, Compagnia di San Paolo di Torino, Programma Oncologia 2001; the German Federal Office for Radiation Protection (grants No. StSch4261 and StSch4420); the Health Research Board (Ireland) (EpiLymph); the National Research Council (CNR) Applied Project “Clinical Applications of Oncological Research” and the Italian Association for Cancer Research (northern Italy study); grant CA51086 from NCI, the European Community (Europe Against Cancer Programme), and the Lega Italiana per la Lotta Contro i Tumori (Italy study); Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan (HERPACC1 & 2).
References
- 1.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001 May;2(2):131–40. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 2.Samartin S, Chandra RK. Obesity, overnutrition and the immune system. Nutrition Research. 2001;21(1–2):243–62. [Google Scholar]
- 3.Holly EA, Lele C, Bracci PM, McGrath MS. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay Area, California. Am J Epidemiol. 1999 Aug 15;150(4):375–89. doi: 10.1093/oxfordjournals.aje.a010017. [DOI] [PubMed] [Google Scholar]
- 4.Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, Adami HO. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001 Jan;12(1):13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004 Feb 1;159(3):259–68. doi: 10.1093/aje/kwh041. [DOI] [PubMed] [Google Scholar]
- 7.Bahl S, Cotterchio M, Kreiger N, Klar N. Antidepressant medication use and non-Hodgkin’s lymphoma risk: no association. Am J Epidemiol. 2004 Sep 15;160(6):566–75. doi: 10.1093/aje/kwh234. [DOI] [PubMed] [Google Scholar]
- 8.Cerhan JR, Bernstein L, Severson RK, Davis S, Colt JS, Blair A, Hartge P. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control. 2005 Dec;16(10):1203–14. doi: 10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 9.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005 Jul 20;23(21):4742–54. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 10.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005 Oct 31;93(9):1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett EV, Skibola CF, Adamson P, Skibola DR, Morgan GJ, Smith MT, Roman E. Non-Hodgkin’s lymphoma, obesity and energy homeostasis polymorphisms. Br J Cancer. 2005 Oct 3;93(7):811–6. doi: 10.1038/sj.bjc.6602762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu BC, Gapstur SM, Greenland P, Wang R, Dyer A. Body mass index, abnormal glucose metabolism, and mortality from hematopoietic cancer. Cancer Epidemiol Biomarkers Prev. 2006 Dec;15(12):2348–54. doi: 10.1158/1055-9965.EPI-06-0007. [DOI] [PubMed] [Google Scholar]
- 13.Engeland A, Tretli S, Hansen S, Bjorge T. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. Am J Epidemiol. 2007 Jan 1;165(1):44–52. doi: 10.1093/aje/kwj353. [DOI] [PubMed] [Google Scholar]
- 14.Chiu BC, Soni L, Gapstur SM, Fought AJ, Evens AM, Weisenburger DD. Obesity and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2007 May 7; doi: 10.1007/s10552-007-9013-9. [DOI] [PubMed] [Google Scholar]
- 15.Lim U, Morton LM, Subar AF, Baris D, Stolzenberg-Solomon R, Leitzmann M, Kipnis V, Mouw T, Carroll L, Schatzkin A, Hartge P. Alcohol, Smoking, and Body Size in Relation to Incident Hodgkin’s and Non-Hodgkin’s Lymphoma Risk. Am J Epidemiol. 2007 Jun 27; doi: 10.1093/aje/kwm122. [DOI] [PubMed] [Google Scholar]
- 16.Paffenbarger RS, Jr, Wing AL, Hyde RT. Characteristics in youth predictive of adult-onset malignant lymphomas, melanomas, and leukemias: brief communication. J Natl Cancer Inst. 1978 Jan;60(1):89–92. doi: 10.1093/jnci/60.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Whittemore AS, Paffenbarger RS, Jr, Anderson K, Lee JE. Early precursors of site-specific cancers in college men and women. J Natl Cancer Inst. 1985 Jan;74(1):43–51. [PubMed] [Google Scholar]
- 18.Franceschi S, Serraino D, Bidoli E, Talamini R, Tirelli U, Carbone A, La Vecchia C. The epidemiology of non-Hodgkin’s lymphoma in the north-east of Italy: a hospital-based case-control study. Leuk Res. 1989;13(6):465–72. doi: 10.1016/0145-2126(89)90168-9. [DOI] [PubMed] [Google Scholar]
- 19.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A(3):344–50. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Hunter DJ, Rosner BA, Colditz GA, Fuchs CS, Speizer FE, Willett WC. Dietary fat and protein in relation to risk of non-Hodgkin’s lymphoma among women. J Natl Cancer Inst. 1999 Oct 20;91(20):1751–8. doi: 10.1093/jnci/91.20.1751. [DOI] [PubMed] [Google Scholar]
- 21.Cerhan JR, Janney CA, Vachon CM, Habermann TM, Kay NE, Potter JD, Sellers TA, Folsom AR. Anthropometric characteristics, physical activity, and risk of non-Hodgkin’s lymphoma subtypes and B-cell chronic lymphocytic leukemia: a prospective study. Am J Epidemiol. 2002 Sep 15;156(6):527–35. doi: 10.1093/aje/kwf082. [DOI] [PubMed] [Google Scholar]
- 22.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF., Jr Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004 Feb;15(1):35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 23.Bosetti C, Dal Maso L, Negri E, Talamini R, Montella M, Franceschi S, La Vecchia C. Re: Body mass index and risk of malignant lymphoma in Scandinavian men and women. J Natl Cancer Inst. 2005 Jun 1;97(11):860–1. doi: 10.1093/jnci/dji150. [DOI] [PubMed] [Google Scholar]
- 24.Chang ET, Hjalgrim H, Smedby KE, Akerman M, Tani E, Johnsen HE, Glimelius B, Adami HO, Melbye M. Body mass index and risk of malignant lymphoma in Scandinavian men and women. J Natl Cancer Inst. 2005 Feb 2;97(3):210–8. doi: 10.1093/jnci/dji012. [DOI] [PubMed] [Google Scholar]
- 25.Lukanova A, Bjor O, Kaaks R, Lenner P, Lindahl B, Hallmans G, Stattin P. Body mass index and cancer: Results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006 Jan 15;118(2):458–66. doi: 10.1002/ijc.21354. [DOI] [PubMed] [Google Scholar]
- 26.MacInnis RJ, English DR, Hopper JL, Giles GG. Body size and composition and the risk of lymphohematopoietic malignancies. J Natl Cancer Inst. 2005 Aug 3;97(15):1154–7. doi: 10.1093/jnci/dji209. [DOI] [PubMed] [Google Scholar]
- 27.Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Adami J. Tobacco use, body mass index and the risk of malignant lymphomas--a nationwide cohort study in Sweden. Int J Cancer. 2006 May 1;118(9):2298–302. doi: 10.1002/ijc.21617. [DOI] [PubMed] [Google Scholar]
- 28.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006 Sep;17(7):901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 29.Willett EV, Smith AG, Dovey GJ, Morgan GJ, Parker J, Roman E. Tobacco and alcohol consumption and the risk of non-Hodgkin lymphoma. Cancer Causes Control. 2004 Oct;15(8):771–80. doi: 10.1023/B:CACO.0000043427.77739.60. [DOI] [PubMed] [Google Scholar]
- 30.Besson H, Brennan P, Becker N, De SS, Nieters A, Font R, Maynadie M, Foretova L, Cocco PL, Staines A, Vornanen M, Boffetta P. Tobacco smoking, alcohol drinking and Hodgkin’s lymphoma: a European multi-centre case-control study (EPILYMPH) Br J Cancer. 2006 Aug 7;95(3):378–84. doi: 10.1038/sj.bjc.6603229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker N, Deeg E, Nieters A. Population-based study of lymphoma in Germany: rationale, study design and first results. Leuk Res. 2004 Jul;28(7):713–24. doi: 10.1016/j.leukres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 32.de Sanjose S, Shah KV, Domingo-Domenech E, Engels EA, Fernandez dS, Alvaro T, Garcia-Villanueva M, Romagosa V, Gonzalez-Barca E, Viscidi RP. Lack of serological evidence for an association between simian virus 40 and lymphoma. Int J Cancer. 2003 Apr 20;104(4):522–4. doi: 10.1002/ijc.10993. [DOI] [PubMed] [Google Scholar]
- 33.Talamini R, Montella M, Crovatto M, Dal Maso L, Crispo A, Negri E, Spina M, Pinto A, Carbone A, Franceschi S. Non-Hodgkin’s lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004 Jun 20;110(3):380–5. doi: 10.1002/ijc.20137. [DOI] [PubMed] [Google Scholar]
- 34.Tajima K, Hirose K, Inoue M, Takezaki T, Hamajima N, Kuroishi T. A Model of Practical Cancer Prevention for Out-patients Visiting a Hospital: the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) Asian Pac J Cancer Prev. 2000;1(1):35–47. [PubMed] [Google Scholar]
- 35.Suzuki T, Matsuo K, Ito H, Hirose K, Wakai K, Saito T, Sato S, Morishima Y, Nakamura S, Ueda R, Tajima K. A past history of gastric ulcers and Helicobacter pylori infection increase the risk of gastric malignant lymphoma. Carcinogenesis. 2006 Jul;27(7):1391–7. doi: 10.1093/carcin/bgi334. [DOI] [PubMed] [Google Scholar]
- 36.Spinelli JJ, Ng C, Weber JP, Connors JM, Gascoyne RD, Lai A, Brooks-Wilson A, Le ND, Berry B, Gallagher RP. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007 doi: 10.1002/ijc.23005. In press. [DOI] [PubMed] [Google Scholar]
- 37.Jaffe E, Harris N, Stein H, Vardiman J. Tumours of the Haemopoietic and Lymphoid Tissues. IARC Press; 2001. [Google Scholar]
- 38.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, Jack A, Cozen W, Maynadie M, Spinelli JJ, Costantini AS, Rudiger T, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007 Mar 27; doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO Expert Committee on Physical Status. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995. Report No.: 854. [PubMed] [Google Scholar]
- 40.Morton LM, Hartge P, Holford TR, Holly EA, Chiu BC, Vineis P, Stagnaro E, Willett EV, Franceschi S, La Vecchia C, Hughes AM, Cozen W, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph) Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):925–33. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 41.Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, Spinelli JJ, Willett E, de Sanjose S, Cocco P, Berndt SI, Brennan P, et al. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: a report from the InterLymph Consortium. Lancet Oncol. 2006 Jan;7(1):27–38. doi: 10.1016/S1470-2045(05)70434-4. [DOI] [PubMed] [Google Scholar]
- 42.Morton LM, Zheng T, Holford TR, Holly EA, Chiu BC, Costantini AS, Stagnaro E, Willett EV, Dal Maso L, Serraino D, Chang ET, Cozen W, et al. Alcohol consumption and risk of non-Hodgkin lymphoma: a pooled analysis. Lancet Oncol. 2005 Jul;6(7):469–76. doi: 10.1016/S1470-2045(05)70214-X. [DOI] [PubMed] [Google Scholar]
- 43.Wang SS, Slager SL, Brennan P, Holly EA, De SS, Bernstein L, Boffetta P, Cerhan JR, Maynadie M, Spinelli JJ, Chiu BC, Cocco PL, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007 Apr 15;109(8):3479–88. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, Spinelli JJ, De SS, Hartge P, Melbye M, Willett EV, Becker N, et al. Personal sun exposure and risk of non Hodgkin lymphoma: A pooled analysis from the Interlymph Consortium. Int J Cancer. 2007 Aug 20; doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 45.Breslow NE, Day NE. The Analysis of Case-Control Studies. Vol. 1. Lyon: International Agency for Research on Cancer; 1980. Statistical Methods in Cancer Research. [PubMed] [Google Scholar]
- 46.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 47.Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol. 1995 Aug 15;142(4):371–82. doi: 10.1093/oxfordjournals.aje.a117644. [DOI] [PubMed] [Google Scholar]
- 48.Delgado-Rodriguez M. Glossary on meta-analysis. J Epidemiol Community Health. 2001 Aug;55(8):534–6. doi: 10.1136/jech.55.8.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stata Statistical Software: Release 9 [computer program] College Station, Texas: Stata Corporation; 2005. [Google Scholar]
- 50.Skibola CF, Holly EA, Forrest MS, Hubbard A, Bracci PM, Skibola DR, Hegedus C, Smith MT. Body mass index, leptin and leptin receptor polymorphisms, and non-hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004 May;13(5):779–86. [PubMed] [Google Scholar]
- 51.Larsson SC, Wolk A. Obesity and risk of non-Hodgkin’s lymphoma: A meta-analysis. Int J Cancer. 2007 Apr 18; doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- 52.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007 Jul;8(4):307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 53.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002 Aug;5(4):561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 54.WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva, Switzerland: World Health Organization; 2000. Report No.: 894. [PubMed] [Google Scholar]
- 55.WHO Expert Committee on Physical Status. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995. Report No.: 854. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.