Abstract
During gastrulation, distinct lineage specification into three germ layers, the mesoderm, endoderm and ectoderm, occurs through an elaborate harmony between signaling molecules along the embryonic proximo-distal and anterior-posterior axes, and Nodal signaling plays a key role in the early embryonic development governing embryonic axis formation, mesoderm and endoderm specification, and left-right asymmetry determination. However, the mechanism by which Nodal expression is regulated is largely unknown. Here, we show that Meteorin regulates Nodal expression and is required for mesendoderm development. It is highly expressed in the inner cell mass of blastocysts and further in the epiblast and extra-embryonic ectoderm during gastrulation. Genetic ablation of the Meteorin gene resulted in early embryonic lethality, presumably due to impaired lineage allocation and subsequent cell accumulation. Embryoid body culture using Meteorin-null embryonic stem (ES) cells showed reduced Nodal expression and concomitant impairment of mesendoderm specification. Meteorin-null embryos displayed reduced levels of Nodal transcripts before the gastrulation stage, and impaired expression of Goosecoid, a definitive endoderm marker, during gastrulation, while the proximo-distal and anterior-posterior axes and primitive streak formation were preserved. Our results show that Meteorin is a novel regulator of Nodal transcription and is required to maintain sufficient Nodal levels for endoderm formation, thereby providing new insights in the regulation of mesendoderm allocation.
Introduction
During embryonic axis formation, lineage specification is governed by the reciprocal signaling including Wnt, Bone morphogenetic protein (BMP), and Nodal signaling pathways among epiblast, extra-embryonic ectoderm, and visceral endoderm cell populations [1], [2], [3], [4], [5], [6], [7], [8]. The proximo-distal (P–D) axis in the epiblast is primarily established by Nodal signaling that activates its antagonists Lefty1 and Cerberus-like (Cerl) in the distal region of the visceral endoderm [9], [10]. Activation of these antagonists in the distal visceral endoderm restricts Nodal target gene expression to the proximal region. The movement of the distal visceral endoderm toward the anterior side of the conceptus further generates the anterior-posterior (A–P) axis [5], [11]. The differential gene expression patterns of several signaling pathways along the P–D and A–P axes subsequently guide proper embryonic pattern formation and successful embryogenesis [5], [12]. Although the significance of embryonic axes formation is well documented in genetic studies, the mechanisms by which these embryonic axes cause the emergence of further lineage specification remain unclear.
Nodal, a member of the Transforming growth factor-beta (TGF-β) superfamily [1], binds type I and II activin receptors and the co-receptor Cripto, which leads to phosphorylation of Smad2/3. Phosphorylated Smad2/3 interacts with Smad4, translocates to the nucleus, and acts as a transcriptional activator with several regulatory factors such as Forkhead box protein H1 (FoxH1) [13]. This Nodal signaling plays a key role in the early embryonic development governing P–D and A–P axes formation [5], [12], [14], endoderm and mesoderm specification [7], [15], [16], and left-right asymmetry determination [17], through positive regulation of its own expression as well as that of Lefty2, a negative regulator of Nodal signaling [18], [19]. Although Nodal plays a critical role in early pattern formation, a more in-depth understanding of the regulation of Nodal expression has yet to be obtained.
Meteorin was initially identified as a secreted protein. During embryonic neurogenesis, it is expressed in neural progenitors and astrocyte precursors, and may play roles in glial cell differentiation and axonal network formation [20]. Jorgensen et al. reported that Meteorin is also broadly expressed in astrocytes, Bergmann glial cells, and some interneurons in the adult brain, suggesting that it may be implicated in gliogenesis and angiogenesis [21], [22]. Moreover, recent studies suggested that it has a neurotrophic potential in several in vivo injury models [23], [24]. However, its physiological roles in neurogenesis, gliogenesis, and angiogenesis have not yet been determined through genetic models yet. In this study, we disrupted the Meteorin gene by homologous recombination. Unexpectedly, we found that Meteorin−/− mice show early embryonic lethality. Consistent with this, Meteorin was highly expressed during the preimplantation and gastrulation stages, suggesting that Meteorin may play a role in early embryogenesis. Experiments using cultured embryoid bodies (EBs), which recapitulate early embryonic developmental processes [25], revealed that the levels of Nodal and its downstream target genes, Lefty1 and Cerl, were decreased in Meteorin−/Δ EB cultures. Reduced expression of Nodal target genes was rescued by exogenous Nodal recombinant protein, suggesting that Nodal signaling itself is intact in the Meteorin−/Δ cells. Reduced levels of Nodal signaling and subsequent impairment in mesendoderm allocation were consistently observed upon disruption of Meteorin in both in vivo and in vitro systems, in agreement with the previous concept that graded Nodal signaling governs cell fate decisions during gastrulation [7], [26]. Our data show that regulation of Nodal signaling by Meteorin is required for mouse embryonic development.
Materials and Methods
Ethics statement
All the mice used in this study were maintained in the specific pathogen-free facility of Seoul National University, and the experiments followed the guidelines of the animal ethics committee. All animal experiments were approved by the Seoul National University Institutional Animal Care and Use Committee (Approval number: SNU120501-9).
Generation of Meteorin-knockout mice
Part of Meteorin exon 4 and its 3′ downstream sequence were substituted with an IRES-LacZ-Puro cassette, and the diphtheria toxin A (DTA) gene was introduced for negative selection. After electroporation of E14Tg2A embryonic stem (ES) cells with the targeting vector, clones were selected by puromycin treatment. Several clones were verified by Southern blot with a flanking probe (Fig. S1), and a number of positive clones were injected into C57BL/6J blastocysts to generate chimeras. After germline transmission, Meteorin+/−(puro) mice were bred with Protamine-Flpe mice for excision of the flippase recognition target (FRT)-flanked puromycin resistance gene. In this study, more than 6 rounds of breeding were carried out with C57BL/6J mice to obtain congenic mice.
Generation of Meteorin−/ΔES cells
DNA fragments containing the third and fourth exons of the Meteorin gene were flanked by loxP sequences in the targeting vector, and the neomycin resistance gene flanked by FRT sequences was introduced between the loxP sequences. The DTA gene was also inserted in the vector for negative selection. E14Tg2A ES cells were sequentially electroporated with targeting vectors. After neomycin and puromycin double selection, one ES cell line was identified by Southern blot screening. Cre expression was induced by transient expression of the pCAGGS-Cre vector, and the resulting Meteorin−/Δ cells were selected for further experiments (see Fig. S2). The pCAGGS-Cre and pCAGGS-mock vector were kindly provided by Ken-ichi Yamamura (Kumamoto University, Japan).
Embryo preparation, section, and hematoxylin and eosin (H&E) staining
Embryos at each developmental stage were collected from timed-pregnant mice. The noon of the day of plug detection was designated as E0.5. For histological sectioning, the embryos were obtained in ice-cold PBS and fixed in 4% paraformaldehyde at 4°C overnight. After the samples were washed with distilled water overnight, they were subjected to serial dehydration steps and paraffin embedding. Four-micrometer-thick sections were cut for staining with hematoxylin and eosin. All images were captured with a Zeiss Axio Imager A2 microscope and Olympus DP70 camera or Diagnostic SPOTFlex camera.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described previously [27]. Gene-specific riboprobe vectors were generated by cloning a nucleotide fragment of each gene into the pGEM-T Easy vector. The sequences of the primers used to amplify the cDNA fragments have been provided in Table S1. The Meteorin, Lefty1/2, Cerl, Sox17, and BMP4 probes were kindly provided by Kyu-Won Kim (Seoul National University, South Korea), Hiroshi Hamada (Osaka University, Japan), José António Belo (University of Algarve, Portugal), Yoshiakira Kanai (The University of Tokyo, Japan) and Paul Overbeek (Baylor College of Medicine, USA), respectively. All images were captured with a Leica MZ16 and Olympus DP70 camera or Diagnostic SPOTFlex camera.
ES cell culture and EB culture
ES cells were maintained in ES cell media supplemented with or without 300 µg/ml G418 or 3 µg/ml puromycin on mitomycin C-treated mouse embryonic fibroblasts. Recombinant mouse Nodal (rmNodal; R&D, USA) or recombinant mouse Meteorin (kindly provided from Dr. Kyu-Won Kim; Seoul National University, South Korea) were applied to the culture medium to rescue the defects of Meteorin-deficient ES cells. Meteorin cDNA was cloned into the pCAGGS vector (pCAGGS-mMeteorin), and Meteorin−/Δ ES cells were transfected using Lipofectamine™ 2000 reagent (Invitrogen, USA) to induce the transient expression of Meteorin. EBs were cultured as previously described [28].
qRT-PCR analysis
Total RNA isolation, reverse transcription, and real-time qRT-PCR were conducted as previously described [29]. The sequences of the primers used in this study have been provided in Table S1. P-values were calculated using the Student's t-test.
Western blots
For western blot assays, cells were harvested and sonicated in IP buffer (1% Triton X-100, 80 mM NaCl, 3 mM EDTA, and 50 mM HEPES with protease inhibitors). Generally, 5∼20 µg of protein from lysate supernatants was separated by size, blotted with each primary and secondary antibody, and detected with WEST-ZOL plus (Intron). Rabbit anti-β-actin (Sigma), rabbit anti-p-Smad2 (Cell Signaling, #3101), rabbit anti-Smad2 (Cell Signaling, #5339), and rabbit anti-p-Smad2 (Cell Signaling, #3101) were used for this research.
Results
Meteorin expression in early embryos
To determine whether Meteorin is expressed earlier than reported [20], we analyzed mRNA expression of Meteorin in early embryos through in situ hybridization. We found that Meteorin was expressed as early as the blastocyst stage and that its expression was restricted to the inner cell mass (Fig. 1A). After implantation, it was ubiquitously expressed throughout epiblast and extra-embryonic ectoderm cells, but not in the visceral endoderm at E6.5 (Fig. 1B, B′) and was maintained in these areas as embryonic development progressed. It was highly expressed in the epiblast close to the proamniotic cavity with a gradient pattern and vague in the outer epiblast adjacent to visceral endoderm (Fig. 1C, D, and D′).
Figure 1. Expression of Meteorin in early embryogenesis.
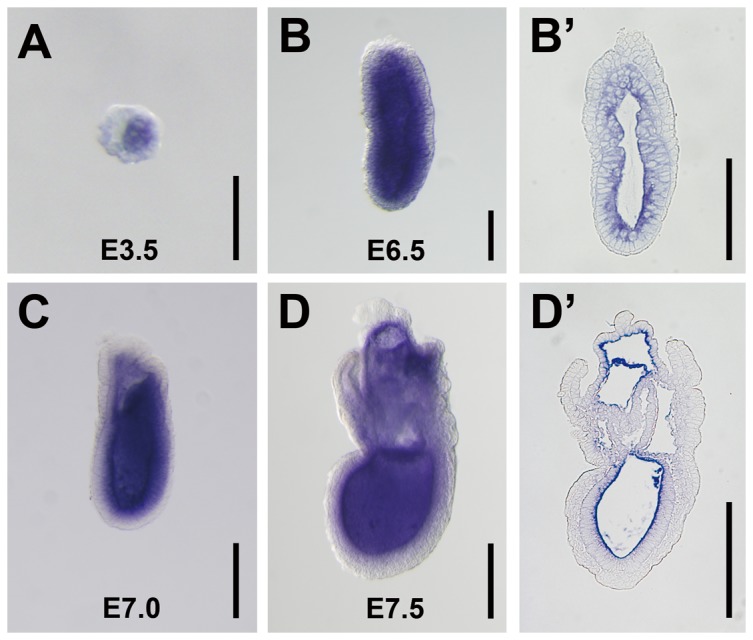
(A–D) Expression of Meteorin mRNA at different stages of development was analyzed by whole-mount in situ hybridization with embryos from a timed-pregnant mouse. (A) Meteorin was expressed in the inner cell mass at the E3.5 blastocyst stage. (B, B′) At E6.5, the pre-steak stage, Meteorin was expressed in the extra-embryonic ectoderm and epiblast, but not in the visceral endoderm. (C, D, D′) During E7.0–E7.5, the mid- to late-gastrulation stages, Meteorin expression was sustained in extra-embryonic ectoderm and the epiblast, but not in extra-embryonic tissue. (B′ and D′) After in situ hybridization with a probe specific for Meteorin at each developmental stage, embryos were fixed, embedded in paraffin, and then sectioned sagittally. The expression level was relatively high in cells close to the proamniotic cavity. All experiments were conducted with more than 5 embryos at each developmental stage and the representative images were captured. Scale bars in A, B, and B′: 100 µm. Scale bars in C, D, D′: 200 µm.
Impaired gastrulation and early embryonic lethality in Meteorin-null embryos
To elucidate the biological function of Meteorin in early embryonic development, we generated Meteorin-null mice using a gene-targeting method. Meteorin+/− mice were viable and fertile with no apparent differences compared to wild-type mice, but no viable Meteorin−/− mice were observed from heterozygous matings at birth, suggesting that Meteorin deficiency results in embryonic lethality. To determine when Meteorin−/− embryos died, embryos from timed-pregnant heterozygous females at differential gestational stages were genotyped. At earlier than E7.0, Meteorin−/− embryos appeared in the expected Mendelian ratio. At E7.5, several Meteorin−/− embryos were obtained but their proportions were beyond the Mendelian ratio. At E8.5, no Meteorin−/− embryos were observed, indicating that Meteorin−/− embryos die between E7.0 and E8.5 (Table 1).
Table 1. Early embryonic lethality in Meteorin−/− embryos.
| Number of embryos for each genotype (%) | ||||
| Age of embryos | Meteorin+/+ | Meteorin+/− | Meteorin−/− | Total n |
| E8.5 | 13 (33.33) | 26 (66.67) | 0 (0)A | 39 |
| E7.5 | 20 (35.71) | 30 (53.57) | 6 (10.71)B | 56 |
| E7.0 | 16 (23.18) | 40 (57.97) | 13 (18.84) | 69 |
| E6.5 | 9 (23.68) | 21 (55.26) | 8 (21.05) | 38 |
Chi-square test: A; p = 0.0015, B; p = 0.0261.
To determine whether the early lethality of Meteorin−/− embryos is due to abnormality prior to implantation, we collected E3.5 blastocysts from heterozygote intercrosses and cultured them individually for 5 days. Two days after culture, all blastocysts had successfully attached to the bottom of the culture dish and hatched from the zona pellucida. Five days after culture, all the blastocysts displayed apparently normal outgrowth of trophoblast giant cells and inner cell mass (Fig. S2A–C). In situ hybridization of Pou domain class 5 transcription factor 1 (Pou5f1) (also known as Oct3/4), a marker of inner cell mass also shows that there is no difference between wild-type and Meteorin-null embryos (Fig. S2D–G). Taken together, it suggests that Meteorin−/− embryos develop normally prior to implantation.
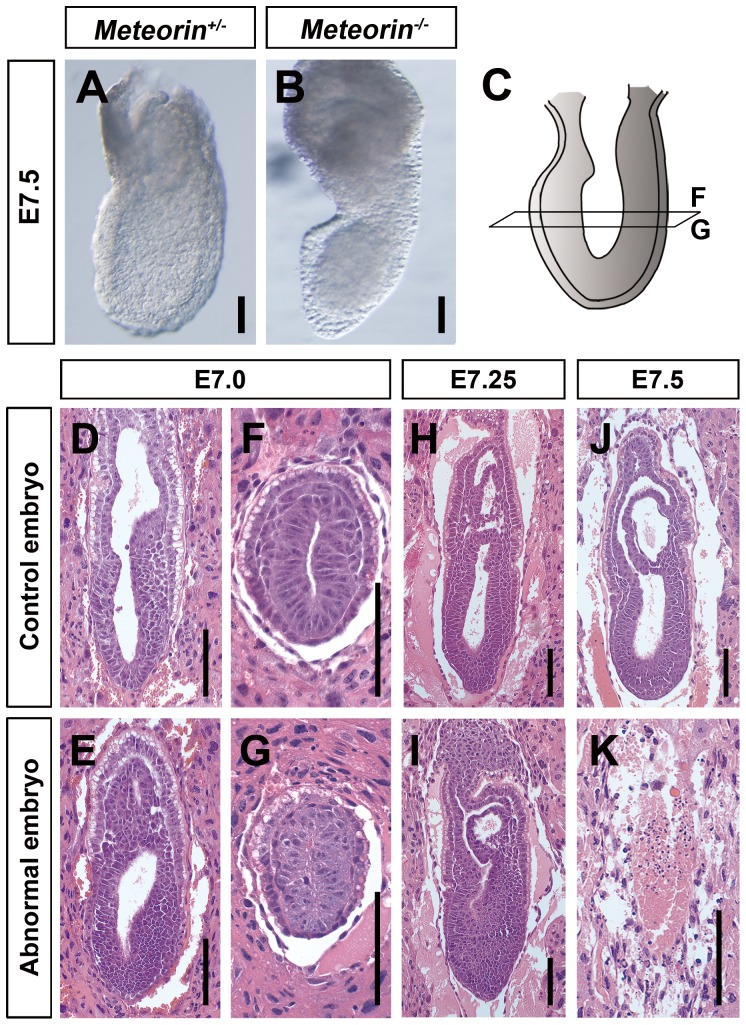
As blastocyst outgrowth experiments revealed normal Meteorin−/− embryo development prior to implantation, we examined whether there were developmental defects during the gastrulation stage in Meteorin−/− embryos. As shown in Fig. 2A and B, viable Meteorin−/− embryos at E7.5 were much smaller than controls, especially in the embryonic region, suggesting that Meteorin may play a role in embryonic development. Histological analysis showed that there was no apparent difference between embryos from intercrosses until E6.75 (data not shown). Mesendoderm cells derived from the epiblast are specified between E7.0 and E7.5 and migrate through the primitive streak [8]. Between E7.0 and E7.25, around one-fourth of embryos from heterozygous matings displayed abnormal accumulation of cells in the presumptive primitive streak toward the posterior or distal region of the embryos, suggesting that impaired mesendoderm development and disorganized embryonic integrity might lead to lethality and empty deciduae at E7.5 (Fig. 2D–K).
Figure 2. Developmental defects in Meteorin−/− embryos during gastrulation.
(A, B) Gross morphology of Meteorin+/+ and Meteorin−/− embryos at E7.5. A fraction of Meteorin−/− embryos survived at E7.5 and these were markedly smaller than Meteorin+/+ embryos. (C) A schematic view indicates where the transverse section images of panels F and G were obtained. (D–K) Paraffin sections of each decidua from Meteorin heterozygous matings were stained with hematoxylin and eosin. At E7.0, slightly thickened cell layers were observed in the presumptive primitive streak in abnormal embryos (E and I) and this accumulation of cells became more apparent at E7.25 (I). At E7.5, some embryos were resorbed and were no longer seen in deciduae (K). All scale bars: 100 µm.
Meteorin-null EB culture displays defective mesendodermal differentiation
To investigate the nature of Meteorin−/− embryo abnormalities, we adopted the in vitro EB culture system, which recapitulates the differentiation process during gastrulation. To first generate Meteorin-null ES cells, we sequentially targeted both alleles of the Meteorin gene by homologous recombination (Fig. S3A and B). After the forced expression of Cre recombinase, Meteorin transcripts were no longer expressed in Meteorin−/Δ ES cells (Fig. S3D and Fig. 3B). There was no apparent difference in morphology or proliferation rate between Meteorin−/Δ and control ES cells (data not shown).
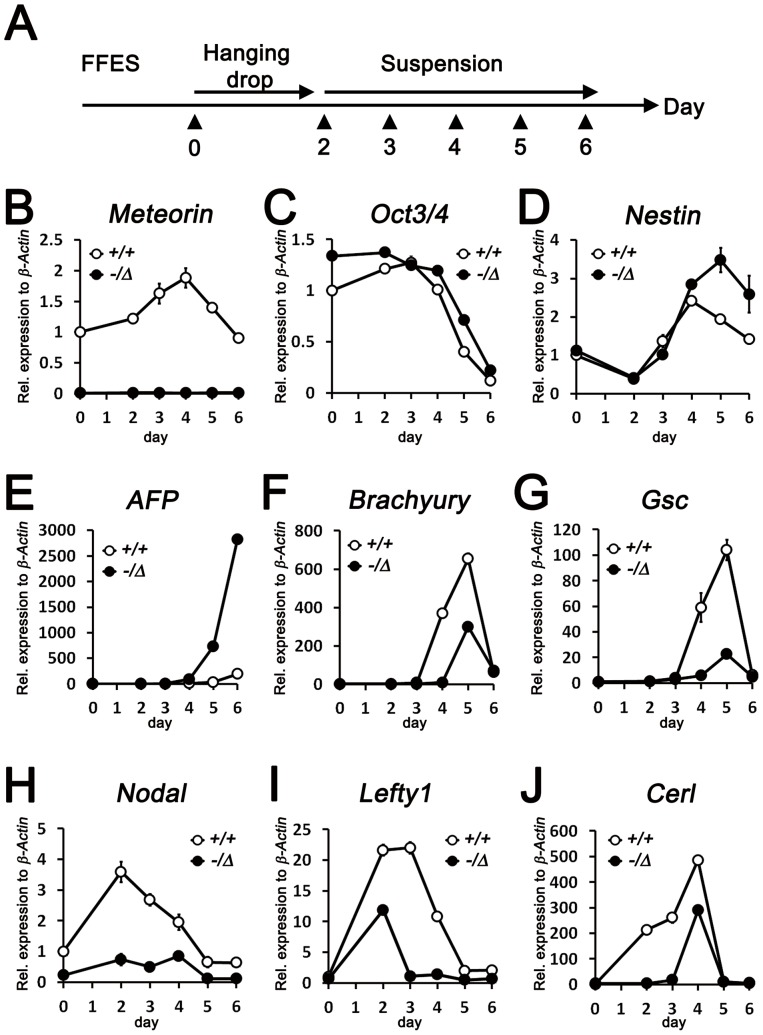
Figure 3. Impaired mesendodermal development in EB culture of Meteorin−/Δ cells.
(A) Schematic view of EB culture. After 2 passages maintained in feeder-free embryonic stem cells, cells were dissociated and cultured using hanging drops for 2 days. Then, they were collected and cultured on bacterial-grade dishes. At each day of culture, indicated as arrowheads in the schematic view, cells were harvested for subsequent qRT-PCR experiments. (B–H) The expression of early developmental lineage markers on each day of culture was analyzed by qRT-PCR. (B) Successful gene deletion in Meteorin−/Δ ES cells was confirmed by observing the transcript level of Meteorin. (C) Expression of Oct3/4, a pluripotency marker, was similarly maintained in Meteorin−/Δ EB culture compared to Meteorin+/+ EB culture. Expression levels of Nestin, an early neuroectoderm marker (D), and AFP, a visceral endoderm marker (E), were higher in Meteorin−/Δ EB culture than in Meteorin+/+ EB culture. The expression levels of Brachyury, an early mesendoderm marker (F), Gsc, an endoderm marker (G), and Nodal, a posterior epiblast marker (H), significantly decreased in Meteorin−/Δ EB culture throughout the culture period. The expression of Lefty1 (I) and Cerl (J), downstream molecules of Nodal signaling, was also lower in Meteorin−/Δ EB culture than in the control. Error bars indicate standard error of the mean (s.e.m.). All experiments were conducted more than 3 times and the representative graphs are shown.
Using these ES cells, we performed EB culture experiments. When ES cells are grown in medium with leukemia inhibitory factor (LIF), they can proliferate indefinitely while retaining their self-renewing activity. However, upon the removal of LIF, differentiation into three germ layers, the endoderm, mesoderm, and ectoderm, occurs spontaneously as in embryonic development [30]. After two days in hanging drop culture, cells were re-plated on bacterial-grade dishes in suspension for 4 consecutive days, and mRNA was harvested on each day of culture for subsequent qRT-PCR experiments (Fig. 3A). As EBs developed, the expression of the pluripotency markers, Oct3/4 and Nanog, gradually decreased in the control EB culture, while the expression of the differentiation markers Nestin, Neurofilament-M (NFM), Alpha-fetoprotein (AFP), Transthyretin (Ttr), Brachyury (T), Fetal liver kinase 1 (Flk1), Goosecoid (Gsc), and Mix1 homeobox-like 1 (Mixl1) was elevated (Fig. 3C–G and Fig. S4A–E) as reported earlier [31]. In the Meteorin−/Δ EB culture, the Oct3/4 and Nanog levels were comparable to those of the controls, suggesting that pluripotency is not influenced by Meteorin disruption (Fig. 3C and Fig. S4A). However, the expression of neuroectoderm markers, Nestin and NFM, and visceral endoderm markers, AFP and Ttr was significantly higher in the Meteorin−/Δ EB culture than in the controls (Fig. 3D–E and Fig. S4B–C). In contrast, the expression of mesoderm markers, Brachyury and Flk1, and definitive endoderm markers, Gsc and Mixl1, was significantly lower in Meteorin−/Δ EB culture than in the controls (Fig. 3F–G and Fig. S4D–E), suggesting that the differentiation potential toward the mesendoderm is specifically affected by the disruption of Meteorin.
Reduced Nodal signaling in Meteorin-null ES cells and embryos
When Nodal signaling, an important event in early embryonic patterning, is inhibited, mesendoderm formation is abrogated, while epiblast cells precociously differentiate toward the neuroectoderm [7], [16], [32]. To elucidate whether the defective differentiation in the Meteorin−/Δ EB culture is due to impaired Nodal signaling, we examined the expression of Nodal and its downstream genes, Lefty1 and Cerl. Intriguingly, all of these molecules were significantly downregulated in the Meteorin−/Δ EB culture compared to the controls (Fig. 3H–J), suggesting that the defects in mesendoderm differentiation in the Meteorin−/Δ EB culture are a result of decreased Nodal signaling.
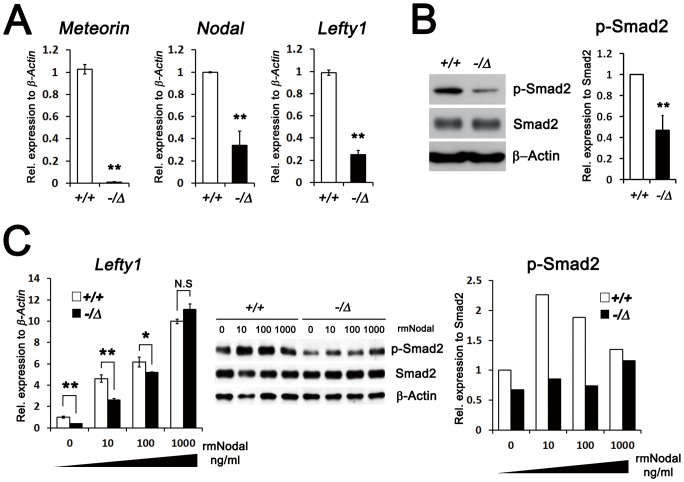
To investigate whether the decreased Nodal signaling is caused by reduced expression of Nodal in the Meteorin−/Δ ES cells themselves, we assessed the expression of Nodal and Lefty1 and found that it was lower in Meteorin−/Δ ES cells than in the controls (Fig. 4A). In vertebrates, Nodal signaling is transmitted through Smad2 phosphorylation and its subsequent translocation into the nucleus acting as a transcription factor [16]. Indeed, the phosphorylated level of Smad2 in Meteorin−/Δ ES cells was significantly lower than that of the control ES cells (Fig. 4B). To examine whether Meteorin−/Δ ES cells show any defect in the Nodal signaling pathway itself, we stimulated Meteorin−/Δ ES cells with recombinant mouse Nodal (rmNodal) protein. The expression of Lefty1 and the phosphorylation of Smad2 were rescued by the addition of exogenous rmNodal in Meteorin−/Δ ES cells (Fig. 4C), indicating that the Nodal signaling pathway remains intact in Meteorin−/Δ ES cells and that the reduced expression of Nodal itself might be regulated by Meteorin.
Figure 4. Reduced, but intact, Nodal signal transduction pathway in Meteorin−/Δ ES cells.
(A) Relative levels of Meteorin, Nodal, and Lefty1 in Meteorin+/+ and Meteorin−/Δ ES cells were analyzed by qRT-PCR. In Meteorin−/Δ ES cells, the transcript levels of these genes decreased. Error bars indicate standard deviation (s.d.). **p<0.01. (B) Smad2 and its phosphorylated level (p-Smad2) were investigated by western blotting, and the protein loading was normalized to β-Actin. In Meteorin−/Δ ES cells, the phosphorylated level of Smad2 significantly decreased. Scanned blot images were measured by Scion image. Error bars indicate s.d. **p<0.01. (C) Expression of Lefty1, a downstream molecule of Nodal signaling, was assessed by qRT-PCR at 24 hours after addition of recombinant mouse Nodal (rmNodal) protein to Meteorin+/+ or Meteorin−/Δ ES cells. Levels of Smad2 and p-Smad2 were also measured by western blotting at 2 hours after rmNodal treatment. The reduced levels of Lefty1 transcript and p-Smad2 were rescued by rmNodal treatment in Meteorin−/Δ ES cells. Scanned blot images were measured by Scion image.
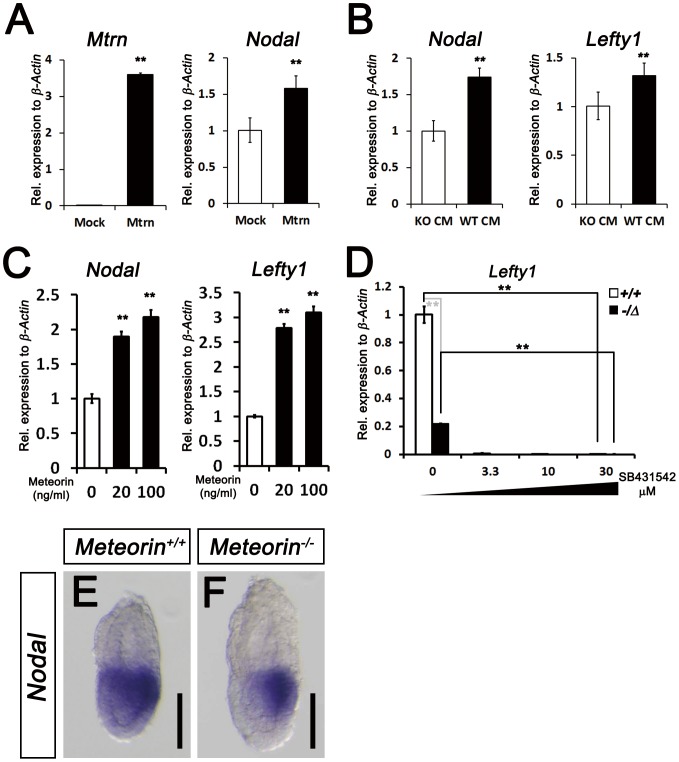
We next examined whether reduced Nodal expression in the Meteorin−/Δ ES cells can be rescued by introduction of Meteorin. When Meteorin−/Δ ES cells were transfected with a Meteorin-expressing vector, the expression of Nodal increased (Fig. 5A). Since Meteorin is a secreted protein, we applied the conditioned medium (CM) obtained from control ES cells to Meteorin−/Δ ES cells. As expected, the addition of control CM to Meteorin−/Δ ES cells led to increase in Nodal and Lefty1 expression (Fig. 5B). To rule out the possibility that this result was caused by Nodal protein in the conditioned medium, not by Meteorin protein, we directly applied recombinant mouse Meteorin (rmMeteorin) protein to Meteorin−/Δ ES cells. The addition of rmMeteorin to Meteorin-deficient ES cells also rescued the expression of Nodal and Lefty1, directly suggesting that Meteorin is required to produce sufficient amounts of Nodal signaling for normal development (Fig. 5C). On the other hand, residual Lefty1 expression in the Meteorin−/Δ ES cells was further decreased by SB431542, an inhibitor of type I TGF-beta receptors, (Fig. 5D), indicating that TGF-beta/Nodal signaling in the Meteorin−/Δ ES cells, albeit decreased, is functional. Indeed, in Meteorin−/− embryos, Nodal expresses, but the level was relatively lower than that in Meteorin+/+ embryos, especially in the proximal region of embryos (Fig. 5E–F).
Figure 5. Restoration of Nodal signaling in Meteorin+/Δ ES cells by Meteorin expression and reduced expression of Nodal in Meteorin−/− embryos.
(A) At 48-mMeteorin plasmid into Meteorin−/Δ ES cells, Meteorin and Nodal expression was higher than that of pCAGGS mock-transfected Meteorin−/Δ ES cells. (B) When conditioned medium obtained from Meteorin+/+ ES cells (WT CM) was applied to Meteorin−/Δ ES cells, the expression levels of Nodal and Lefty1 were higher than those seen when conditioned medium from Meteorin−/Δ (KO CM) was applied. (C) Nodal and Lefty1 expressions were analyzed by qRT-PCR 48 hrs after the addition of recombinant mouse Meteorin (rmMeteorin) to Meteorin−/Δ ES cells. Both gene expressions were induced by Meteorin protein addition. Error bars indicate s.e.m. **p<0.01. (D) Expression of Lefty1 transcript was assessed by qRT-PCR after the addition of SB431542, an inhibitor of type I TGF-beta receptors, to Meteorin+/+ or Meteorin−/Δ ES cell lines. The residual activity of TGF-beta/Nodal signaling in Meteorin−/Δ ES cells was further inhibited by SB431542 treatment. Error bars indicate s.e.m. **p<0.01. (E–F) Expression of Nodal transcripts was analyzed by in situ hybridization in Meteorin+/+ and Meteorin−/− embryos at each developmental stage. At E6.5, the expression level of Nodal was significantly lower in Meteorin−/− embryos than in Meteorin+/+ embryos, especially in the proximal epiblast. All scale bars: 100 µm.
The expression of endoderm-specific markers is severely affected in Meteorin−/− embryos
When Nodal signaling is completely blocked, the entire primitive streak is not formed [15], [16]. However, in mouse genetic models in which Nodal signaling is reduced, mesendoderm specification is selectively compromised, whereas primitive streak formation is preserved [7], [26], [33], [34]. Since Nodal transcripts were downregulated in Meteorin−/− embryos, we investigated whether the embryos displayed the developmental abnormalities frequently observed in Nodal signaling-compromised mutants [7], [26], [33]. As expected, the expression of Brachyury (T) and BMP4 in the Meteorin−/− embryos was comparable to that in wild-type embryos (Fig. 6A–B and Fig. S5A–B). Meteorin−/− embryos also showed comparable expression of both Lefty1 and Lefty2 in the anterior visceral endoderm and posterior primitive streak, respectively (Fig. 6C–F). In addition, FoxH1 and FoxA2, anterior primitive streak markers, were expressed in Meteorin−/− embryos (Fig. 6G–J), suggesting that mesodermal, extra-embryonic ectodermal, and visceral endodermal development occurred properly in the absence of Meteorin.
Figure 6. Selective reduction of the early endoderm markers Cerl and Gsc in Meteorin− /− embryos.
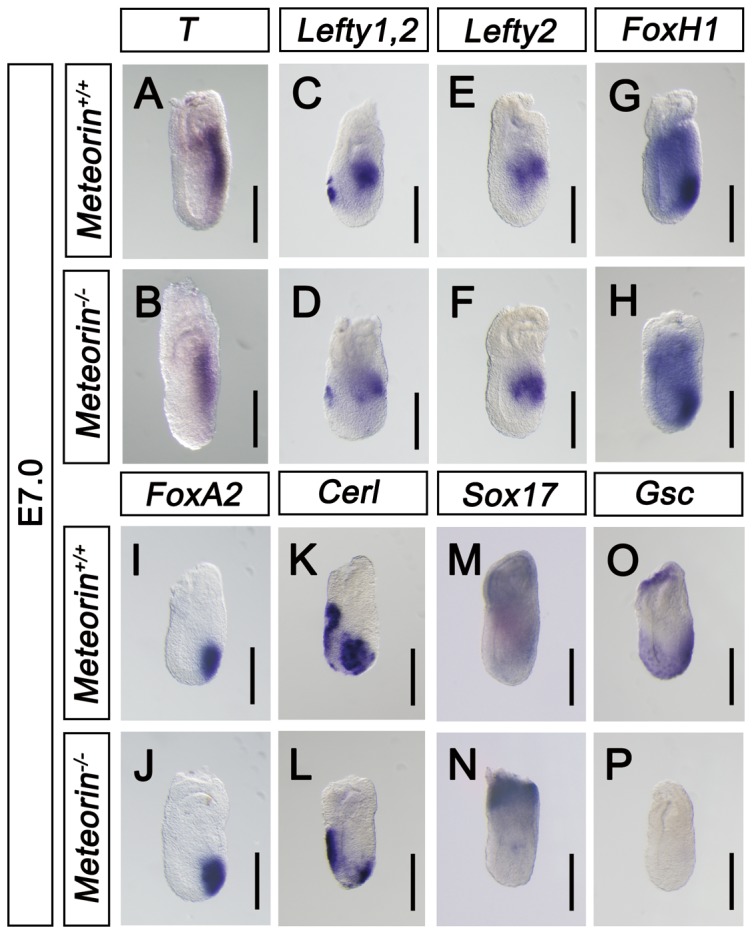
At E7.0, the expression of various developmental markers and Nodal signaling downstream molecules was analyzed by in situ hybridization with each specific probe in Meteorin+/+ and Meteorin−/− embryos. (A and B) Brachyury (T) expression levels were comparable in Meteorin−/− and Meteorin+/+ embryos. (C–F) The expression levels of Lefty1 and Lefty2, downstream molecules of Nodal signaling, were analyzed. The expression levels of Lefty1 in the anterior visceral endoderm and Lefty2 in the posterior primitive streak in Meteorin−/− embryos were similar to those of control embryos. (G and H) The expression level of FoxH1, a transcriptional coregulator of Nodal, was not changed in Meteorin−/− embryos compared to controls. (I and J) Both the expression domain and the level of FoxA2, a marker of the anterior primitive streak, were maintained in Meteorin−/− embryos. (K and L) Expression of Cerl, a marker of the anterior visceral endoderm and definitive endoderm, was downregulated specifically in the anterior primitive streak region of Meteorin−/− embryos compared to that of control embryos. (M and N) Sox17 was expressed in extra-embryonic ectoderm and definitive endoderm, and its expression in definitive endoderm was markedly reduced in Meteorin−/− embryos compared to controls. (O and P) Also, the expression of Gsc, another marker of definitive endoderm, was completely absent in Meteorin−/− embryos. For all experiments, at least three samples for each genotype were analyzed. All scale bars: 200 µm.
Cerl is expressed in the anterior visceral endoderm and definitive endoderm in the mid-gastrulation stage [5] (Fig. 6K). While the expression of Cerl in the anterior visceral endoderm is maintained in Meteorin−/− embryos, its expression in the definitive endoderm was specifically reduced (Fig. 6K and L). A marker of definitive endoderm, Sox17 was also down-regulated in Meteorin−/− embryos. (Fig. 6M and N). Consistently, the expression of Gsc was also absent in the Meteorin−/− embryos, suggesting that endoderm specification is selectively inhibited in Meteorin−/− embryos (Fig. 6O and P). From these data, we conclude that the specification of the definitive endoderm is blocked because of impaired Nodal signaling in Meteorin−/− embryos.
Discussion
To date, several studies have suggested Meteorin as a secreted protein which might be implicated in neurogenesis, gliogenesis, and angiogenesis [20], [21], [22]. In this study, we unexpectedly found that disruption of the Meteorin gene resulted in early embryonic lethality around E7.5. Indeed, in situ analysis revealed that Meteorin is expressed in the inner cell mass of blastocysts even before implantation and further in the epiblast and extra-embryonic ectoderm throughout peri-gastrulation stages, suggesting that Meteorin should play a critical role in early embryonic development. The EB culture experiments using Meteorin−/Δ ES cells showed that differentiation into the mesendoderm is selectively inhibited due to decrease in Nodal signaling. Through further experiments using Meteorin−/Δ ES cells and Meteorin−/− embryos, we found that Meteorin regulates the expression of Nodal, which is required for definitive endoderm development during gastrulation.
Although previous studies showed that Nodal signaling is crucial for mesendoderm allocation [35], [36] and we found that Nodal signaling is inhibited in Meteorin−/ΔES cells, we couldn't clearly address whether the reduced Nodal signaling is entirely responsible for all the defects in Meteorin−/Δ EB culture because we cannot impeccably mimic the Nodal signaling of control EB culture in Meteorin-deficient EB culture. Considering our results that the rmNodal addition to Meteorin-null ES cells rescues the expression of target gene of Nodal signaling and phosphorylation of Smad2, and the previous data from Takenaga et al. that the Nodal-overexpressing ES cells can generate mesoderm and endoderm cells at the expense of neuroectoderm cell [35], we believe that the decline in Nodal signaling in Meteorin-deficient ES cells is responsible for the reduction of the mesendoderm formation and the increase of the neuroectoderm lineage cells. However, we cannot completely rule out the possibility of existence of other Nodal-independent signaling pathways.
Nodal is initially expressed throughout the epiblast prior to gastrulation [15], which is required for the induction of distal visceral endoderm expression of Cerl and Lefty1 at the distal tip of the embryo [10], [12] as well as the movement of this visceral endoderm toward the anterior part of the embryo [11]. Cerl and Lefty1 act as negative regulators of Nodal to generate a gradient of Nodal signaling along the A–P axis, restricting the formation of the primitive streak to the posterior part of the embryo [5], [11]. Although Nodal signaling before gastrulation is important for A–P axis and proper primitive streak formation at the posterior region of the embryo, the development of these regions occurred properly in Meteorin−/− embryos, as Cerl and Lefty1 were well expressed in the anterior visceral endoderm and Brachyury in the posterior region, respectively. Indeed, the expression of Nodal was not completely blocked in Meteorin-null embryos, as evident by in situ hybridization at E6.5, and in vitro experiments also showed that SB431542, an inhibitor of TGF-beta/Nodal signaling, further inhibited the expression of a downstream target of Nodal signaling in Meteorin−/Δ ES cells, suggesting that the remaining signaling activity of Nodal is sufficient for proper A–P axis and primitive streak formation.
As the primitive streak elongates to the distal tip of embryos, extra-embryonic mesoderm first arises from the posterior primitive streak, followed by the paraxial and lateral plate mesoderm in the middle and finally the anterior primitive streak derivatives, including axial mesoderm and definitive endoderm in the anterior-most region of the primitive streak [37]. Recent studies have demonstrated that the level of Nodal signaling is important for these cell lineage allocations during gastrulation. Smad2+/−/Smad3−/− and Sox2Cre/Smad2Robm1/CA embryos, in which Nodal signaling is reduced, exhibited formation of the primitive streak, but not of anterior primitive streak derivatives such as the definitive endoderm and prechordal plate [26], [33]. Nodal hypomorph mutant Nodalfl/Δ and NodalΔPEE/413.d embryos also showed defects in definitive endoderm and prechordal plate formation because of the decreased level of Nodal signaling [33], [34]. Nodalnr/nr mice, in which the mutant protein Nr cannot be potentiated by subtilisin-like proprotein convertases, also displayed selective mesendoderm defects due to reduced Nodal signaling activity [7]. Studies through genetic models in which Nodal signaling strength is abrogated to varying degrees suggest a model for mesendoderm allocation by Nodal signaling strength that the signaling strength governs the discrete cell fate decisions along the primitive streak. Specification into anterior primitive streak derivatives requires higher Nodal signaling, while lower levels of Nodal signaling are sufficient for the generation of paraxial and lateral plate mesoderm [7], [26]. In Meteorin−/− embryos, the expression of Gsc, Sox17 and Cerl, markers of the definitive endoderm, was specifically downregulated at the anterior primitive streak, whereas the mesoderm was well specified, as evident by the expression of Brachyury in the elongating primitive streak possibly due to the reduced expression level of Nodal in epiblast. Thus, our data suggest that Meteorin is essential for Nodal expression levels to reach the threshold required to secure endodermal cell allocation.
In Xenopus laevis and Zebrafish, induction into the mesoderm and endoderm is dependent upon the strength of Nodal signaling. Low levels of Nodal signaling are sufficient for the expression of mesoderm markers, while higher levels induce endoderm marker expression [38], [39], [40], [41], [42]. Although it is not clear whether the discrete region of graded Nodal signaling is established during primitive streak elongation and how a subset of cells ingressing at the primitive streak attains increased Nodal signaling, it seems that the requirement of dose-dependent Nodal signaling for mesendoderm allocation is well conserved among species. To date, there is no report about Meteorin homologs in other species, but it seems that there are homologs of Meteorin in human, rat, and zebrafish based on sequence homology. Thus, further studies on whether the regulation of Nodal signaling by Meteorin is conserved among species would broaden our knowledge on how distinct lineage allocation is regulated during vertebrate development.
Supporting Information
Generation of Meteorin-null mice. (A) Schematic view of the targeting strategy. Black boxes indicate exons and an open arrow depicts diphtheria toxin A (DTA). The position of the flanking probe is indicated by a grey dash and the expected fragment sizes after BamHI digestion for Southern blotting are also provided. (B) Southern blotting of the BamHI-digested gDNA derived from Meteorin+/+ and Meteorin+/− mice. The flanking probe detects a 7.6-kb band for the wild-type allele (+) and an 11.1-kb band for the null allele (-). (C) Genotyping of blastocysts was conducted using primers corresponding to wild-type and null alleles. D.W: distilled water, S.M: size marker.
(TIF)
Normal development of Meteorin−/− embryos until the blastocyst stage. (A–C) Blastocysts obtained from heterozygous matings were cultured on gelatin-coated dishes for 5 days. All cells were lysed and genotyped after culture images were taken. All the blastocysts hatched normally. Inner cell mass aggregations on large, flat, and polyploidy trophoblast cells and scattered extra-embryonic endoderm cells were grown from each of blastocysts. (D–E) Expression of Oct3/4, an inner cell mass marker, was analyzed by in situ hybridization. Its expression in Meteorin−/− blastocyst was similar to that of Meteorin+/+ blastocyst. All scale bars: 200 µm.
(TIF)
Generation of Meteorin−/Δ ES cells through sequential targeting and Cre expression. (A) Schematic of Meteorin targeting used for Meteorin−/Δ ES cell generation. The targeting construct was generated by flanking exons 3 and 4 with loxP sequences, and a neomycin-resistance cassette was used for subsequent selection of targeted ES cells. Black boxes indicate exons and an open arrow depicts DTA. (B) Schematic diagram of Meteorin−/Δ ES cell generation. (C) Southern blotting of the BamHI-digested gDNA derived from ES cells at each targeting step. The flanking probe detected a 7.6-kb band from the wild-type allele (+), a 11.1-kb band from the null allele (−), an 8.9-kb band from the conditionally targeted allele (f), and a 7.1-kb band from the lox (Δ) allele obtained upon excision by Cre-recombinase. (D) Meteorin mRNA expression in each ES cell line was analyzed by qRT-PCR. In Meteorin+/f and Meteorin−/f ES cells, the level was reduced to half of that in Meteorin+/+ ES cells, and no Meteorin expression was observed in Meteorin−/Δ ES cells.
(TIF)
Defected mesendoderm development in EB culture of Meteorin−/Δ cells. (A–E) Expression level of markers of several developmental lineages was analyzed by qRT-PCR. (A) Expression of Nanog, a pluripotency marker, was normal in Meteorin−/Δ EB culture compared to Meteorin+/+ EB culture. Expression levels of Neurofilament M (NFM), an early neuroectoderm marker (B), and Transthyretin (Ttr), a visceral endoderm marker (C), were higher in Meteorin−/Δ EB culture than those in Meteorin+/+ EB culture. The expression levels of Fetal liver kinase 1 (Flk1), a mesoderm marker (D), and Mix1 homeobox-like 1 (Mixl1), an endoderm marker (E), were significantly decreased in Meteorin−/Δ EB culture. Error bars indicate standard error of the mean (s.e.m.). All experiments were conducted more than 3 times and the representative graphs are shown.
(TIF)
Normal development of extra-embryonic ectoderm in Meteorin-deficient embryos. (A–B) BMP4, a marker for extra-embryonic ectoderm, expression is analyzed by in situ hybridization at E7.0. The expression of BMP4 in Meteorion−/− embryos (A) was comparable with that of Meteorin+/+ control embryos (B).
(TIF)
Primer sets for qRT-PCR analysis and in situ hybridization probe cloning. Sequences of primer sets used in this study are listed in the table.
(DOCX)
Funding Statement
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (RND no. 2013003731); National R&D Program for Cancer Control, Ministry of Health & Welfare, the Republic of Korea (RND no. 0920310); Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (RND no. 2013037841); and a Global Frontier Project grant (RND no. 2013045940) of the National Research Foundation funded by the Ministry of Education, Science and Technology of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR (1993) Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature 361: 543–547. [DOI] [PubMed] [Google Scholar]
- 2. Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, et al. (1994) Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev 8: 174–189. [DOI] [PubMed] [Google Scholar]
- 3. Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP (1999) T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev 13: 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, et al. (2002) Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3: 745–756. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez TA, Srinivas S, Clements MP, Smith JC, Beddington RS (2005) Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development 132: 2513–2520. [DOI] [PubMed] [Google Scholar]
- 7. Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, et al. (2006) The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11: 313–323. [DOI] [PubMed] [Google Scholar]
- 8. Tam PP, Loebel DA (2007) Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet 8: 368–381. [DOI] [PubMed] [Google Scholar]
- 9. Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, et al. (1999) Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell 4: 287–298. [DOI] [PubMed] [Google Scholar]
- 10. Mesnard D, Guzman-Ayala M, Constam DB (2006) Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development 133: 2497–2505. [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, et al. (2004) Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428: 387–392. [DOI] [PubMed] [Google Scholar]
- 12. Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, et al. (2001) Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411: 965–969. [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto M, Meno C, Sakai Y, Shiratori H, Mochida K, et al. (2001) The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev 15: 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varlet I, Collignon J, Robertson EJ (1997) nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development 124: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 15. Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, et al. (1994) A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120: 1919–1928. [DOI] [PubMed] [Google Scholar]
- 16. Nomura M, Li E (1998) Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature 393: 786–790. [DOI] [PubMed] [Google Scholar]
- 17. Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, et al. (1996) Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 381: 158–161. [DOI] [PubMed] [Google Scholar]
- 18. Saijoh Y, Adachi H, Sakuma R, Yeo CY, Yashiro K, et al. (2000) Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol Cell 5: 35–47. [DOI] [PubMed] [Google Scholar]
- 19. Adachi H, Saijoh Y, Mochida K, Ohishi S, Hashiguchi H, et al. (1999) Determination of left/right asymmetric expression of nodal by a left side-specific enhancer with sequence similarity to a lefty-2 enhancer. Genes Dev 13: 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishino J, Yamashita K, Hashiguchi H, Fujii H, Shimazaki T, et al. (2004) Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J 23: 1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee HS, Han J, Lee SH, Park JA, Kim KW (2010) Meteorin promotes the formation of GFAP-positive glia via activation of the Jak-STAT3 pathway. J Cell Sci 123: 1959–1968. [DOI] [PubMed] [Google Scholar]
- 22. Jorgensen JR, Thompson L, Fjord-Larsen L, Krabbe C, Torp M, et al. (2009) Characterization of Meteorin–an evolutionary conserved neurotrophic factor. J Mol Neurosci 39: 104–116. [DOI] [PubMed] [Google Scholar]
- 23. Jorgensen JR, Emerich DF, Thanos C, Thompson LH, Torp M, et al. (2010) Lentiviral delivery of meteorin protects striatal neurons against excitotoxicity and reverses motor deficits in the quinolinic acid rat model. Neurobiol Dis 41: 160–168. [DOI] [PubMed] [Google Scholar]
- 24. Jorgensen JR, Xu XJ, Arnold HM, Munro G, Hao JX, et al. (2012) Meteorin reverses hypersensitivity in rat models of neuropathic pain. Exp Neurol 237: 260–266. [DOI] [PubMed] [Google Scholar]
- 25. Keller GM (1995) In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 7: 862–869. [DOI] [PubMed] [Google Scholar]
- 26. Dunn NR, Vincent SD, Oxburgh L, Robertson EJ, Bikoff EK (2004) Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development 131: 1717–1728. [DOI] [PubMed] [Google Scholar]
- 27.Nagi A, Gertsenstein M, Vintersten K, Behringer R (2003) Manipulating the mouse embryo: A laboratory manual. New York: Cold Spring Harbor Laboratory Press.
- 28. Hescheler J, Fleischmann BK, Lentini S, Maltsev VA, Rohwedel J, et al. (1997) Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res 36: 149–162. [DOI] [PubMed] [Google Scholar]
- 29. Jeong HW, Jeon US, Koo BK, Kim WY, Im SK, et al. (2009) Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest 119: 3290–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller G (2005) Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev 19: 1129–1155. [DOI] [PubMed] [Google Scholar]
- 31. Mansergh FC, Daly CS, Hurley AL, Wride MA, Hunter SM, et al. (2009) Gene expression profiles during early differentiation of mouse embryonic stem cells. BMC Dev Biol 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camus A, Perea-Gomez A, Moreau A, Collignon J (2006) Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol 295: 743–755. [DOI] [PubMed] [Google Scholar]
- 33. Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ (2003) Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev 17: 1646–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lowe LA, Yamada S, Kuehn MR (2001) Genetic dissection of nodal function in patterning the mouse embryo. Development 128: 1831–1843. [DOI] [PubMed] [Google Scholar]
- 35. Takenaga M, Fukumoto M, Hori Y (2007) Regulated Nodal signaling promotes differentiation of the definitive endoderm and mesoderm from ES cells. J Cell Sci 120: 2078–2090. [DOI] [PubMed] [Google Scholar]
- 36. Pfendler KC, Catuar CS, Meneses JJ, Pedersen RA (2005) Overexpression of Nodal promotes differentiation of mouse embryonic stem cells into mesoderm and endoderm at the expense of neuroectoderm formation. Stem Cells Dev 14: 162–172. [DOI] [PubMed] [Google Scholar]
- 37. Lawson KA (1999) Fate mapping the mouse embryo. Int J Dev Biol 43: 773–775. [PubMed] [Google Scholar]
- 38. Hudson C, Clements D, Friday RV, Stott D, Woodland HR (1997) Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell 91: 397–405. [DOI] [PubMed] [Google Scholar]
- 39. Thisse B, Wright CV, Thisse C (2000) Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature 403: 425–428. [DOI] [PubMed] [Google Scholar]
- 40. Chen Y, Schier AF (2001) The zebrafish Nodal signal Squint functions as a morphogen. Nature 411: 607–610. [DOI] [PubMed] [Google Scholar]
- 41. Yasuo H, Lemaire P (1999) A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr Biol 9: 869–879. [DOI] [PubMed] [Google Scholar]
- 42. Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM (2000) Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 127: 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of Meteorin-null mice. (A) Schematic view of the targeting strategy. Black boxes indicate exons and an open arrow depicts diphtheria toxin A (DTA). The position of the flanking probe is indicated by a grey dash and the expected fragment sizes after BamHI digestion for Southern blotting are also provided. (B) Southern blotting of the BamHI-digested gDNA derived from Meteorin+/+ and Meteorin+/− mice. The flanking probe detects a 7.6-kb band for the wild-type allele (+) and an 11.1-kb band for the null allele (-). (C) Genotyping of blastocysts was conducted using primers corresponding to wild-type and null alleles. D.W: distilled water, S.M: size marker.
(TIF)
Normal development of Meteorin−/− embryos until the blastocyst stage. (A–C) Blastocysts obtained from heterozygous matings were cultured on gelatin-coated dishes for 5 days. All cells were lysed and genotyped after culture images were taken. All the blastocysts hatched normally. Inner cell mass aggregations on large, flat, and polyploidy trophoblast cells and scattered extra-embryonic endoderm cells were grown from each of blastocysts. (D–E) Expression of Oct3/4, an inner cell mass marker, was analyzed by in situ hybridization. Its expression in Meteorin−/− blastocyst was similar to that of Meteorin+/+ blastocyst. All scale bars: 200 µm.
(TIF)
Generation of Meteorin−/Δ ES cells through sequential targeting and Cre expression. (A) Schematic of Meteorin targeting used for Meteorin−/Δ ES cell generation. The targeting construct was generated by flanking exons 3 and 4 with loxP sequences, and a neomycin-resistance cassette was used for subsequent selection of targeted ES cells. Black boxes indicate exons and an open arrow depicts DTA. (B) Schematic diagram of Meteorin−/Δ ES cell generation. (C) Southern blotting of the BamHI-digested gDNA derived from ES cells at each targeting step. The flanking probe detected a 7.6-kb band from the wild-type allele (+), a 11.1-kb band from the null allele (−), an 8.9-kb band from the conditionally targeted allele (f), and a 7.1-kb band from the lox (Δ) allele obtained upon excision by Cre-recombinase. (D) Meteorin mRNA expression in each ES cell line was analyzed by qRT-PCR. In Meteorin+/f and Meteorin−/f ES cells, the level was reduced to half of that in Meteorin+/+ ES cells, and no Meteorin expression was observed in Meteorin−/Δ ES cells.
(TIF)
Defected mesendoderm development in EB culture of Meteorin−/Δ cells. (A–E) Expression level of markers of several developmental lineages was analyzed by qRT-PCR. (A) Expression of Nanog, a pluripotency marker, was normal in Meteorin−/Δ EB culture compared to Meteorin+/+ EB culture. Expression levels of Neurofilament M (NFM), an early neuroectoderm marker (B), and Transthyretin (Ttr), a visceral endoderm marker (C), were higher in Meteorin−/Δ EB culture than those in Meteorin+/+ EB culture. The expression levels of Fetal liver kinase 1 (Flk1), a mesoderm marker (D), and Mix1 homeobox-like 1 (Mixl1), an endoderm marker (E), were significantly decreased in Meteorin−/Δ EB culture. Error bars indicate standard error of the mean (s.e.m.). All experiments were conducted more than 3 times and the representative graphs are shown.
(TIF)
Normal development of extra-embryonic ectoderm in Meteorin-deficient embryos. (A–B) BMP4, a marker for extra-embryonic ectoderm, expression is analyzed by in situ hybridization at E7.0. The expression of BMP4 in Meteorion−/− embryos (A) was comparable with that of Meteorin+/+ control embryos (B).
(TIF)
Primer sets for qRT-PCR analysis and in situ hybridization probe cloning. Sequences of primer sets used in this study are listed in the table.
(DOCX)