Abstract
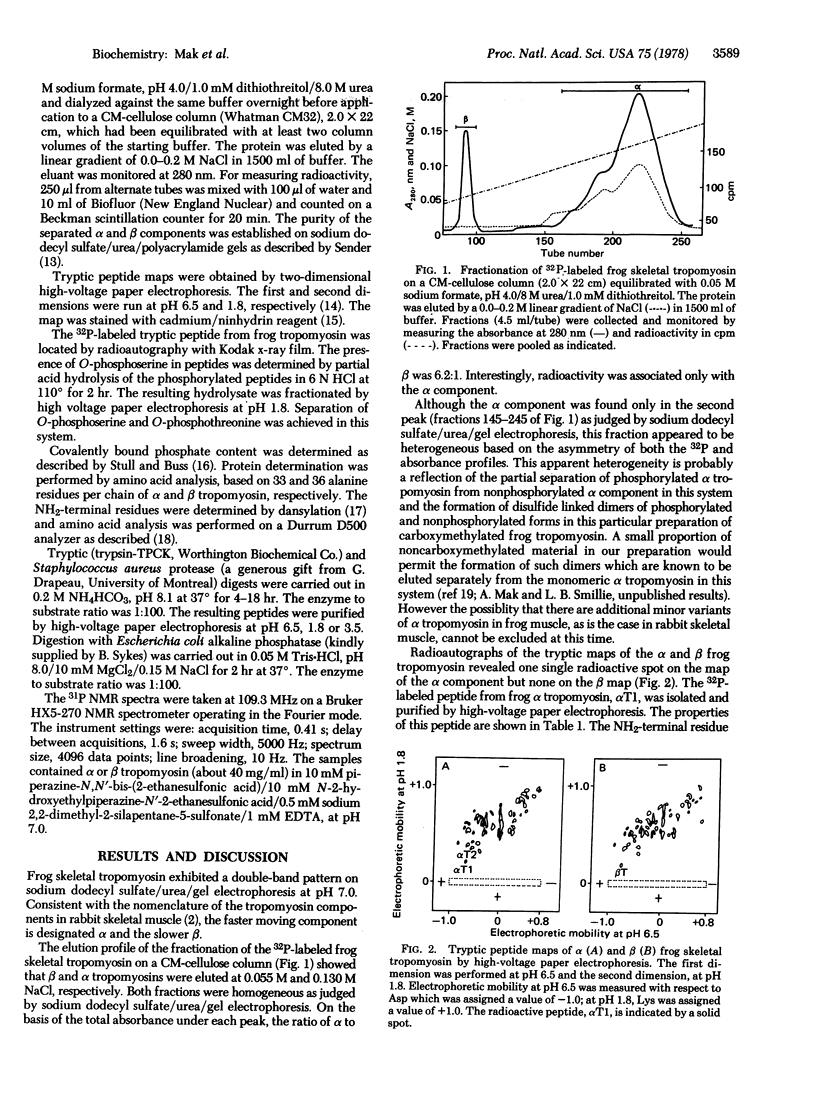
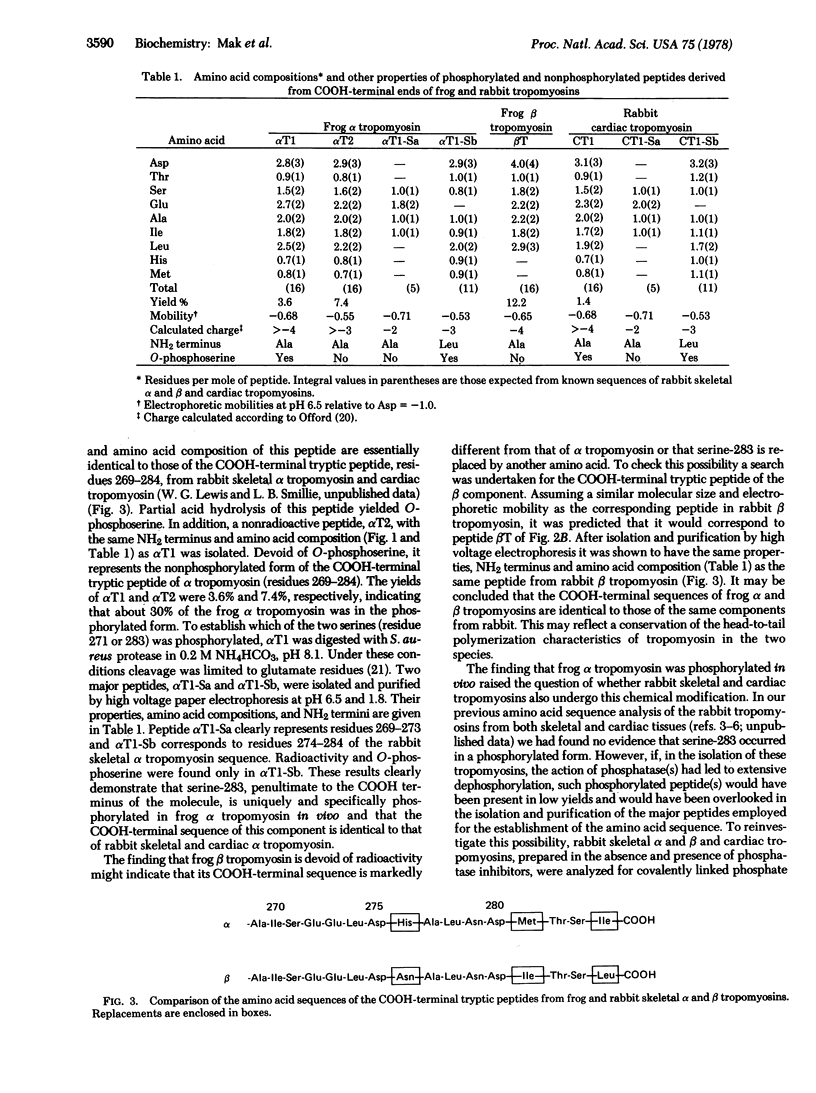
Tropomyosin, extracted from the leg muscle of frogs that had been injected with [32P]orthophosphate, was fractionated into two components, alpha and beta, on a CM-cellulose column. Radioactivity was associated only with the alpha component. A single phosphorylation site was located at serine-283 (pentultimate at the COOH-terminal end) of the frog alpha tropomyosin. The same phosphorylated peptide was recovered in low yields from both rabbit skeletal alpha and cardiac tropomyosin. The presence of covalently bound phosphate in alpha tropomyosin and its absence in the beta component of rabbit skeletal muscle was suggested by 31P NMR spectroscopy. The amino acid sequences around the phosphorylation sites of frog and rabbit tropomyosin are identical. Because this sequence is not similar to any other known phosphorylation site in proteins, this indicates the existence of either specific kinase or phosphatase that can distinguish between alpha and beta tropomyosins. In a model proposed for the head-to-tail overlap of alpha tropomyosin molecules, one O-phosphoserine-283 residue could form a salt linkage with lysine-6 on one side of the overlap region and another with lysine-12 on the other side. This would predict a difference in the stability of polymers of phosphorylated and nonphosphorylated alphaalpha and alphabeta dimers of tropomyosin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen C., Caspar D. L., Parry D. A., Lucas R. M. Tropomyosin crystal dynamics. Cold Spring Harb Symp Quant Biol. 1972;36:205–216. doi: 10.1101/sqb.1972.036.01.028. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. The histidine and methionine sequences of rabbit skeletal tropomyosin. Can J Biochem. 1972 Mar;50(3):312–329. doi: 10.1139/o72-044. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F., Smillie L. B. Rabbit skeletal alpha-tropomyosin chains are in register. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1316–1322. doi: 10.1016/0006-291x(75)90836-0. [DOI] [PubMed] [Google Scholar]
- Johnson P., Smillie L. B. Polymerizability of rabbit skeletal tropomyosin: effects of enzymic and chemical modifications. Biochemistry. 1977 May 17;16(10):2264–2269. doi: 10.1021/bi00629a035. [DOI] [PubMed] [Google Scholar]
- Leger J., Bouveret P., Schwartz K., Swynghedauw B. A comparative study of skeletal and cardiac tropomyosins: subunits, thiol group content and biological activities. Pflugers Arch. 1976 Apr 6;362(3):271–277. doi: 10.1007/BF00581181. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3377–3381. doi: 10.1073/pnas.72.9.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975 Oct 25;98(2):293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Ookubo N. Intramolecular disulfide linked alphabeta and alphaalpha in oxidized tropomyosin: separation, identification, and process of formation. J Biochem. 1977 Apr;81(4):923–931. doi: 10.1093/oxfordjournals.jbchem.a131557. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Carpenter M. R., Johnson P., Smillie L. B. Amino-acid sequence of tropomyosin-binding component of rabbit skeletal muscle troponin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1902–1906. doi: 10.1073/pnas.73.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Heilmeyer L. M.G. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- Ribolow H., BARANY M. Phosphorylation of tropomyosin in live frog muscle. Arch Biochem Biophys. 1977 Mar;179(2):718–720. doi: 10.1016/0003-9861(77)90162-x. [DOI] [PubMed] [Google Scholar]
- Sender P. M. Muscle fibrils: Solubilization and gel electrophoresis. FEBS Lett. 1971 Sep 15;17(1):106–110. doi: 10.1016/0014-5793(71)80575-6. [DOI] [PubMed] [Google Scholar]
- Sodek J., Hodges R. S., Smillie L. B. Amino acid sequence of rabbit skeletal muscle alpha-tropomyosin. The COOH-terminal half (residues 142 to 284). J Biol Chem. 1978 Feb 25;253(4):1129–1136. [PubMed] [Google Scholar]
- Stewart M. Tropomyosin: evidence for no stagger between chains. FEBS Lett. 1975 Apr 15;53(1):5–7. doi: 10.1016/0014-5793(75)80668-5. [DOI] [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Stull J. T., Buss J. E. Phosphorylation of cardiac troponin by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Feb 10;252(3):851–857. [PubMed] [Google Scholar]
- Tawada Y., Oara H., Ooi T., Tawada K. Non-polymerizable tropomyosin and control of the superprecipitation of actomyosin. J Biochem. 1975 Jul;78(1):65–72. [PubMed] [Google Scholar]
- Ueno H., Tawada Y., Ooi T. Properties of non-polymerizable tropomyosin obtained by carboxypeptidase A digestion. J Biochem. 1976 Aug;80(2):283–290. doi: 10.1093/oxfordjournals.jbchem.a131275. [DOI] [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]
- Yamaguchi M., Greaser M. L., Cassens R. G. Interactions of troponin subunits with different forms of tropomyosin. J Ultrastruct Res. 1974 Jul;48(1):33–58. doi: 10.1016/s0022-5320(74)80043-2. [DOI] [PubMed] [Google Scholar]



