Abstract
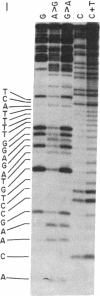
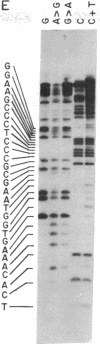
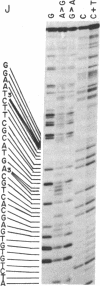
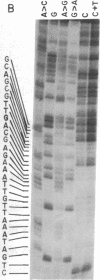
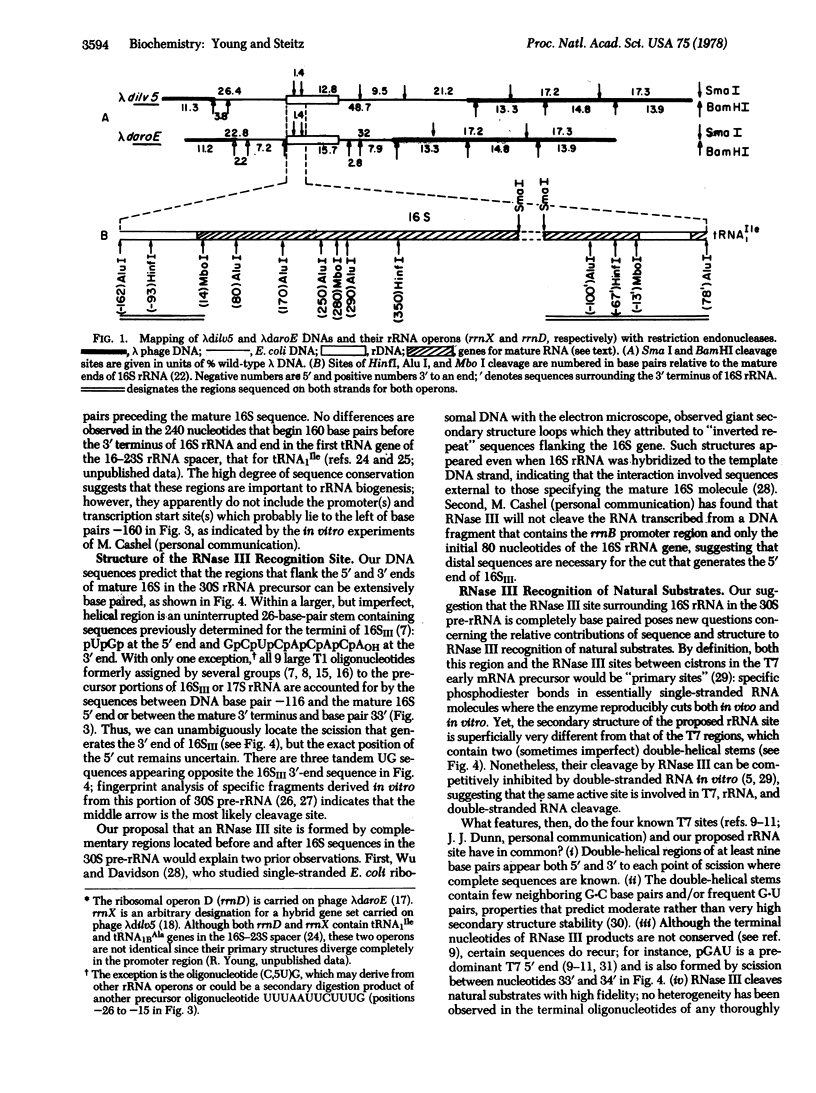
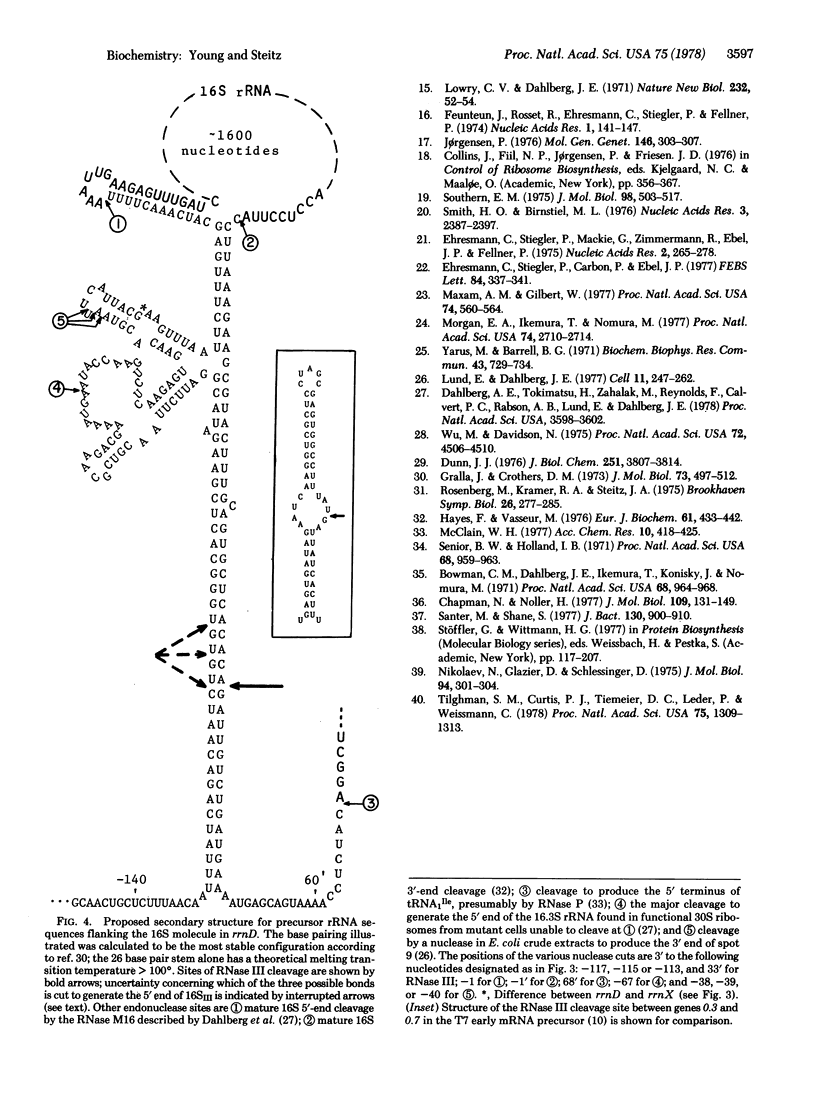
The nucleotide sequence of Escherichia coli DNA at both ends of the gene for 16S rRNA has been determined for two rRNA operons, rrnD and rrnX. The 400 nucleotides we have examined exhibit only one base change between rrnD and rrnX. Within the 160 nucleotides that precede mature 16S rRNA sequences are cleavage sites for several E. coli endonucleases, including RNase III. A 240-nucleotide segment encompassing the 16S 3' end contains another RNase III site and the point of presumed RNase P scission at the 5' end of tRNA1Ile, the first tRNA appearing in the 16-23S spacer region of rrnD and rrnX. Most importantly, the DNA sequences predict that regions flanking the 16S gene in the rRNA primary transcript extensively base pair to form a double-helical structure whose hairpin loop includes the entire mature 16S molecule; within this structure is a 26-base-pair stem containing the two sequences at which RNase III action generates the 5' and 3' ends of a previously characterized precursor to 16S rRNA. Although our proposed secondary structure for this RNase III site is superficially dissimilar to previously described cleavage sites in the T7 early mRNA precursor, certain common features may constitute signals for RNase III recognition. The suggestion that distant portions of an RNA molecule can form a secondary structure within which specific endonucleolytic cleavages occur may have mechanistic implications for the joining of noncontiguous portions of gene sequences evident in several eukaryotic mRNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. Sequence studies on precursor 16S ribosomal RNA of Escherichia coli. Nat New Biol. 1971 Jul 14;232(28):50–52. doi: 10.1038/newbio232050a0. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E., Lund E., Tokimatsu H., Rabson A. B., Calvert P. C., Reynolds F., Zahalak M. Processing of the 5' end of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3598–3602. doi: 10.1073/pnas.75.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J. RNase III cleavage of single-stranded RNA. Effect of ionic strength on the fideltiy of cleavage. J Biol Chem. 1976 Jun 25;251(12):3807–3814. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Carbon P., Ebel J. P. Recent progress in the determination of the primary sequence of the 16 S RNA of Escherichia coli. FEBS Lett. 1977 Dec 15;84(2):337–341. doi: 10.1016/0014-5793(77)80720-5. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Rosset R., Ehresmann C., Stiegler P., Fellner P. Abnormal maturation of precursor 16S RNA in a ribosomal assembly defective mutant of E. coli. Nucleic Acids Res. 1974 Jan;1(1):141–147. doi: 10.1093/nar/1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Hayes F., Hayes D., Fellner P., Ehresmann C. Additional nucleotide sequences in precursor 16S ribosomal RNA from Escherichia coli. Nat New Biol. 1971 Jul 14;232(28):54–55. doi: 10.1038/newbio232054a0. [DOI] [PubMed] [Google Scholar]
- Hayes F., Vasseur M., Nikolaev N., Schlessinger D., Sri Widada J., Krol A., Branlant C. Structure of a 30 S pre-ribosomal RNA of E. coli. FEBS Lett. 1975 Aug 1;56(1):85–91. doi: 10.1016/0014-5793(75)80117-7. [DOI] [PubMed] [Google Scholar]
- Hayes F., Vasseur M. Processing of the 17-S Escherichia coli precursor RNA in the 27-S pre-ribosomal particle. Eur J Biochem. 1976 Jan 15;61(2):433–442. doi: 10.1111/j.1432-1033.1976.tb10037.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. A ribosomal RNA gene of Escherichia coli (rrnD) on lamnda daro E specialized transducing phages. Mol Gen Genet. 1976 Aug 2;146(3):303–307. doi: 10.1007/BF00701255. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Dahlberg J. E. Structural differences between the 16S ribosomal RNA of E. coli and its precursor. Nat New Biol. 1971 Jul 14;232(28):52–54. doi: 10.1038/newbio232052a0. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Spacer transfer RNAs in ribosomal RNA transcripts of E. coli: processing of 30S ribosomal RNA in vitro. Cell. 1977 Jun;11(2):247–262. doi: 10.1016/0092-8674(77)90042-3. [DOI] [PubMed] [Google Scholar]
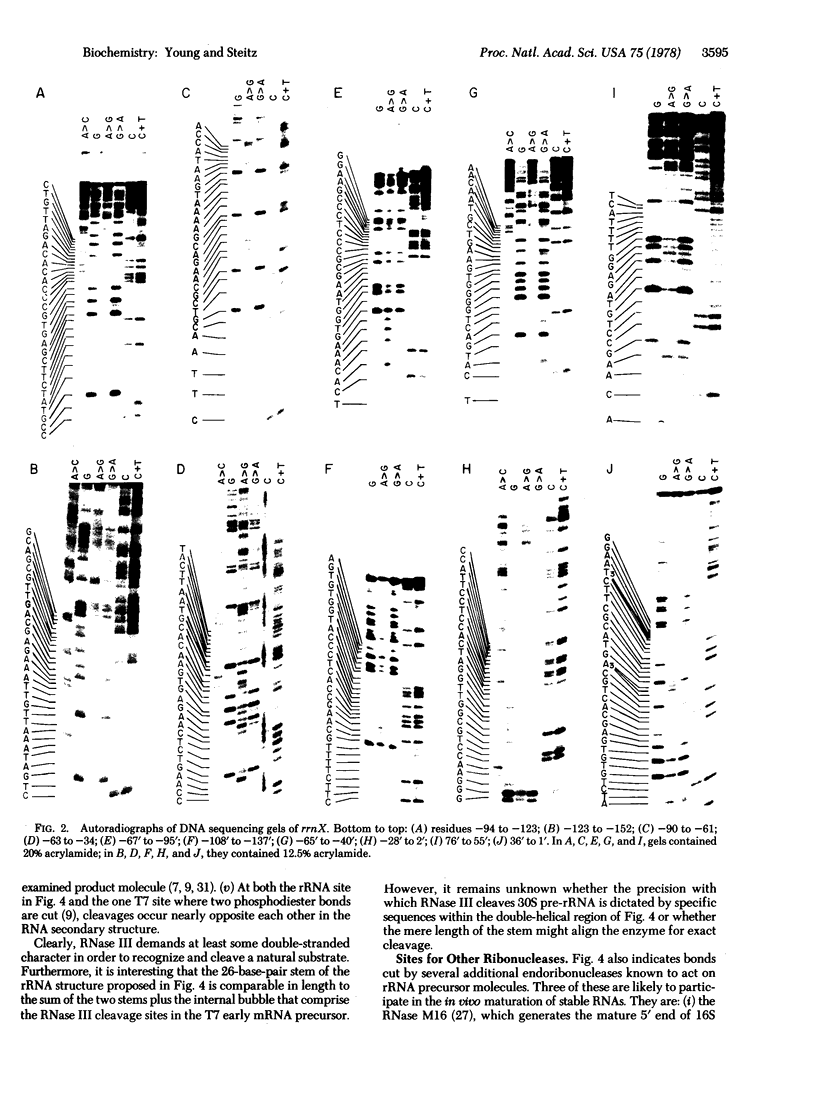
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Glazier D., Schlessinger D. Cleavage by ribonuclease III of the complex of 30 S pre-ribosomal RNA and ribosomal proteins of Escherichia coli. J Mol Biol. 1975 May 15;94(2):301–304. doi: 10.1016/0022-2836(75)90085-6. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley J. L., Coleman J. E. Structure of a promoter for T7 RNA polymerase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4266–4270. doi: 10.1073/pnas.74.10.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Dunn J. J. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A. Nucleotide sequence surrounding a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):984–988. doi: 10.1073/pnas.74.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. The specificity of RNase III cleavage of bacteriophage T7 early messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):277–285. [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M., Pace B., Pace N. R., Woese C. R. Primary structural relationship of p16 to m16 ribosomal RNA. Nat New Biol. 1971 Jul 14;232(28):48–49. doi: 10.1038/newbio232048a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Davidson N. Use of gene 32 protein staining of single-strand polynucleotides for gene mapping by electron microscopy: application to the phi80d3ilvsu+7 system. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4506–4510. doi: 10.1073/pnas.72.11.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Barrell B. G. The sequence of nucleotides in tRNA Ile from E. coli B. Biochem Biophys Res Commun. 1971 May 21;43(4):729–734. doi: 10.1016/0006-291x(71)90676-0. [DOI] [PubMed] [Google Scholar]