Abstract
Objective
Early decompressive craniectomy (DC) has been used as the first stage treatment to prevent secondary injuries in cases of severe traumatic brain injury (TBI). Postoperative management is the major factor that influences outcome. The aim of this study is to investigate the effect of postoperative management, using intracranial pressure (ICP) monitoring and including consecutive DC on the other side, on the two-week mortality in severe TBI patients treated with early DC.
Methods
Seventy-eight patients with severe TBI [Glasgow Coma Scale (GCS) score <9] underwent early DC were retrospectively investigated. Among 78 patients with early DC, 53 patients were managed by conventional medical treatments and the other, 25 patients were treated under the guidance of ICP monitoring, placed during early DC. In the ICP monitoring group, consecutive DC on the other side were performed on 11 patients due to a high ICP of greater than 30 mm Hg and failure to respond to any other medical treatments.
Results
The two-week mortality rate was significantly different between two groups [50.9% (27 patients) and 24% (6 patients), respectively, p=0.025]. After adjusting for confounding factors, including sex, low GCS score, and pupillary abnormalities, ICP monitoring was associated with a 78% lower likelihood of 2-week mortality (p=0.021).
Conclusion
ICP monitoring in conjunction with postoperative treatment, after early DC, is associated with a significantly reduced risk of death.
Keywords: Brain injuries, Intracranial pressure monitoring, Decompressive craniectomy, Mortality
INTRODUCTION
Traumatic brain injury (TBI) is the leading cause of death and disability around the world, despite improvements in emergency care, imaging, critical care, medical and surgical treatment options, and rehabilitation15,32). In US alone, an estimated 1.5 million people sustain TBI each year, resulting in more than 50000 deaths and another 500000 individuals with permanent neurological sequelae14). Despite more than 25 pharmaceutical trials for TBI that have failed to show efficacy, the mainstay of treatment for severe TBI targets the reduction of elevated intracranial pressure (ICP) and the maintenance of adequate cerebral blood flow and oxygenation7).
Decompressive craniectomy (DC) has been perceived as a last report for treating uncontrollable ICP17,19,22). However, the time from injury to DC was reported to be the factor with the greatest influence on the outcomes of severe TBI19,22,25). Authors of several studies advocate that DC should be performed as soon as possible after trauma, in order to prevent secondary injuries that may occur during unsuccessful ICP or other medical treatments2,12,22,25,35). Even in case of early DC and maximal postoperative medical treatments, uncontrollable ICP due to post-traumatic edema may progress. In this case, better decompression and suitable ICP decrease may be achieved if consecutive DC is performed on the other side of the brain as well25,36).
Evidence-based guidelines for severe TBI recommended ICP monitoring in patients with severe TBI and link the degree as well as duration of intracranial hypertension with increased mortality rates4). Patients who responded to ICP-lowering treatment showed a significantly reduced risk of death at 2 weeks than those who did not respond after adjusting for factors that independently predict risk of death7). ICP monitoring on the initial decompression area may provide pertinent diagnostic information leading to more appropriate postoperative medication reducing ICP or to timely treatment with invasive procedures like consecutive DC on the other side.
The purpose of this study is to evaluate the influence of postoperative ICP monitoring, placed during early DC, on two-week mortality in patients who underwent early DC for severe TBI.
MATERIALS AND METHODS
Study design and assessment
From January 2010 through December 2012, A retrospective analysis was made of 92 patients with severe traumatic brain injury [Glasgow Coma Scale (GCS) score <9] underwent early DC. On admission, they received an initial cranial computed tomography (CT) scan and were diagnosed as having type III, IV, or non-evacuated mass lesion of Marshall CT classification18). Within this group, we excluded patients according to the following criteria : under 18 years old, severe extra-cranial lesions such as cardiovascular, pulmonary or abdominal injuries, hemodynamic shock, transfer to our hospital more than 12 hours after the initial injury, or admission with the diagnosis of brain death. Ultimately, 78 patients were included in this study. The subjects were arranged into two groups. Group 1 contained 53 patients who were given postoperative management, without ICP monitoring. Group 2 contained 25 patients treated postoperatively with subdural ICP monitoring, placed during DC. With the hospital ethics committee's approval, we reviewed medical records and radiology of all patients. The following parameters were recorded and analyzed : patient's age, sex, initial GCS score, pupillary response, and two-week mortality. The two week time frame was selected because over 85% of deaths occur within two weeks after injury8).
Decompressive surgical procedures
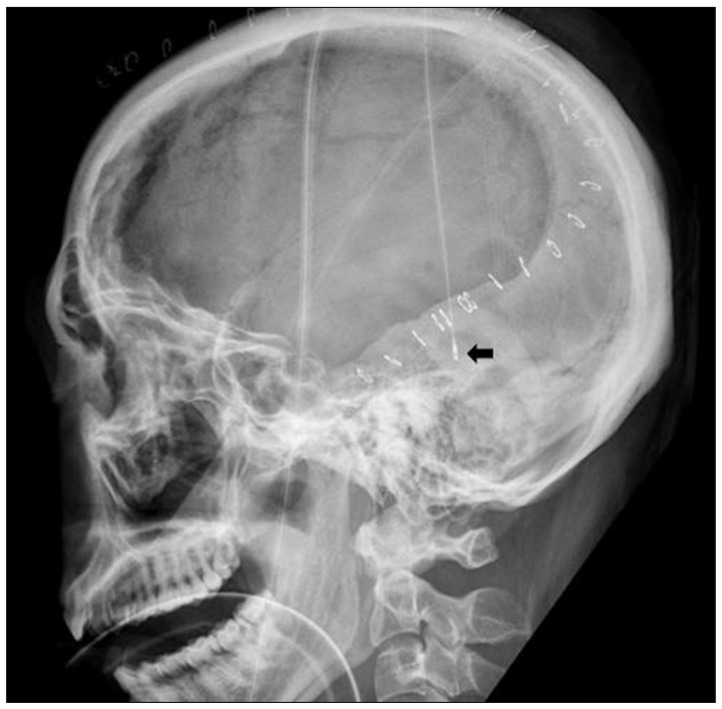
The patient was induced for general anesthesia endotracheally. The unilateral decompression procedure involved making a large curvilinear incision in the fronto-temporo-parietal region. This was followed by preparing a myocutaneous flap and craniectomy, elevating large bone flaps, with a diameter of at least 12 cm, including the frontal, parietal, temporal, and parts of the occipital squama. Additional bone was removed at the temporal region, down to the floor of the middle fossa, to release the compression of the basal cistern. The dura mater was attached to the craniotomy edge, to prevent epidural bleeding. The dura was then opened at the temporal base, in a stellate fashion, to provide additional space for brain swelling. When the dura was opened, the underlying brain or hematoma typically herniated outward. The contused brain tissue was gently removed and extra-cerebral clots were also evacuated. After meticulous hemostasis around injured lesions, a subdural ICP monitoring catheter (Integra NeuroSciences, San Diego, CA, USA), zeroed relative to atmospheric pressure, was placed underneath the incised dura, at the posterior temporal bone margin and secured with dura sutures, to prevent displacement (Fig. 1). The dura was then expanded with the synthetic dural substitute to allow the brain to bulge outward. The temporalis muscle and skin flap were then re-approximated with sutures. The bone flap was maintained in wet gauze at -70℃ and cranioplasty was performed 3-6 months after surgery for surviving patients.
Fig. 1.
Lateral plain radiograph of the skull shows subdural Intracranial pressure monitoring sensor (arrow), placed at the posterior temporal bone margin, after the initial decompressive craniectomy.
Postoperative management
All patients underwent primary resuscitation and stabilization, according to the Brain Trauma Foundation (BTF) guidelines4), and were assessed for age, gender, GCS, and pupillary response. These patients were transferred to an operating room within a maximum of 12 hours after their initial CT scans. After the decompressive surgery, conventional medical therapies, including hyper-osmotic agents, hyperventilation, or sedation were used as needed to reduce intracranial hypertension in both groups. These medical treatments were determined, according to the CT scan, in correlation with neurological deterioration for Group 1 patients, while ICP monitoring was added to the considerations for Group 2 patients. Consecutive DC on the other side of the brain was performed, when ICP increased to greater than 30 mm Hg, for a period longer than 15 minutes, and when the patient failed to respond to the maximal medical treatment mentioned above (Fig. 2). Body temperature, respiratory rate, heart rate, blood pressure, cardiac rhythm, oxygen saturation, and ICP for group 2 patients were monitored continuously. ICP measurements were recorded using a monitoring device (Integra NeuroSciences, San Diego, CA, USA) and ICP values were collected on an hourly basis, with additional values included if there were any meaningful changes. Normal ICP was defined as an ICP of 0-20 mm Hg.
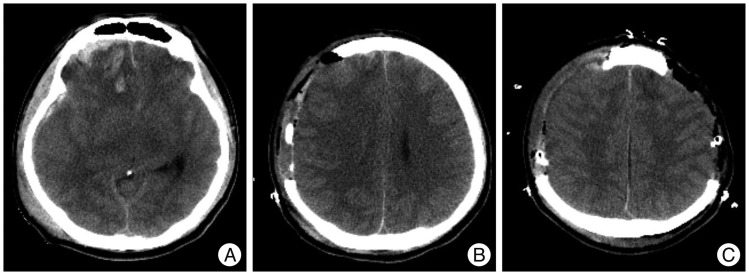
Fig. 2.
Pre- and postoperative computed tomography (CT) scans of an illustrative case. A : Preoperative CT scan showing diffuse brain swelling and obliterated basal cisterns. B : Initial postoperative CT scan showing early decompressive craniectomy (DC). C : Postoperative CT scan showing consecutive bilateral DC at an interval of eight hours.
Statistical analysis
Comparisons between groups were analyzed using the chi-square test, Fisher's exact test, and Student's t-test. The means are expressed as mean±standard deviation. Logistic regression analyses, controlled for sex, pupillary status, and initial GCS scores, were used to evaluate the association between ICP monitoring status and two-week mortality. The odds ratios, 95% confidence intervals, and p values of the covariates were reported. All statistical tests were two-sided, and p<0.05 was considered statistically significant. Analyses were performed using the statistical software SPSS (ver. 17.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of the study population
Sixty-four patients were male (82.0%). Motor vehicle related accidents were the cause of 38 cases (48.7%). The patients were 44±17.6 years old (range 18-80 years). The GCS scores ranged from 3 to 8, including 38 patients with GCS scores of 3-5, and 40 patients with a GCS score of 6-8. Twenty-five patients had unilaterally fixed and dilated pupils, with no response to light, and 14 patients had bilaterally fixed pupils at admission. A total of 25 patients (32.1%) had ICP monitoring placed during their early DC.
Use and outcomes of ICP monitoring
Patients were further divided into two groups after early DC; one included patients treated with an ICP monitor and the other included patients not treated an ICP monitor. The clinical characteristics of the two groups are listed in Table 1. For group 1, which was patients treated without an ICP monitor, the mean age was 45.2±18.24 years, 44 patients were male (83%), twenty-eight patients (52.8%) had initial GCS scores lower than 6, and twenty-six patients (49.1%) had fixed and dilated pupils with no response to light, regardless of bilateralism. For group 2, which included patients treated with an ICP monitor, the mean age was 42.9±16.65 years, twenty patients were male (80.0%), fifteen patients (60.0%) had GCS scores lower than 6, and thirteen patients (52.0%) had fixed and dilated pupils with no response to light. The differences between the groups in terms of age, initial GCS score, and pupillary response were not statistically significant. The 2-week mortality rate, however, was 50.9% (27 patients) for group 1, compared with 24.0% (6 patients) for group 2 with a statistically significant difference (p=0.025).
Table 1.
Characteristics of the study population
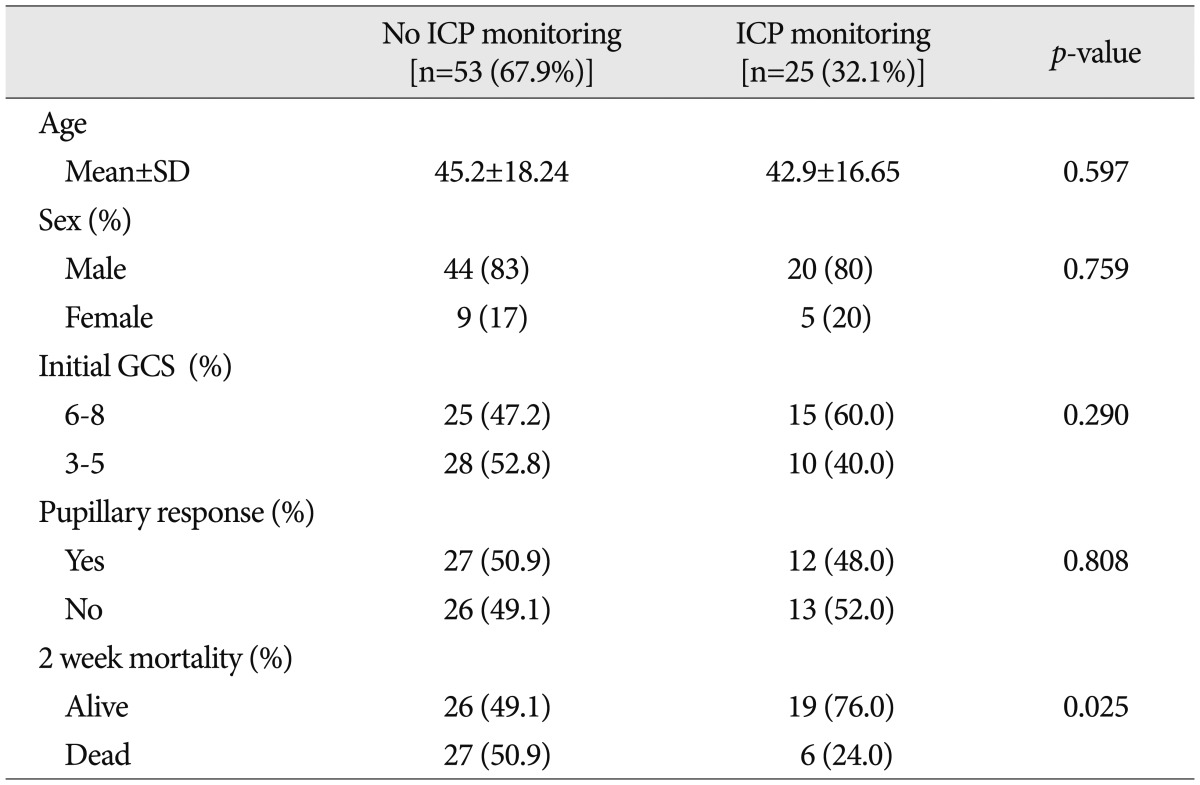
GCS : Glasgow Coma Scale, ICP : intracranial pressure, SD : standard deviation
Factors predicting two-week mortality
Multivariable logistic regression models predicting two-week mortalities are shown in Table 2. After adjusting for confounding factors, including sex, low GCS score, and pupillary abnormalities, ICP monitoring was associated with a 78% lower likelihood of 2-week mortality (adjusted odds ratio 0.22, 95% confidence interval 0.059-0.790, p=0.021). To identify factors independently associated with the two-week mortality, including using an ICP monitor, differences in all available covariates were examined, and we concluded that women were associated with significantly increased mortality.
Table 2.
Logistic regression analyses predicting two-week mortalities for all 78 patients
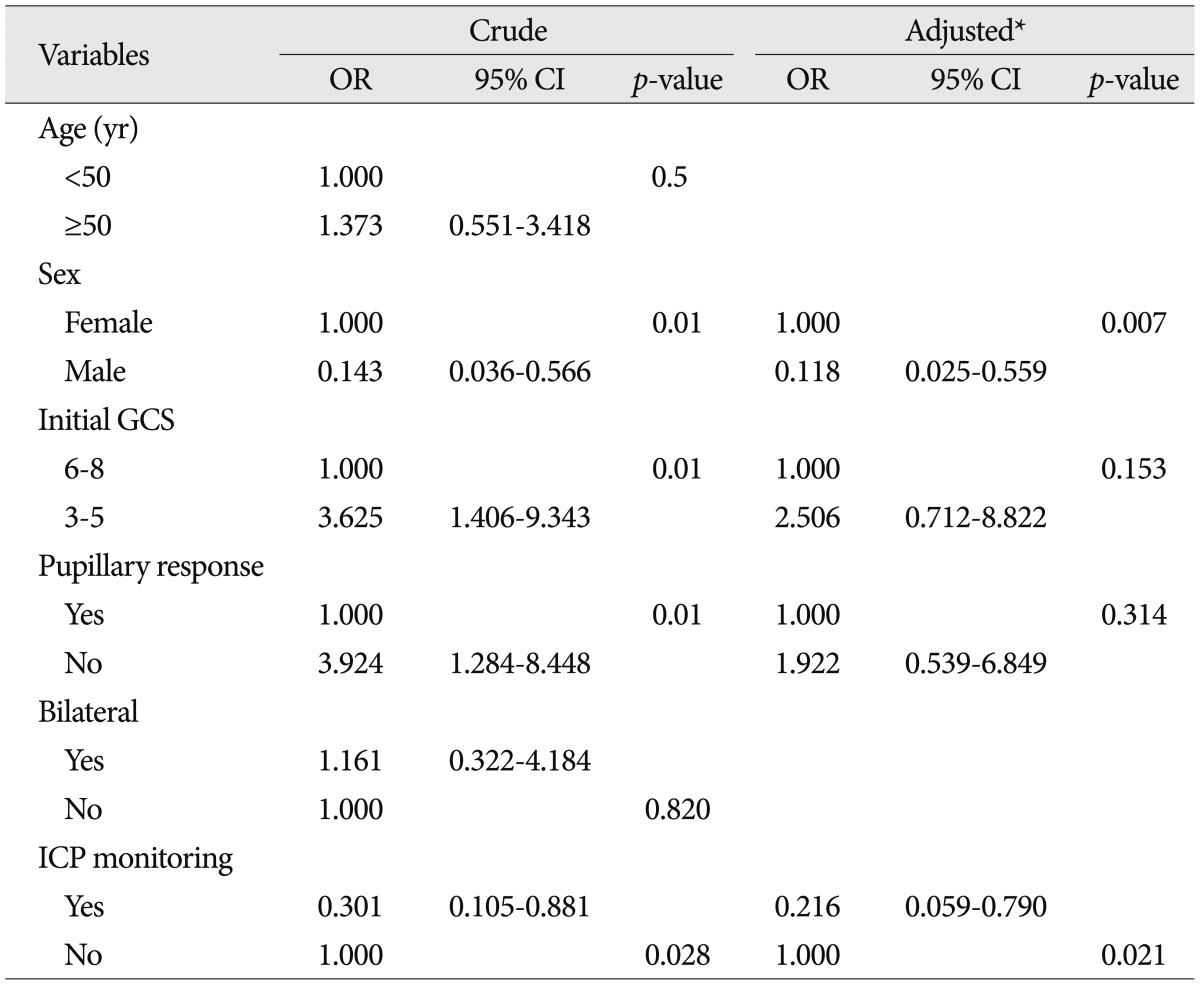
*Adjusted by Sex, Pupil, GCS. OR : odds ratio, CI : confidence interval, GCS : Glasgow Coma Scale, ICP : intracranial pressure
Outcome of consecutive decompressive surgery
In group 2, where patients were treated with the ICP monitor, patients were further divided into two groups, depending on whether they received consecutive DC on the other side. Table 3 shows the clinical characteristics of the two groups. Patients who had consecutive DC on the other side, showed significantly higher mean ICP values right after early DC (p<0.001) and significantly lower initial GCS values (<6, p<0.049) than those who did not receive additional surgeries. The two-week mortality rate of the consecutive decompression group was 45.5% (five patients). The result was lower than that for patients with GCS score of 3-5 (57.8%) although there was no statistical significance (p>0.05). The median interval of time between initial and consecutive DC was 23.2±22.1 hours (range 4 to 76).
Table 3.
Characteristics of the group with intracranial pressure monitoring
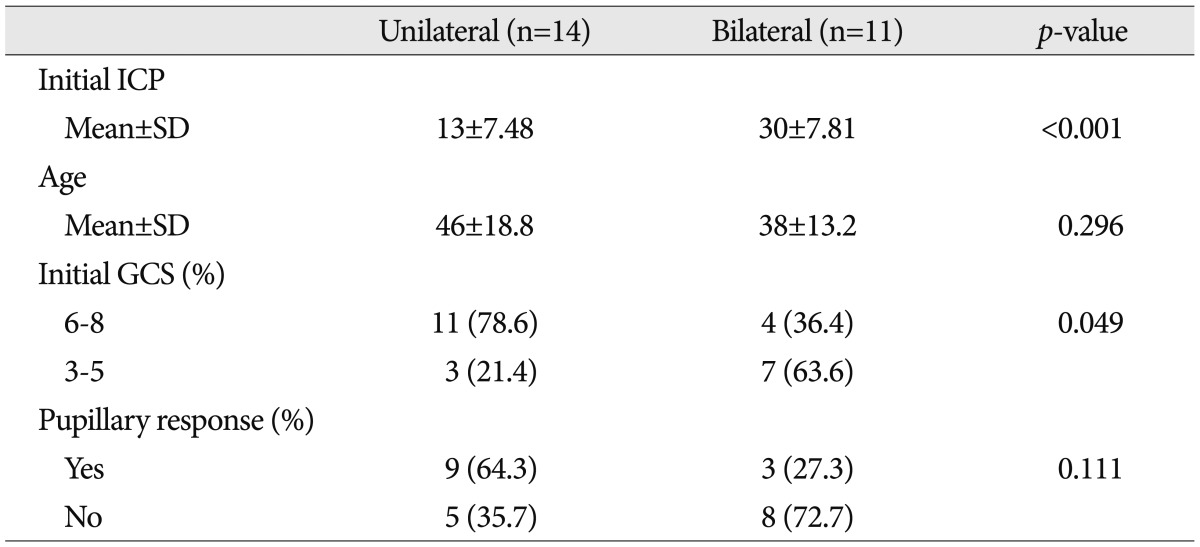
ICP : intracranial pressure, GCS : Glasgow Coma Scale, SD : standard deviation
DISCUSSION
Significance of early decompression
DC has become a valuable tool in managing severe head injury since Kocher3) first introduced decompressive surgery to alleviate intracranial hypertension. The optimal technique, timing, indications, and outcomes for decompressive craniectomy remain controversial. The role of DC in severe neurotrauma has not been proven scientifically, as the pathophysiological process of post-traumatic brain swelling is complicated and not fully understood1,11,28,29). In many neurosurgical guidelines, indication for DC includes dilated pupils, unresponsiveness to light, uncontrollable ICP values (greater than 30 mm Hg) for a period longer than 15 minutes and failure to respond to medical treatments10,22,25). DC is often perceived as a last resort for treating uncontrollable ICP, which restricts many of its positive effects. Several clinical reports demonstrate that early evacuation of a hematoma and decompression reduce mortality. Additionally, accumulating experimental evidence shows that very early decompression significantly reduces secondary brain injury5,22,24,37). Thus, it seems reasonable to assume that in patients with a severe brain injury, an early decompression may prevent secondary injuries that would occur during unsuccessful ICP treatment with conventional methods. Thus patients given early decompressions will have a better outcome than those that have a later decompression. In this study, we performed early DC in severe TBI patients who had abnormal findings in their CT scans.
Postoperative ICP monitoring
Measuring ICP is a milestone in TBI management. ICP monitors maintain adequate cerebral perfusion and oxygenation in situations of intracranial hypertension, in order to avoid secondary brain injuries during the recovery period. According to the best practice, evidence-based guidelines of the BTF4), using an ICP monitor is recommended for all patients with head injuries who have a GCS of 3 to 8 after resuscitation and an abnormal head CT scan, as well as in patients with severe TBI and normal head CT scans, if two or more of the following features are present on admission : age greater than 40, unilateral or bilateral posturing, or systolic blood pressure less than 90 mm Hg. Several evidence-based studies show that ICP monitoring, when used in a protocol-based manner in neurosurgical intensive care units, leads to improved outcomes in adjusted mortality rates for patients with severe TBI6,9,23). In addition, a meta-analysis of clinical studies since 1970 found that patients who were aggressively monitored for ICP, after severe TBI, had a 12% lower mortality rate, and 6% more favorable outcomes, when compared with patients who were less intensely monitored30). There is still debate over the effect of ICP monitoring on outcomes, despite increasing evidence that its implementation leads to improved outcomes. The findings of this study suggest that post-decompression treatment with subdural ICP monitoring improves outcome, as measured by the two-week adjusted mortalities. Two-week mortality was chosen as the end point of this study because this early time point accounts for over 85% of all TBI-related mortality and more appropriately reflects the severity of the injury as well as the efficacy of early intervention26), whereas later time points such as 30-day mortality include complications or associated comorbidities due to ICU and hospital length of stay27). The age and GCS of the patient are major factors that influence the DC effectiveness. It is generally reported that outcomes for patients younger than 50, or those with an initial GCS score of 6 or more, are significantly better than that for older patients, or those with an initial GCS score lower than 611,16,21,22,25). The characteristics of the patients in this study that were treated for intracranial hypertension with monitoring, versus those treated without a monitor did not differ with regard to age, sex, initial GCS score, or pupillary response. The two-week mortalities, however, were significantly improved if the post-decompression treatment was coupled with the use of an ICP monitor. We speculate that the reason for this improvement is that ICP monitoring ensures that the patients receive treatment with a better speed and accuracy.
Consecutive decompressive surgery
Bilateral DC was first described by Miyazakib et al.20) in 1966, and was popularized in 197113). For patients with diffuse brain swelling or global brain edema who fail to respond to more conservative medical treatments, bilateral DC may be the ideal treatment3). After unilateral decompressive surgery, several mechanisms such as vasogenic and cytotoxic edema, as well as cerebral auto-regulatory dysfunction, may still contribute to post traumatic brain swelling following severe TBI31). Thus, additional DC on the other side of the brain should be considered as a last-resort therapy for patients at risk of developing brain swelling or edema, after unilateral decompressive surgery, to interrupt this vicious cycle and hopefully avoid further complications. Wolfla et al.33,34) reported that brain tissue pressure gradients indicating ICP increases for any lesion inside the head occur bilaterally. Consequently, using global ICP management strategies may be effective for certain focal brain injuries. In this study, we used a consecutive bilateral fronto-temporo-parietal DC, including dura opening and duraplasty, with a short time lag if necessary. This procedure provides maximal supratentorial bilateral decompression, and more space for later brain swelling, ultimately, decreasing the mortality rate, although not at a level of statistical significance.
Limitations of this study
This study has some limitations mostly stemming from its small sample size and retrospective design. Methodological weaknesses include the following : 1) retrospectively collected data, 2) lack of selection parameters influencing the decision to monitor, 3) baseline differences between groups in other disease characteristics and therapeutic factors known to affect outcome following TBI. Knowing the rationale for decisions to monitor or not is a topic requiring investigation. Besides, Two-week adjusted mortality rather than 6-month Glasgow Outcome Scale score was used as the primary outcome measure. Although our data indicate that ICP monitoring after early DC yields good outcomes, the retrospective design and short term outcome are prone to bias. Therefore, future research of corresponding randomized controlled trials and outcome assessment to include long-term functional status is warranted to further explore the role of this procedure.
CONCLUSION
The present study suggests that ICP monitoring, in conjunction with postoperative treatment after early DC, is associated with a significantly reduced risk of death. This is the first study that statistically evaluated two-week mortalities in patients who had ICP monitoring as part of their postoperative treatment, compared to patients treated without ICP monitoring. In addition, we speculate that consecutive DC on the other side may be a favorable treatment for patients who show high ICP's of greater than 30 mm Hg, and fail to respond to maximal medical treatment after early DC.
References
- 1.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469–479. doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- 2.Akyuz M, Ucar T, Acikbas C, Kazan S, Yilmaz M, Tuncer R. Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010;20:382–389. doi: 10.5137/1019-5149.JTN.2785-09.1. [DOI] [PubMed] [Google Scholar]
- 3.Bao YH, Liang YM, Gao GY, Pan YH, Luo QZ, Jiang JY. Bilateral decompressive craniectomy for patients with malignant diffuse brain swelling after severe traumatic brain injury : a 37-case study. J Neurotrauma. 2010;27:341–347. doi: 10.1089/neu.2009.1040. [DOI] [PubMed] [Google Scholar]
- 4.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, et al. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma. 2007;24(Suppl 1):S7–S13. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- 5.Cremer OL, Moons KG, van Dijk GW, van Balen P, Kalkman CJ. Prognosis following severe head injury : development and validation of a model for prediction of death, disability, and functional recovery. J Trauma. 2006;61:1484–1491. doi: 10.1097/01.ta.0000195981.63776.ba. [DOI] [PubMed] [Google Scholar]
- 6.Fakhry SM, Trask AL, Waller MA, Watts DD. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma. 2004;56:492–499. doi: 10.1097/01.ta.0000115650.07193.66. discussion 499-500. [DOI] [PubMed] [Google Scholar]
- 7.Farahvar A, Gerber LM, Chiu YL, Carney N, Härtl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012;117:729–734. doi: 10.3171/2012.7.JNS111816. [DOI] [PubMed] [Google Scholar]
- 8.Farahvar A, Gerber LM, Chiu YL, Härtl R, Froelich M, Carney N. Response to intracranial hypertension treatment as a predictor of death in patients with severe traumatic brain injury. J Neurosurg. 2011;114:1471–1478. doi: 10.3171/2010.11.JNS101116. [DOI] [PubMed] [Google Scholar]
- 9.Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline : testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma. 2007;63:1271–1278. doi: 10.1097/TA.0b013e3181493080. [DOI] [PubMed] [Google Scholar]
- 10.Gerl A, Tavan S. [Bilateral craniectomy in the treatment of severe traumatic brain edema] Zentralbl Neurochir. 1980;41:125–138. [PubMed] [Google Scholar]
- 11.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling : indications and results. J Neurosurg. 1999;90:187–196. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 12.Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir (Wien) 1988;90:111–116. doi: 10.1007/BF01560563. [DOI] [PubMed] [Google Scholar]
- 13.Kjellberg RN, Prieto A., Jr Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34:488–493. doi: 10.3171/jns.1971.34.4.0488. [DOI] [PubMed] [Google Scholar]
- 14.Langlois JA, Kegler SR, Butler JA, Gotsch KE, Johnson RL, Reichard AA, et al. Traumatic brain injury-related hospital discharges. Results from a 14-state surveillance system, 1997. MMWR Surveill Summ. 2003;52:1–20. [PubMed] [Google Scholar]
- 15.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury : a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Iannotti F, et al. European Brain Injury Consortium. EBIC-guidelines for management of severe head injury in adults. Acta Neurochir (Wien) 1997;139:286–294. doi: 10.1007/BF01808823. [DOI] [PubMed] [Google Scholar]
- 17.Marshall LF. Head injury : recent past, present, and future. Neurosurgery. 2000;47:546–561. doi: 10.1097/00006123-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–S292. [PubMed] [Google Scholar]
- 19.McKinley BA, Parmley CL, Tonneson AS. Standardized management of intracranial pressure : a preliminary clinical trial. J Trauma. 1999;46:271–279. doi: 10.1097/00005373-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki Y, Hirai H, Hachisu Y, Takada I. [Bifrontal external decompression for traumatic brain edema] Shujutsu. 1966;20:845–852. [PubMed] [Google Scholar]
- 21.Morgalla MH, Krasznai L, Buchholz R, Bitzer M, Deusch H, Walz GU, et al. Repeated decompressive craniectomy after head injury in children : two successful cases as result of improved neuromonitoring. Surg Neurol. 1995;43:583–589. doi: 10.1016/0090-3019(95)00034-8. discussion 589-590. [DOI] [PubMed] [Google Scholar]
- 22.Münch E, Horn P, Schürer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315–322. doi: 10.1097/00006123-200008000-00009. discussion 322-323. [DOI] [PubMed] [Google Scholar]
- 23.Palmer S, Bader MK, Qureshi A, Palmer J, Shaver T, Borzatta M, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. Americans Associations for Neurologic Surgeons. J Trauma. 2001;50:657–664. doi: 10.1097/00005373-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Plesnila N. Decompression craniectomy after traumatic brain injury : recent experimental results. Prog Brain Res. 2007;161:393–400. doi: 10.1016/S0079-6123(06)61028-5. [DOI] [PubMed] [Google Scholar]
- 25.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–92. doi: 10.1097/00006123-199707000-00018. discussion 92-94. [DOI] [PubMed] [Google Scholar]
- 26.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial) : randomised placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 27.Roberts SR, Hamedani B. Benefits and methods of achieving strict glycemic control in the ICU. Crit Care Nurs Clin North Am. 2004;16:537–545. doi: 10.1016/j.ccell.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;(1):CD003983. doi: 10.1002/14651858.CD003983.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Servadei F, Compagnone C, Sahuquillo J. The role of surgery in traumatic brain injury. Curr Opin Crit Care. 2007;13:163–168. doi: 10.1097/MCC.0b013e32807f2a94. [DOI] [PubMed] [Google Scholar]
- 30.Stein SC, Georgoff P, Meghan S, Mirza KL, El Falaky OM. Relationship of aggressive monitoring and treatment to improved outcomes in severe traumatic brain injury. J Neurosurg. 2010;112:1105–1112. doi: 10.3171/2009.8.JNS09738. [DOI] [PubMed] [Google Scholar]
- 31.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 32.van Baalen B, Odding E, Maas AI, Ribbers GM, Bergen MP, Stam HJ. Traumatic brain injury : classification of initial severity and determination of functional outcome. Disabil Rehabil. 2003;25:9–18. doi: 10.1080/dre.25.1.9.18. [DOI] [PubMed] [Google Scholar]
- 33.Wolfla CE, Luerssen TG, Bowman RM. Regional brain tissue pressure gradients created by expanding extradural temporal mass lesion. J Neurosurg. 1997;86:505–510. doi: 10.3171/jns.1997.86.3.0505. [DOI] [PubMed] [Google Scholar]
- 34.Wolfla CE, Luerssen TG, Bowman RM, Putty TK. Brain tissue pressure gradients created by expanding frontal epidural mass lesion. J Neurosurg. 1996;84:642–647. doi: 10.3171/jns.1996.84.4.0642. [DOI] [PubMed] [Google Scholar]
- 35.Yamakami I, Yamaura A. Effects of decompressive craniectomy on regional cerebral blood flow in severe head trauma patients. Neurol Med Chir (Tokyo) 1993;33:616–620. doi: 10.2176/nmc.33.616. [DOI] [PubMed] [Google Scholar]
- 36.Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK. Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg. 1999;91:953–959. doi: 10.3171/jns.1999.91.6.0953. [DOI] [PubMed] [Google Scholar]
- 37.Zweckberger K, Erös C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006;23:1083–1093. doi: 10.1089/neu.2006.23.1083. [DOI] [PubMed] [Google Scholar]