Abstract
Deaf children have been characterized as being impulsive, distractible, and unable to sustain attention. However, past research has tested deaf children born to hearing parents who are likely to have experienced language delays. The purpose of this study was to determine whether an absence of auditory input modulates attentional problems in deaf children with no delayed exposure to language. Two versions of a continuous performance test were administered to 37 deaf children born to Deaf parents and 60 hearing children, all aged 6–13 years. A vigilance task was used to measure sustained attention over the course of several minutes, and a distractibility test provided a measure of the ability to ignore task irrelevant information – selective attention. Both tasks provided assessments of cognitive control through analysis of commission errors. The deaf and hearing children did not differ on measures of sustained attention. However, younger deaf children were more distracted by task-irrelevant information in their peripheral visual field, and deaf children produced a higher number of commission errors in the selective attention task. It is argued that this is not likely to be an effect of audition on cognitive processing, but may rather reflect difficulty in endogenous control of reallocated visual attention resources stemming from early profound deafness.
Keywords: deafness, visual selective attention, sustained attention, neuroplasticity, cognitive control
1. Introduction
Recently, there has been much interest in the relationship between audition and cognition. The new field of cognitive hearing science (Arlinger et al., 2009) has highlighted the important role of domain-general cognitive processes, such as working memory (Rönnberg, et al., 2008), attention (Wild et al., 2012), and sequence processing (Conway et al., 2009) in supporting spoken language comprehension and production. In instances where auditory systems are compromised (for example, in age-related hearing loss, or noisy environments), these cognitive systems have been shown to play a pivotal role in supporting successful spoken language processing. One approach to identifying which cognitive processes support auditory processing in the context of language comprehension is to study individuals who are profoundly deaf. Indeed, such studies have lead to theories that articulate the role of audition in shaping those cognitive processes (Conway et al., 2009). This has lead to the claim that the deleterious effect of profound deafness on spoken language development is compounded –deafness makes access to the sound structure of the language difficult, and at the same time leads to deficits in the cognitive skills needed to support spoken language comprehension under adverse conditions (Conway et al., 2009).
However, there are some profoundly deaf children who do not struggle to acquire language. These are deaf children born into culturally Deaf families where they are exposed in infancy to a natural signed language such as American Sign Language (ASL). Sign languages are the natural languages of Deaf communities and possess phonological systems, morphological systems and syntactic rules, operating within complex grammatical systems (Sandler and Lillo-Martin, 2006). Whatever cognitive processes are required for modality-independent language processing are clearly not impaired by deafness in these children, who achieve typical language and social milestones in infancy (Bonvillian et al., 1983; Marschark, 1993; Peterson & Siegal, 2000; Petitto & Marentette, 1991). However, it is remains possible that the cognitive processes required to support spoken language are negatively impacted by a lack of auditory stimulation. One such process that has been demonstrated to play a role in audio-visual speech comprehension (Kushnerenko et al., 2013) and word-to-world mapping (Yu & Smith, 2011) is visual attention. Here we focus upon two aspects of visual attention thought to be compromised in deaf children: the ability to sustain attention over a significant period of time, and the ability to select task-relevant stimuli and avoid distraction –selective attention.
1.1 Attentional Deficits in Deaf Children
Deaf children have been reported to have behavioral problems related to impulse control, distractibility, and an inability to sustain attention in the visual modality. Quittner et al. (1990) reported that parents of deaf children indicated that their children had greater distractibility-hyperactivity problems compared with the parents of hearing children. In a study of teacher-identified problem behaviors in deaf children, Reivich and Rothrock (1972) suggested that impulsivity and a lack of inhibitory control accounted for a significant amount of the problem behaviors reported. Chess and Fernandez (1980) reported elevated levels of impulsive behavior in deaf children manifest as aggressive acts such as kicking, hitting, and biting. Theirs was a study of deaf children whose mothers had Rubella during gestation, and the aggressive behaviors were more prevalent in those with multiple disabilities, than in the healthy children with deafness alone.
Parental and teacher reports, however, are by nature a subjective approach. Other researchers have adopted clinical measures that assess cognitive control by measuring how long it takes a child to complete a task, and how many errors they make -- fast completion coupled with a large number of errors is taken as an indicator of an impulsive response style. Several studies have shown that deaf children of hearing parents perform more poorly than hearing children on these types of clinical measures, including the Porteus Maze Test (Best, 1974; Eabon, 1984; O’Brien, 1987), the Matching Familiar Figures Test (Eabon, 1984; O’Brien, 1987), and the Draw-a-Man Test (Harris, 1978). Interestingly, the study by Harris (1978) revealed an effect of parental hearing status on the Matching Familiar Figures and Draw-a-Man Test, with deaf children born to deaf parents outperforming those born to hearing parents.
1.2 Continuous Performance Tests
More recently, deficits in visual continuous performance tasks (CPTs) have been reported in deaf children (Horn et al., 2005; Mitchell & Quittner, 1996; Quittner et al., 2004; Quittner et al., 1994; Smith et al., 1998; Yucel & Derim, 2008). CPTs are computerized measures of attention that typically require children to attend to a rapidly changing stream of stimuli. They have advantages over the clinical measures discussed in the previous section, including less subjectivity in the rating of performance and determination of errors, ease of administration, and the existence of large data sets providing norms across a large range of ages.
In one commonly used CPT, the Gordon Diagnostic System (GDS; Gordon & Mettleman, 1987), digits appear rapidly, one at a time, in the center of an LED display. Children are usually required to make a response to a target digit or to a specific sequence of target digits. The GDS can be administered as a visual task, with no auditory component, and has therefore been used with deaf children. In one version of the task, correctly pressing a button in response to the digit 9, but only when a 1 precedes it, is an index of sustained attention. Pushing the button at any other time (a commission error) is taken as being indicative of impulse control problems, reflecting poor cognitive control. In another version, irrelevant digits appear to the left and right of the central target digit stream. Poor performance is attributed to the child being distracted by the flanking digits; in other words, a failure of visual selective attention. In studies using these tasks, deaf children have been reported to have poorer cognitive control (Quittner et al., 1994) and to suffer from an inability to select targets appropriately (Mitchell & Quittner, 1996) relative to hearing age-matched controls. Furthermore, Smith et al. (1998) reported data suggesting that cochlear implantation alleviates these deficits, although the children with cochlear implants (CIs) did not achieve the performance levels of hearing controls. The authors suggested that their data indicate a deficit in visual selective attention stemming from poor multimodal sensory integration as a result of early, profound hearing loss. Such a position can be termed a deficiency hypothesis and, generally stated, it proposes that integration of information from the different senses is an essential component to the development of normal attentional functioning within each individual sensory modality.
An alternative view holds that attention-related deficits in deaf children may be related to their limited exposure to language and impoverished social communication early in life (Dye & Bavelier, 2013). Whether auditory loss, delays in language exposure, or abnormal socio-emotional development leads to attention deficits in deaf children remains a poorly understood issue. Other confounds are also worthy of consideration. For example, Parasnis et al. (2003) administered the Test of Variables of Attention (T.O.V.A.; Leark et al., 1999) to deaf and hearing college students. Their data suggested that deaf observers had decreased cognitive control when selecting the appropriate response, accompanied by decreased perceptual sensitivity. Parasnis et al. (2003) argued that this reflected appropriate adaptations to the environment for someone who cannot hear and was not an attentional pathology. Specifically, they argued, a less conservative response criterion reflects reliance upon vision for alerting in the absence of auditory input. The decreased perceptual discrimination ability, they argued, resulted from redistribution of attention away from the center and toward peripheral vision, as initially proposed by Neville and her collaborators (Neville & Lawson, 1987a, 1987b; Neville et al., 1983). In the absence of audition, a key modality in the detection of events in an individual’s immediate environment, visual selection attention becomes enhanced in deaf individuals in the periphery of their visual field (Bavelier et al., 2006). This possibility should also be entertained when considering the Mitchell and Quittner (1996) findings. In sum, the existing body of evidence points to weaker cognitive control and poor visual selective attention in deaf individuals, but the source of these effects remains controversial.
1.3 Continuous Performance Tests and Cochlear Implantation
Horn et al. (2005) reported a retrospective longitudinal study of CPT performance in deaf children who had undergone CI surgery. These implanted children demonstrated poor sustained attention, which improved little with increasing years of CI use. A study by Yucel and Derim (2008) looked at the effect of age of implantation on sustained attention in 6–11 year old deaf children. They reported elevated levels of inattention and impulsivity in deaf children compared to hearing controls, with performance poorer in those deaf children who received CIs after the age of 4 years compared to those who received their implants at a younger age. Interestingly, Shin et al. (2007) reported the opposite in a prospective longitudinal study of Korean deaf children receiving a CI at 6–7 years of age: they demonstrated more inattention and impulsivity following surgery than they did pre-implant.
In studies of recovery of function following cochlear implantation there is a confound between restoration of auditory input, age of implantation, and the acquisition of language. It is unclear to what extent any remediative effects of cochlear implantation are due to improved access to audition or to exposure to (spoken) language, with earlier implantation leading to better language development than later implantation (Niparko et al., 2010; Tomblin et al., 2005). Interestingly, a study by Tharpe et al. (2002) failed to find any differences in vigilance performance between hearing children and deaf children who either used hearing aids or CIs. Whilst they did not find any group differences as a function of preferred communication mode, their sample sizes were relatively small. Tharpe et al. (2002) concluded that further research was needed to determine how preferred communication mode shapes the relationship between hearing status and performance of tests of visual attention.
1.4 Deafness, Audition and Language Delay
Deafness cannot be easily separated from social and linguistic experience (Dye & Bavelier, 2013). All of the studies that report attentional deficits as a consequence of deafness are based upon deaf children born to hearing parents, many of whom received CIs, and most of whom are taught spoken language or a form of manual communication based upon a visual representation of spoken language that is co-produced with speech. This latter mode of communication is sometimes called Total Communication, and has been reported to limit spoken language acquisition due to segmentation difficulties (Ting et al., 2012) and limit sign language acquisition due to input that cannot be nativized (Wilbur, 2008). Without confirmation from studies recruiting deaf children born to Deaf parents, who acquire ASL and achieve typical language and social milestones in infancy, it is possible that the attentional problems indicated in deaf children are the result of early communicative deficits stemming from language acquisition delays.
In this study, we administered two forms of the GDS CPT to 37 deaf children born to Deaf parents from whom they acquired ASL as a native language, and to 60 hearing children born into hearing families. Deficit hypotheses (Conway et al., 2009; Mitchell, 1996) predict weaker sustained attention, less ability to allocate selective attention, and decreased cognitive control in deaf children compared to hearing peers of the same age, regardless of age of exposure to natural language and early socio-communicative environments. Thus any observed differences in performance between our groups, all exposed to a natural language from birth but differing in access to audition, could be attributed to the effects of auditory deprivation per se and not to language delay. The GDS has been used in previous studies of attention in deaf children (see above). It presents target stimuli at a fixed spatial location, requiring temporal sequence processing in order to successfully respond to the 1–9 target sequence. As a task that imposes few spatial demands but which has high temporal demands, it is an ideal instrument to test the types of deficit predicted by hypotheses such as the auditory scaffolding hypothesis (Conway et al., 2009).
2. Materials and Methods
2.1 Participants
The Institutional Review Board at the University of Illinois at Urbana-Champaign approved this study. Written, informed consent was obtained from both parents and children before data collection procedures were initiated. Inclusion/exclusion criteria and sample characteristics for deaf and hearing children are summarized below. All participants were required to have normal or corrected-to-normal vision, no reported learning disability (such as SLI or ADD/ADHD), nor any cognitive deficits (based upon parental and/or teacher reports). Due to reports of elevated attentional abilities in children who play action video games (Dye & Bavelier, 2010b; Dye, Green, et al., 2009), children who reported playing such games were also excluded from the study. Testing time did not permit the administration of tests of non-verbal IQ or language skill. However, no general cognitive or language difficulties were reported for any of the children by their parents or teachers. More detailed demographic data for the deaf children is reported in Table 1.
Table 1.
Demographic characteristics of hearing and deaf children
| Hearing | Deaf | |||
|---|---|---|---|---|
|
| ||||
| 6–8 years | 9–13 years | 6–8 years | 9–13 years | |
|
| ||||
| N | 19 | 41 | 12 | 25 |
|
| ||||
| Age M (SD) months | 89 (11) | 133 (18) | 90 (7) | 138 (18) |
| Range | 74–106 | 109–167 | 77–101 | 109–165 |
|
| ||||
| # males (%) | 12 (63%) | 17 (41%) | 7 (58%) | 10 (40%) |
|
| ||||
| SES M (SD) | 56 (8) | 55 (10) | 51 (11) | 49 (9) |
| Range | 39–66 | 22–66 | 22–64 | 28–61 |
|
| ||||
| Racial identity | ||||
|
| ||||
| White | 14 | 30 | 12 | 25 |
|
| ||||
| Black | 1 | 2 | 0 | 0 |
|
| ||||
| Asian or PI | 3 | 8 | 0 | 0 |
|
| ||||
| No Response | 1 | 1 | 0 | 0 |
|
| ||||
| Ethnicity | ||||
|
| ||||
| Non-Hispanic | 17 | 39 | 9 | 20 |
|
| ||||
| Hispanic | 1 | 1 | 3 | 5 |
|
| ||||
| No Response | 1 | 1 | 0 | 0 |
|
| ||||
| Reported Language Fluency | ||||
|
| ||||
| English | 19 | 41 | 2 | 9 |
|
| ||||
| ASL | 0 | 0 | 12 | 25 |
|
| ||||
| Spanish | 0 | 1 | 0 | 0 |
|
| ||||
| Hebrew | 1 | 0 | 0 | 0 |
|
| ||||
| Mandarin | 0 | 1 | 0 | 0 |
2.1.1 Hearing Children
Sixty hearing children were recruited from public schools in Champaign, Illinois in the United States. This included 29 boys and 31 girls, all aged between 6 and 13 years of age, None of these children had a diagnosed hearing loss, learning disability, or any other sensory impairment. Hearing status and the presence or absence of learning disabilities was ascertained via parent or teacher report. All were monolingual native speakers of English, and had no knowledge of ASL. Socio-economic status (SES) was assessed using the Hollingshead four-factor method (Hollingshead, 1975) that weights paternal and maternal occupation and education levels to derive a single SES score that can range from 8 to 62. Higher scores indicate a higher socio-economic status. The mean SES score for the hearing children was 55.5 (SD = 9.3), which can be interpreted as having parents with a college degree and a professional occupation.
2.1.2 Deaf Children
Thirty-seven deaf children were recruited from residential schools for the Deaf in Texas, California, Maryland, and Indiana. All of these schools employed a bilingual-bicultural approach to deaf education. There were 17 boys and 20 girls, all with at least a severe-to-profound hearing loss (HL > 75dB PTA in the better ear). Hearing status was ascertained via parent or teacher report. All had acquired ASL as infants from their Deaf parents, and none had received a CI. As with the hearing children, none had any other diagnosed sensory impairment or learning disability. Their mean SES computed using the Hollingshead method was 50.0 (SD = 9.8), interpretable as having parents with a college degree and managerial/administrative occupations.
The deaf and hearing children differed significantly on this SES measure: F (1, 93) = 6.38, p = .013, partial eta-squared = .06. Due to the significantly lower SES of the deaf children, this measure was used as a covariate in all analyses.
2.2 Design
Measures were obtained of sustained attention, selective attention, and cognitive control from all children using the vigilance and distractibility forms of the GDS CPT. Children were divided into younger and older groups based upon previous findings in the literature (Quittner et al., 1994; Smith et al., 1998). The effect of age group (6–8 years, 9–13 years) and hearing status (hearing, deaf) was determined for each of the three dependent measures.
2.3 Procedure
Children were tested individually and in a quiet setting free from auditory and visual distraction, either at home or in their school. After explaining the study to children in their preferred language and obtaining written consent, the experimental procedures were explained. A native hearing speaker gave instructions in spoken English to hearing children, and a native ASL signer gave instructions in ASL to deaf children. No practice trials were given. For all children, the vigilance test was administered first, followed by a 5-minute break and then the distractibility test.
2.3.1 Sustained Attention
The children were shown the GDS CPT apparatus (Figure 1) and told that they were required to watch a stream of digits appearing on the red LED display. Their task was to look for a specific sequence of digits - a 9 preceded by a 1 (see Figure 2A for a diagrammatic representation of the sustained attention task). They were instructed to rest their hand on a blue button below the red LED display, and to press that blue button as quickly as possible whenever they saw a 9 that was preceded by a 1. Children were specifically instructed not to press when they saw a 1, and not to respond to a 9 if any digit other than a 1 preceded it. The test consisted solely of visual stimuli, with no auditory component. Children were seated with their eyes approximately 30 cm from the LED display, such that each digit was 1.9 degrees of visual angle high and 0.95 degrees of visual angle wide. A total of 540 digits appeared at a rate of 1 per second. These digits appeared in 3 blocks of 180 digits (although children were unaware of this) and the target sequence (1 -> 9) occurred 15 times within each block.
Figure 1.

The Gordon Diagnostic System has an LED display upon which stimuli are presented, and a large blue button for making responses. The red and green lights do not provide feedback on performance, but only indicate whether a data collection session is ready to proceed or has terminated.
Figure 2.
(A) Schematic representation of sustained attention task. Digits appeared one at a time in the center of the LED display at a rate of one digit per second. The observer was required to respond to a target sequence (here, a 1 followed by a 9), and withhold responses to non-target sequences (here, a 6 followed by a 9); (B) Schematic representation of selective attention task. The central digit sequence is identical to that in the sustained attention task. However, distractor digits appear to the left and right of the LED display, sometimes concurrently with the central target. Dashed boxes indicate the target stream, and were not visible to participants in the task.
D-prime scores were computed using the method reported by Green and Swets (1966). Children who did not make any misses were assigned a hit rate of 44/45, and those who made no false alarms were assigned a false alarm rate of 494/495. The vigilance d-prime scores were negatively skewed, and therefore a log-transformed variable for this measure was computed to allow parametric statistical analysis. These log-transformed d-prime scores were used as an index of sustained attention. Failure to sustain attention to the target stream would result in an increase in the number of misses, which would not be offset by a higher overall response rate.
2.3.2 Cognitive Control – Sustained Attention Task
To obtain a measure of cognitive control, the total number of commission errors in the sustained attention task was computed for each child. These errors included responses to the first digit of the target sequence (XX1 or X1X responses), or responses to the second digit of the sequence (9) when it was not in a target sequence (XX9 and X9X responses). Errors that were considered to be due to a delayed response (19X responses) were not included. Due to experimenter error, commission error data for the sustained attention task was not collected for 5 deaf children (one 6–8 year old, and four 9–13 year olds). The number of commission errors was compared to published norms (Gordon and Mettleman, 1987) in order to determine the proportion of children in each group who were normal or borderline-abnormal.
2.3.3 Selective Attention
After completing the sustained attention task, children performed the selective attention task. They were instructed to respond in the same way as in the sustained attention task, ignoring flanking distractor digits that appeared to the left of right of the center target digits. Again, there were a total of 540 digits appearing in the center of the display at a rate of 1 per second and subtending 1.9 by 0.95 degrees of visual angle. The sequence of digits was identical to that presented in the sustained attention task. Distractor digits appeared randomly 1.9 degrees of visual angle to the left or right of the central target digits. See Figure 2B for a diagrammatic representation of the selective attention task.
To compute an index of selective attention, each individual’s d-prime score from this task was subtracted from their d-prime score for the sustained attention task. In the GDS, the stream of central digits is identical in both tasks. This subtractive measure therefore provides an index of the extent to which the flanking distractor digits impaired performance1. The selective attention measure was normally distributed and therefore not subjected to a transformation prior to parametric statistical analysis.
2.3.4 Cognitive Control – Selective Attention Task
To obtain a measure of cognitive control, the total number of commission errors in the selective attention task was computed for each child. These errors included responses to the first digit of the target sequence (XX1 or X1X responses), or responses to the second digit of the sequence (9) when it was not in a target sequence (XX9 and X9X responses). Errors that were considered to be due to a delayed response (19X responses) were not included. Due to experimenter error, commission error data for the selective attention task was not collected for 13 deaf children (four 6–8 year olds, and nine 9–13 year olds). The number of commission errors was compared to published norms (Gordon and Mettleman, 1987) in order to determine the proportion of children in each group who were normal or borderline-abnormal.
3. Results
For all statistical analyses, an alpha criterion of .05 was used to determine statistical significance. All p-values reported below are two-tailed and uncorrected for multiple comparisons unless otherwise stated.
3.1 Sustained Attention
Means and standard deviations for sustained attention performance are reported in Table 2. As expected, younger children (M = 3.36) demonstrated poorer sustained attention than did older children (M = 4.26). In addition, boys (M = 3.74) appeared to display weaker sustained attention than girls (M = 4.18). However, no large differences were apparent between the performance of deaf and hearing children, despite a sample size significantly larger than that used in previous studies reporting sustained attention differences on the basis of hearing status.
Table 2.
Performance measures on (a) sustained attention and (b) selective attention forms of the Gordon Diagnostic System continuous performance test
| (a) Sustained attention | Hearing | Deaf | ||
|---|---|---|---|---|
| 6–8 years | 9–13 years | 6–8 years | 9–13 years | |
| N | 19 | 41 | 12 | 25 |
| Mean (SD) sensitivity (d′) | 3.47 (0.78) | 4.36 (0.55) | 3.18 (0.75) | 4.09 (0.66) |
| Mean (SD) criterion (c) | 0.59 (0.25) | 0.45 (0.17) | 0.51 (0.21) | 0.37 (0.21) |
| Median (Range) commission errors | 2 (0–12) | 1 (0–11) | 4 (0–28) | 2 (0–20) |
| Percentage with commission errors in normal range | 68.4% | 78.0% | 54.5% | 61.9% |
| (b) Selective Attention | Hearing | Deaf | ||
|---|---|---|---|---|
| 6–8 years | 9–13 years | 6–8 years | 9–13 years | |
| N | 19 | 41 | 10 | 20 |
| Mean (SD) sensitivity d′ | 3.07 (0.90) | 3.90 (0.76) | 2.11 (1.15) | 3.70 (0.87) |
| Mean (SD) criterion (c) | 0.79 (0.27) | 0.64 (0.21) | 0.81 (0.48) | 0.49 (0.20) |
| Median (Range) commission errors | 2 (0–20) | 2 (0–39) | 10 (2–39) | 5 (0–18) |
| Percentage with commission errors in normal range | 78.9% | 90.2% | 33.3% | 50% |
| Selective attention score* | 0.40 (0.76) | 0.46 (0.68) | 1.01 (0.91) | 0.42 (0.91) |
Computed as (sustained d′ - selective d′)
In order to assess these observations, log-transformed d-prime scores from the sustained attention task were entered into a three-way ANCOVA, with hearing status (hearing, deaf), age group (6–8 years, 9–13 years) and gender (female, male) as between subject factors, and SES as a covariate. This revealed significant main effects of age group (F (1, 88) = 31.14, p < .001, partial eta-squared = .261) and gender (F (1, 88) = 4.79, p = .031, partial eta-squared = .05). No other main effects or interactions reached the criterion for statistical significance (all F < 1, except hearing status: F (1, 88) = 1.59, p = .211, partial eta-squared = .018). SES was not a significant covariate in the analysis (F (1, 88) = 3.85, p = .053, partial eta-squared = .042).
3.2 Cognitive Control (Sustained Attention Task)
The number of commission errors was highly positively skewed for the sustained attention task (see Table 2 and Figure 3). In addition, closer inspection of the data revealed that some deaf children made an unusually large number of commission errors. Rather than rejecting data as outliers, the number of commission errors for each child was categorized as normal or abnormal-borderline, based upon age norms published in Gordon and Mettleman (1987). Chi-squared analyses revealed no differences in vigilance commission error classification as a function of hearing status (Chi-squared = 2.41, df = 1, p = .121) or age group (Chi-squared = 0.82, df = 1, p = .366).
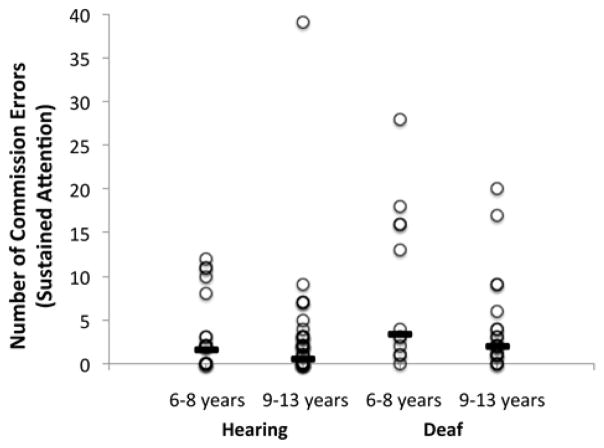
Figure 3.
Number of commission errors made by each child during performance of the sustained attention task. Solid lines indicate the median number of errors for each group.
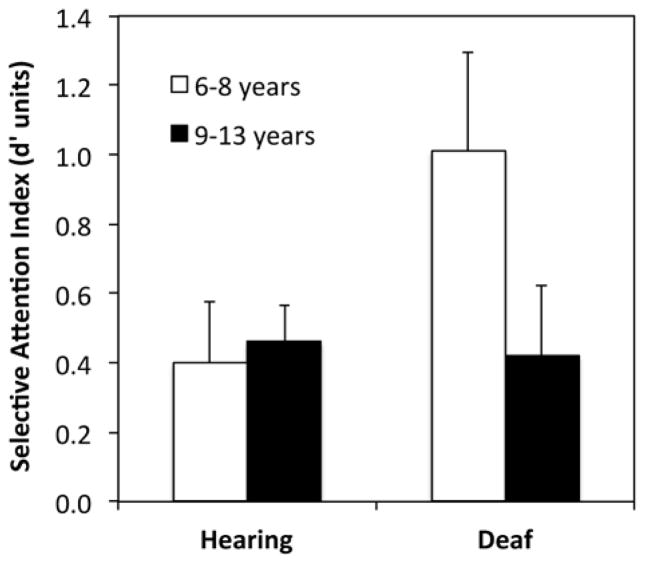
3.3 Selective Attention
Deaf 6–8 year olds (M = 1.01) appeared less able to selectively attend to the target stimulus stream than 9–13 year olds (M = 0.42), who had similar selective attention scores to hearing 9–13 year olds (M = 0.46; Table 2; Figure 4). In order to confirm this observation, selective attention scores were entered into a three-way ANCOVA, with hearing status (hearing, deaf), age group (6–8 years, 9–13 years) and gender (female, male) as between subject factors, and SES as a covariate. This revealed significant main effects of age group (F (1, 81) = 6.67, p = .012) and gender (F (1, 81) = 8.11, p = .006, partial eta-squared = .091). The main effect of gender reflected better selective attention for boys (M = 0.35) than for girls (M = 0.642). The main effect of age group was qualified by a significant two-way interaction between hearing status and age group (F (1, 81) = 6.59, p = .012, partial eta-squared = .075).
Figure 4.
Mean distractibility effect by hearing status and age group. Higher values indicate poorer selective attention (more distraction by task-irrelevant flankers). Error bars represent +/− 1 S.E..M.
The main effect of hearing status was not statistically significant (F (1, 81) = 2.41, p = .124, partial eta-squared = .029), and nor were the two-way interactions between hearing status and gender (F (1, 81) = 2.64, p = .108, partial eta-squared = .032) and between age group and gender (F (1, 81) = 3.73, p = .057, partial eta-squared = .044). Finally, the three-way interaction between hearing status, age group and gender was not statistically significant (F (1, 81) = 2.40, p = .125, partial eta-squared = .029).
In order to unpack the two-way interaction between hearing status and age group, separate ANOVAs were conducted for each participant group. This revealed a significant effect of age group for deaf children (F (1, 25) = 7.21, p = .013, partial eta-squared = .224) but not for hearing children (F (1, 55) = 0.001, p = .982, partial eta-squared < .001), confirming the poorer selective attention performance in deaf 6–8 year old children.
3.3 Cognitive Control (Selective Attention Task)
The number of commission errors was also highly positively skewed for the selective attention task. In addition, as for the sustained attention task, closer inspection of the data revealed that some deaf children made an unusually large number of commission errors. Rather than rejecting data as outliers, the number of commission errors for each child was again categorized as normal or abnormal-borderline, based upon age norms published in Gordon and Mettleman (1987).
For commission error classifications on the selective attention task there was an effect of hearing status (Chi-squared = 16.75, df = 1, p < .001) but not age group (Chi-squared = 2.10, df = 1, p = .147). Deaf children were more likely than hearing children to make enough commission errors to be classified as abnormal or borderline abnormal for their age, suggesting weaker cognitive control in the selective attention task in deaf children (see Table 2 and Figure 5).
Figure 5.
Number of commission errors made by each child during performance of the selective attention task. Solid lines indicate the median number of errors for each group.
4. Discussion
A cross-sectional sample of 6–13 year old hearing and deaf children performed two versions of a visual CPT. The first assessed their ability to sustain attention over a 9 minute time period. The second measured the extent to which their attentional system was able to ignore task-irrelevant stimuli in the near periphery – selective attention. Previous studies using such tests have led to the suggestion that deaf children are inattentive, distractible, and impulsive (Quittner et al., 1994; Smith et al., 1998; Yucel & Derim, 2008), or that they are unable to process sequences as well as hearing children (Horn et al., 2005). This has lent some support to deficit theories which propose that deaf children distribute their attention widely across the visual field in an unfocussed manner (Mitchell, 1996), or that they have impaired domain-general sequencing skills (Conway et al., 2009). In this study, deaf children from Deaf families were recruited. Unlike deaf children who have hearing parents, deaf children from Deaf families are exposed to their native language from birth, and are therefore likely to achieve normal language development. We reasoned that if auditory experience is important for the development of domain-general abilities such as attention and sequence processing, then these deaf children from Deaf families should demonstrate significant impairment in those functions.
On the sustained attention task, the performance of deaf and hearing children was comparable across the age range tested, despite a sample size larger than in previous studies that reported differences between deaf and hearing children in the same age range. This indicates that there is no evidence to suggest sustained attention deficits in deaf children born to Deaf parents who started to acquire ASL in infancy as a first language. However, the addition of task irrelevant stimuli to the left and right of the target sequence location (the selective attention task) was particularly disruptive for the younger deaf children, for whom task-irrelevant stimuli in the near periphery were more likely to impair performance. In the selective attention task, deaf children also demonstrated a greater tendency to make impulsive responses - responding either preemptively to the first digit of a two-digit sequence, or responding to the second digit when the preceding digit was not part of the target sequence. This weaker cognitive control was particularly evident in 6–8 year old deaf children.
4.1 Sustained Attention
The failure to replicate previous findings on sustained attention may be indicative of the important role language plays in the shaping of attentional processes. Indeed, poor performance on this form of CPT has been reported in hearing children with specific language impairments (Ebert & Kohnert, 2011; Finneran et al., 2009; Spaulding et al., 2008) and children with poor social interaction skills such as those with autism (Corbett & Constantine, 2006; Garretson et al., 1990). Both spoken and signed interactions typically require sustained joint attention with the sender, and caregivers are known to shape the attentional behaviors and gaze direction of their infants during interactions (Chavajay & Rogoff, 1999; Loots & Devisé, 2003). This raises the possibility that previous demonstrations of apparent inattentiveness in deaf children may reflect poor early communicative environments, and delays in the acquisition of language needed to support those interactions. None of the children recruited in previous studies had deaf parents, and none had been exposed to a natural signed language, such as ASL, from infancy. Indeed, the improvements in sustained attention performance seen in young deaf children with CIs (Horn et al., 2005) may reflect increased communicative and linguistic competence afforded by the implant and speech-listening training, rather than remediation of hearing loss per se. If this is the case, then it further suggests clinical benefit from early implantation as well as early introduction of natural language – either spoken or signed.
4.2 Selective Attention
The younger deaf children performed very poorly on the selective attention task – poorer than their hearing peers of similar age. However, in 9–13 year olds, these differences were no longer evident. Importantly, these older deaf children were similar to the younger children, in that none had received a CI, all were reported to have severe-to-profound hearing losses, and all preferred to receive test instructions in ASL. Furthermore, the selective attention differences between deaf and hearing children at 6–8 years of age could not be attributed to weaker sustained attention or problems processing sequential or numerical information -- the groups performed at similar levels on the sustained attention task.
One hypothesis is that the younger deaf children struggle on this task because of an inability to control the allocation of their visual attention. Several studies have now shown that deaf individuals have greater visual attention to the periphery than do hearing individuals (Buckley et al., 2010; Chen et al., 2006; Codina et al., 2011; Dye et al., 2007; Dye & Bavelier, 2010a; Dye et al., 2009; Loke & Song, 1991; Proksch & Bavelier, 2002; Sladen et al., 2005). This neural plasticity can be seen as adaptive for an organism that must rely upon vision to monitor events in its periphery, and which cannot use audition to locate events or interlocutors. However, to be successful in navigating its environment, the organism must be able to employ the enhanced peripheral attention in a goal-directed manner. It is possible that, by the age of 9–13 years, deaf children have enhanced attention to their peripheral visual field (relative to hearing peers). However they can inhibit that process when a task requires attention to the central visual field and the processing of peripheral visual stimuli is detrimental to performance. The fronto-parietal cognitive control network has been shown to improve across the age range tested here (Fair et al., 2007; Hwang et al., 2010; Wendelken et al., 2011). On the other hand, 6–8 year olds combine the enhanced peripheral attention with an inability to selectively attend to the central visual field and ignore the periphery when the task requires them to do so. In other words, the neuroplastic changes that shape the spatial allocation of visual attention interact with the development of inhibitory processes and other executive functions to determine performance.
4.3 Cognitive control
Deaf children appeared to demonstrate weaker cognitive control than the hearing children. This was only observed for the selective attention task, where only 33.3% of deaf 6–8 year olds and 50% of deaf 9–13 year olds performed within the range considered typical for hearing children. The fact that cognitive control was similar for hearing and deaf children in the vigilance test suggests caution in considering the deaf children to be more impulsive per se. The relatively large number of commission errors seen in these children may be, at least in part, driven by an inability to ignore the distractor digits in their near periphery due to enhanced peripheral visual attention. This may particularly be the case for the younger deaf children.
4.4 Limitations
While there is no reason to believe that the IQ of the kind of deaf children enrolled in the study would be different from that of hearing children, this cannot be ruled out conclusively. However, none of the deaf children in this study were reported to have linguistic or cognitive disabilities, and all were exposed to natural language from birth. Racial and ethnic factors could also have played a role in the pattern of data observed. The deaf sample was exclusively white Caucasian, with some Hispanic children included, whereas the hearing sample had greater racial diversity and fewer Hispanic children (see Table 2). However, race and ethnicity were well matched across age groups in the sample of deaf children, and thus unlikely to explain any age group differences within the sample of deaf children.
Where there does appear to be a systematic difference between the deaf and hearing is in their language background. Whereas the vast majority of the hearing children were monolingual English speakers, several of the deaf children were reported to be fluent in ASL and English. Indeed, perhaps unsurprisingly, the older deaf children were more likely to be bilingual than the younger deaf children. An alternative interpretation, therefore, is that a lack of audition impairs performance on these tasks, but cognitive benefits stemming from sign-print bilingualism offset this impairment in older deaf children. It has been suggested that bilingualism has an effect on executive function skills (Barac & Bialystock, 2012). Interestingly, however, it remains unclear whether such advantages accrue to sign-print bilinguals in the same way (Emmorey et al., 2008; Kushalnagar et al., 2010), and it has been reported that any bilingual advantages may not generalize to measures of impulse control (Carlson & Meltzoff, 2008). This study cannot address these issues as we did not collect measures of language proficiency across the necessary domains; for now an effect of bilingualism is purely speculative. Further research is required, alongside more detailed characterization of the multilingual abilities of deaf children in bilingual-bicultural settings.
5. Conclusions
Previous research has suggested that deaf children suffer from elevated inattentiveness, distractibility, and impulsiveness. We sought to extend and refine that research by testing deaf children born to Deaf parents from whom they acquired ASL as a first language. The data suggest that these deaf children do not suffer from weaker sustained attention. This raises the possibility that earlier reports may have misattributed inattentiveness to deafness, when the causes may have been related to delayed access to natural language and/or problems with communication. While some difficulties with selective attention were observed, this was restricted to younger deaf children, and not evident in deaf children aged 9–13 years. One suggestion is that this inability to select task-relevant information at fixation stems from greater peripheral attentional resources, as described before in deaf children and adults (Bavelier et al., 2006; Dye, Hauser, et al., 2009). Younger deaf children may still be learning how to control the allocation of their attentional resources, with tasks that require suppression of peripheral information and focus on central targets being especially challenging. The finding of weaker cognitive control, especially in the presence of peripherally distracting information, reinforces this view.
6. Future Directions
More studies are needed to assess the potential influence of developing bilingualism in deaf children who use a sign language such as ASL and also develop oral or written language skills in a spoken language such as English. Future work should also carefully assess IQ, executive function, and language skills in young deaf children and where possible also provide audiometry to determine the extent of deafness. Here we conclude that deafness does not necessarily result in deficits in visual attention, pointing to the need to carefully document the language background and proficiency of deaf children in both the signed and spoken modalities where appropriate.
While the data were collected using a cross-sectional design, all of the children were born into Deaf families and attended residential schools for deaf children that employed bilingual-bicultural educational practices. Nonetheless, there is a strong need for further longitudinal research that addresses the effects of early language acquisition and communicative interaction on the developmental trajectory of attentional abilities. In particular, one argument put forward here is that the cognitive control errors and decrease in perceptual sensitivity we observed in young deaf children (but not older deaf children) in the selective attention task may be a result of interactions between different cognitive-neural systems that are developing at different rates. Johnson (2012) has put forward a similar argument from the perspective of research on children with autism and ADHD. Many studies have reported an enhanced ability to attend to the visual periphery following early, profound deafness. However, little is known about how a deaf child is able to endogenously control this ability in a task-driven manner. Thus, the development of fronto-parietal attentional networks is likely to interact with cross-modal changes in the visual dorsal stream to produce a different developmental time course for attentional behavior in the deaf child. Longitudinal studies that combine behavioral assessment with structural and functional neuroimaging have the potential to shed light on how these neural systems develop and interact across the school-aged years.
HIGHLIGHTS.
Recruited Deaf native signing children with no language delay
In contrast to other studies, sustained attention as good in Deaf as in hearing children
Distraction could be due to interactions between developing neural systems
Early exposure to natural language promotes visual attention more than does audition
Acknowledgments
This research was supported by NSF awards SBE-0541953 and SBE-1041725 to the Science of Learning Center on Visual Language and Visual Learning at Gallaudet University, and grant NIDCD R01 DC004418 to Daphne Bavelier and PH. We wish to thank Geo Kartheiser, Rupert Dubler, Kim Scanlon, and Dani Hagemann for recruitment and data collection efforts.
List of Abbreviations
- ADD/ADHD
Attention Deficit Disorder/Attention Deficit-Hyperactivity Disorder
- ANCOVA
Analysis of Covariance
- ASL
American Sign Language
- CI
cochlear implant
- CPT
continuous performance task
- GDS
Gordon Diagnostic System
- SES
socio-economic status
- SLI
Specific Language Impairment
- T.O.V.A
Test of Variables of Attention
Footnotes
This may be small underestimate of actual distractibility, as order of test administration was fixed and thus distractibility test performance may have been influenced by a learning effect.
As this is a difference score, a higher value reflects greater distractibility in the face of competing distractor digits.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew W. G. Dye, Department of Speech and Hearing Science, University of Illinois at Urbana-Champaign, Champaign, IL 61820, United States of America.
Peter C. Hauser, Department of American Sign Language and Interpreting Education, National Technical Institute for the Deaf, Rochester, NY 14623, United States of America
References
- Arlinger S, Lunner T, Lyxell B, Pichora-Fuller MK. The emergence of Cognitive Hearing Science. Scandinavian Journal of Psychology. 2009;50(5):371–384. doi: 10.1111/j.1467-9450.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- Barac R, Bialystock E. Bilingual effects on cognitive and linguistic development: Role of language, cultural background, and education. Child Development. 2012;83(2):413–422. doi: 10.1111/j.1467-8624.2011.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Dye MWG, Hauser PC. Do deaf individuals see better? Trends in Cognitive Sciences. 2006;10:512–518. doi: 10.1016/j.tics.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best P. PhD. Wayne State University; 1974. Psychological differentiation in the deaf. [Google Scholar]
- Bonvillian JD, Orlansky MD, Novack LL. Early sign language acquisition and its relation to cognitive and motor development. In: Kyle JG, Woll B, editors. Language in sign: An international perspective on sign language. London: Croom Helm; 1983. [Google Scholar]
- Buckley D, Codina C, Bhardwaj P, Pascalis O. Action video game players and deaf observers have larger Goldmann visual fields. Vision Research. 2010;50(5):548–556. doi: 10.1016/j.visres.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Meltzoff AN. Bilingual experience and executive functioning in young children. Developmental Science. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavajay P, Rogoff B. Cultural variation in management of attention by children and their caregivers. Developmental Psychology. 1999;35(4):1079–1090. doi: 10.1037//0012-1649.35.4.1079. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang M, Zhou X. Effects of spatial distribution of attention during inhibition of return (IOR) on flanker interference in hearing and congenitally deaf people. Brain Research. 2006;1109:117–127. doi: 10.1016/j.brainres.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Chess S, Fernandez P. Impulsivity in rubella deaf children: A longitudinal study. American Annals of the Deaf. 1980;125(4):505–509. doi: 10.1353/aad.2012.1329. [DOI] [PubMed] [Google Scholar]
- Codina C, Buckley D, Port M, Pascalis O. Deaf and hearing children: A comparison of peripheral vision development. Developmental Science. 2011;14(4):725–737. doi: 10.1111/j.1467-7687.2010.01017.x. [DOI] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, Kronenberger WG. The importance of sound for cognitive sequencing abilities: The auditory scaffolding hypothesis. Current Directions in Psychological Science. 2009;18(5):275–279. doi: 10.1111/j.1467-8721.2009.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ. Autism and attention deficit hyperactivity disorder: Assessing attention and response control with the integrated visual and auditory continuous performance test. Child Neuropsychology. 2006;12(4–5):335–348. doi: 10.1080/09297040500350938. [DOI] [PubMed] [Google Scholar]
- Dye MWG, Baril DE, Bavelier D. Which aspects of visual attention are changed by deafness? The case of the Attentional Network Test. Neuropsychologia. 2007;45(8):1801–1811. doi: 10.1016/j.neuropsychologia.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MWG, Bavelier D. Attentional enhancements and deficits in deaf populations: An integrative review. Restorative Neurology and Neuroscience. 2010a;28:181–192. doi: 10.3233/RNN-2010-0501. [DOI] [PubMed] [Google Scholar]
- Dye MWG, Bavelier D. Differential development of visual attention skills in school-age children. Vision Research. 2010b;50(4):452–459. doi: 10.1016/j.visres.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MWG, Bavelier D. Visual attention in deaf humans: A neuroplasticity perspective. In: Kral A, Fay RR, Popper AN, editors. Springer handbook of auditory research: Deafness. Berlin, Germany: Walter de Gruyter; 2013. [Google Scholar]
- Dye MWG, Green CS, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009;47:1780–1789. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MWG, Hauser PC, Bavelier D. Is visual attention in deaf individuals enhanced or deficient? The case of the Useful Field of View. PLoS One. 2009;4:e5640. doi: 10.1371/journal.pone.0005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eabon MF. On the relationship between impulsivity and field-dependence in hearing-impaired children. Paper presented at the 56th Annual Meeting of the Midwestern Psychological Association; Chicago, IL. 1984. [Google Scholar]
- Ebert KD, Kohnert K. Sustained attention in children with primary language impairment: A meta-analysis. Journal of Speech, Language, and Hearing Research. 2011;54:1372–1384. doi: 10.1044/1092-4388(2011/10-0231). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Luk G, Pyers JE, Bialystock E. The source of enhanced cognitive control in bilinguals. Psychological Science. 2008;19(12):1201–1206. doi: 10.1111/j.1467-9280.2008.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finneran DA, Francis AL, Leonard LB. Sustained attention in childen with Specific Language Impairment (SLI) Journal of Speech, Language, and Hearing Research. 2009;52:915–929. doi: 10.1044/1092-4388(2009/07-0053). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garretson HB, Fein D, Waterhouse L. Sutained attention in children with autism. Journal of Autism and Developmental Disorders. 1990;20(1):101–114. doi: 10.1007/BF02206860. [DOI] [PubMed] [Google Scholar]
- Gordon M, Mettleman BB. Technical guide to the Gordon Diagnostic System (GDS) DeWitt, NY: Gordon Systems; 1987. [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York, NY: Wiley; 1966. [Google Scholar]
- Harris RI. The relationship of impulse control to parent hearing status, manual communication, and academic achievement in deaf children. American Annals of the Deaf. 1978;123(1):52–67. [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Horn DL, Davis RA, Pisoni David B, Miyamoto Richard T. Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear and Hearing. 2005;26(4):389–408. doi: 10.1097/00003446-200508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. The Journal of Neuroscience. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Executive function and developmental disorders: The flip side of the coin. Trends in Cognitive Sciences. 2012;16(9):454–457. doi: 10.1016/j.tics.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Kushalnagar P, Hannay HJ, Hernandez AE. Bilingualism and attention: A study of balanced and unbalanced bilingual deaf users of American Sign Language and English. Journal of Deaf Studies and Deaf Education. 2010;15(3):263–273. doi: 10.1093/deafed/enq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnerenko E, Tomalski P, Ballieux H, Potton A, Birtles D, Frostick C, Moore DG. Brain responses and looking behavior during audiovisual speech integration in infants predict auditory speech comprehension in the second year of life. Frontiers in Psychology. 2013;4(432) doi: 10.3389/fpsyg.2013.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leark RA, Dupuy TR, Greenberg LM, Corman CL, Kindschi CL. T.O.V.A. Test of Variables of Attention. Los Alamitos, CA: Universal Attention Disorders, Inc; 1999. [Google Scholar]
- Loke WH, Song S. Central and peripheral visual processing in hearing and nonhearing individuals. Bulletin of the Psychonomic Society. 1991;29(5):437–440. [Google Scholar]
- Loots G, Devisé I. The use of visual-tactile communication strategies by deaf and hearing fathers and mothers of deaf infants. Journal of Deaf Studies and Deaf Education. 2003;8(1):31–42. doi: 10.1093/deafed/8.1.31. [DOI] [PubMed] [Google Scholar]
- Marschark M. Psychological development of deaf children. New York, NY: Oxford University Press; 1993. [Google Scholar]
- Mitchell TV. How audition shapes visual attention. (Doctor of Philosophy) Indiana University; Bloomington, IN: 1996. [Google Scholar]
- Mitchell TV, Quittner AL. Multimethod study of attention and behavior problems in hearing-impaired children. Journal of Clinical Child Psychology. 1996;25(1):83–96. [Google Scholar]
- Neville HJ, Lawson D. Attention to central and peripheral visual space in a movement detection task: An event-related and behavioral study. II. Congenitally deaf adults. Brain Research. 1987a;405:268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Lawson D. Attention to central and peripheral visual space in a movement detection task. III. Seperate effects of auditory deprivation and acquisition of a visual language. Brain Research. 1987b;405:284–294. doi: 10.1016/0006-8993(87)90297-6. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Schmidt AL, Kutas M. Altered visual-evoked potentials in congenitally deaf adults. Brain Research. 1983;266:127–132. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, Fink NE. Spoken language development in children following cochlear implantation. Journal of the American Medical Association. 2010;303(15):1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DH. Reflection-impulsivity in Total Communication and oral deaf and hearing children: A developmental study. American Annals of the Deaf. 1987;132(3):213–217. doi: 10.1353/aad.2012.0820. [DOI] [PubMed] [Google Scholar]
- Parasnis I, Samar VJ, Berent GP. Deaf adults without attention deficit hyperactivity disorder display reduced perceptual sensitivity and elevated impulsivity on the Test of Variables of Attention (T.O.V.A.) Journal of Speech, Language, and Hearing Research. 2003;46(5):1165–1183. doi: 10.1044/1092-4388(2003/091). [DOI] [PubMed] [Google Scholar]
- Peterson CC, Siegal M. Insights into theory of mind from deafness and autism. In: Coltheart M, Davies M, editors. Mind and language. Vol. 15. Oxford: Blackwell; 2000. pp. 123–145. [Google Scholar]
- Petitto LA, Marentette PF. Babbling in the manual mode: Evidence for the ontogeny of language. Science. 1991;251(5000):1493–1496. doi: 10.1126/science.2006424. [DOI] [PubMed] [Google Scholar]
- Proksch J, Bavelier D. Changes in the spatial distribution of visual attention after early deafness. Journal of Cognitive Neuroscience. 2002;14(5):687–701. doi: 10.1162/08989290260138591. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Glueckauf RL, Jackson DN. Chronic parenting stress: Moderating vs. mediating effects of social support. Journal of Personality and Social Psychology. 1990;59:1266–1278. doi: 10.1037//0022-3514.59.6.1266. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Leibach P, Marciel K. The impact of cochlear implants on young deaf children: New methods to assess cognitive and behavioral development. Archives of Otolaryngology and Head and Neck Surgery. 2004;130(5):547–554. doi: 10.1001/archotol.130.5.547. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Smith LB, Osberger MJ, Mitchell TV, Katz DB. The impact of audition on the development of visual attention. Psychological Science. 1994;5(6):347–353. [Google Scholar]
- Reivich RS, Rothrock IA. Behavior problems of deaf children and adolescents: A factor-analytic study. Journal of Speech and Hearing Research. 1972;15:93–104. doi: 10.1044/jshr.1501.93. [DOI] [PubMed] [Google Scholar]
- Rönnberg J, Rudner M, Foo C, Lunner T. Cognition counts: A working memory system for ease of language understanding (ELU) International Journal of Audiology. 2008;47:S171–S177. doi: 10.1080/14992020802301167. [DOI] [PubMed] [Google Scholar]
- Sandler W, Lillo-Martin D. Sign language and linguistic universals. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- Shin MS, Kim SK, Kim SS, Park MH, Kim CS, Oh SH. Comparison of cognitive function in deaf children between before and after cochlear implant. Ear and Hearing. 2007;28(2):22S–28S. doi: 10.1097/AUD.0b013e318031541b. [DOI] [PubMed] [Google Scholar]
- Sladen DP, Tharpe AM, Ashmead DH, Wesley Grantham D, Chun Marvin M. Visual attention in deaf and normal hearing adults: effects of stimulus compatibility. Journal of Speech, Language, and Hearing Research. 2005;48(6):1529–1537. doi: 10.1044/1092-4388(2005/106). [DOI] [PubMed] [Google Scholar]
- Smith LB, Quittner AL, Osberger MJ, Miyamoto R. Audition and visual attention: The developmental trajectory in deaf and hearing populations. Developmental Psychology. 1998;34(5):84–850. doi: 10.1037//0012-1649.34.5.840. [DOI] [PubMed] [Google Scholar]
- Spaulding TJ, Plante E, Vance RB. Sustained selective attention skills of preschool children with specific language impairment: Evidence for separate attentional capacities. Journal of Speech, Language, and Hearing Research. 2008;51:16–34. doi: 10.1044/1092-4388(2008/002). [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Ashmead DH, Rothpletz AM. Visual attention in children with normal hearing, children with hearing aids, and children with cochlear implants. Journal of Speech, Language, and Hearing Research. 2002;45:403–413. doi: 10.1044/1092-4388(2002/032). [DOI] [PubMed] [Google Scholar]
- Ting JY, Bergeson TR, Miyamoto RT. Effects of simultaneous speech and sign on infants’ attention to spoken language. The Laryngoscope. 2012;122:2808–2812. doi: 10.1002/lary.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Barker BA, Spencer LJ, Zhang X, Gantz BJ. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. Journal of Speech, Language, and Hearing Research. 2005;48:853–867. doi: 10.1044/1092-4388(2005/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Baym CL, Gazzaley A, Bunge SA. Neural indices of improved attentional modulation over middle childhood. Developmental Cognitive Neuroscience. 2011;1(2):175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur RB. Success with deaf children: How to prevent educational failure. In: Lindgren K, DeLuca D, Napoli DJ, editors. Sign and voices. Washington DC: Gallaudet University Press; 2008. pp. 119–140. [Google Scholar]
- Wild CJ, Yusuf A, Wilson DE, Peelle JE, Davis MH, Johnsrude IS. Effortful listening: The processing of degraded speech depends critically on attention. The Journal of Neuroscience. 2012;32(40):14010–14021. doi: 10.1523/JNEUROSCI.1528-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Smith LB. What you learn is what you see: Using eye movements to study infant cross-situational word learning. Developmental Science. 2011;14(2):165–180. doi: 10.1111/j.1467-7687.2010.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel E, Derim D. The effect of implantation age on visual attention skills. International Journal of Pediatric Otorhinolaryngology. 2008;72:869–877. doi: 10.1016/j.ijporl.2008.02.017. [DOI] [PubMed] [Google Scholar]