Abstract
Background
This study investigated the effects of a series of four hypertonic dextrose injections on the subsynovial connective tissue (SSCT) and median nerve within the carpal tunnel of a rabbit model.
Methods
Twenty New Zealand white rabbits were used. One forepaw carpal tunnel was randomly injected with 0.1 ml of 10 % dextrose solution. The contralateral forepaw was injected with a similar amount of saline. This injection was made once per week for 4 weeks. The animals were killed at 16 weeks after the initial injection and were evaluated by electrophysiology (EP), SSCT mechanical testing, and histology.
Results
Mechanical testing revealed significantly greater ultimate load and energy absorption in the dextrose injection group compared to the saline injection group (P < 0.05). Histological evaluation revealed SSCT fibrosis and thickening and edema in the median nerve bundles in the dextrose injection group. There was a prolongation in the latency of the EP test in the dextrose injection group (P = 0.08).
Conclusions
Previous studies had shown that one or two injections of 10 % dextrose could induce moderate SSCT fibrosis and mild EP changes without nerve histology changes. In this study, we have shown that higher doses create more severe fibrosis and, most importantly, more severe neuropathy, suggesting a dose–response effect, and confirming this as a potentially useful animal model for researching the etiology and treatment of carpal tunnel syndrome.
Keywords: Subsynovial connective tissue, Rabbit, Carpal tunnel syndrome, Hypertonic dextrose
Introduction
Idiopathic carpal tunnel syndrome (CTS) is known to be associated with pathological changes in the carpal tunnel subsynovial connective tissue (SSCT). Non-inflammatory fibrosis and synovial tissue thickening increase the volume of the SSCT and are associated with elevated carpal tunnel pressure [4, 13]. It has thus been hypothesized that the cause of nerve compression might be SSCT fibrosis [7]. Several investigators have reported on the pathophysiology of acute median nerve external compression [3, 8, 9] but reports on in vivo assessment of this chronic disease are sparse.
In previous studies, single or double injections of 10–20 % dextrose were observed to induce SSCT fibrosis in a rabbit model [11, 24, 25]. Double injections had a more pronounced effect than single injections of a higher dextrose concentration. Although those studies showed changes in SSCT morphology, material properties and median nerve electrophysiology, changes in nerve histology were not observed. Considering the context of these interesting preliminary observations, we hypothesized that additional dextrose injections might create more severe SSCT fibrosis and lead to median nerve histological changes. The purpose of this study was, therefore, to investigate the effects of multiple injections of hypertonic dextrose on rabbit carpal tunnel SSCT and on median nerve morphology.
Methods
Surgical Procedure
All procedures were reviewed and approved by our Institutional Animal Care and Use Committee. All institutional and national guidelines for the care and use of laboratory animals were followed. Twenty 4.0–4.5 kg New Zealand white rabbits were used. The method has been described previously [24]. Briefly, a small incision was made proximal to the carpal tunnel. The synovium around the middle digit flexor digitorum superficialis (FDS) tendon was injected, by random allocation, with either 0.1 ml of 10 % dextrose solution (Hospira Inc., Illinois, USA) or 0.9 % saline solution. This treatment was repeated weekly for 4 weeks. At 16 weeks after the initial injection, the rabbits were anesthetized, electrophysiology (EP) was performed (n = 12 for each group), and animals were killed. Following death, 12 paws were harvested, with intact carpal tunnel, for mechanical evaluation and either nerve or SSCT histology was performed on the remaining eight [25].
Evaluation of Subsynovial Connective Tissue
Mechanical Testing
Mechanical testing was performed using techniques described previously [23]. Briefly, the middle digit FDS tendon was resected proximal to the flexor retinaculum and mounted onto a custom-made microtester consisting of a linear servo motor (MX 80 Daedal, Irwin, Pennsylvania, USA) and a load cell (MDB-5, Transducer Techniques, Temecula, California, USA). The proximal end of the middle digit FDS tendon was joined to the load cell with 5-0 Vicryl suture. The connected tendon was then resected distal to the carpal tunnel. The tendon was pulled in the proximal direction through the carpal tunnel under a constant displacement rate of 0.5 mm/s. Specimens were moistened by a saline solution spray during testing. The ultimate tensile force and excursion energy (area under the load/displacement curve) were assessed.
Histological Analysis
The tissue for SSCT analysis was formalin-fixed and paraffin-embedded using a standard protocol. Five-micron sections of the carpal tunnel were made and stained with hematoxylin and eosin (H&E) using standard staining methods. Light microscopy analysis (BLX51, Olympus Co., Tokyo, Japan) was performed to evaluate cellularity, neovascularization, fibrosis, and inflammation. Image J software (National Institute of Mental Health, Bethesda, Maryland, USA) was used to measure the shortest distance between the middle finger FDS tendon and flexor digitorum profundus (FDP) tendon and the shortest distance between index and middle and middle and ring finger FDS tendons, at the mid-carpal tunnel level [25].
Evaluation of the Median Nerve
Electrophysiological Analysis
Under general anesthesia, EP testing was performed on the median nerve of both the treated and control forepaws. Compound muscle action potential (CMAP) of the thenar muscle was recorded while stimulating the median nerve at 3.0 cm proximal to the recording point. EP testing was performed at 12 weeks and immediately prior to killing, 4 weeks later. The distal motor latency and CMAP amplitude were measured.
Histological Analysis
Tissue harvested for median nerve histology was fixed in 4 % paraformaldehyde solution for 24 h, followed by immersion in a fixative solution of 10 % glutaraldehyde and 10 % paraformaldehyde for secondary fixation. Tissue harvested for transmission electron microscopy (TEM) was post-fixed in phosphate buffered 1 % osmium tetroxide, rinsed with distilled water, stained en bloc with 2 % uranyl acetate at 60 °C, and then embedded in plastic resin. The median nerve tissue harvested from the carpal tunnel level was prepared for light microscopy analysis by slicing into the 0.6-μm cross sections and staining with toluidine blue. The sections were evaluated qualitatively using a light microscope for fascicular demyelination and subperineurial edema. For the TEM analysis, thin sections were mounted on copper grids and evaluated (JEOL USA, Peabody, Massachusetts, USA).
Statistical Analysis
The means of selected parameters were compared using the paired t test; P < 0.05 was considered significant. The results were expressed as mean ± standard deviation. All statistical analyses were performed using the statistical package Statcel QC for Excel (OMS Publishing Inc., Japan).
Results
Evaluation of the Subsynovial Connective Tissue
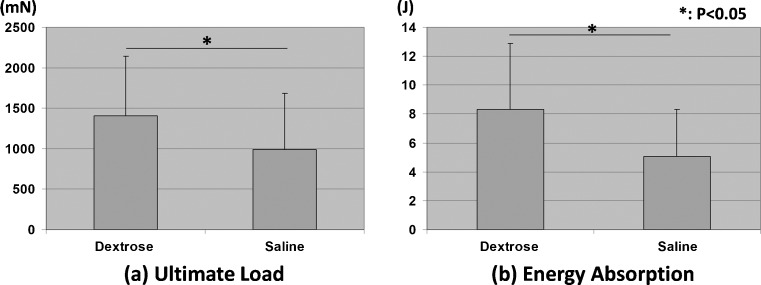
Mechanical testing showed that the ultimate load and energy absorption were significantly greater after dextrose injection than after saline injection (P < 0.05) (Fig. 1).
Fig. 1.
Results of mechanical testing. a Ultimate load. b Energy absorption. Asterisk shows significant difference between each combination (P < 0.05)
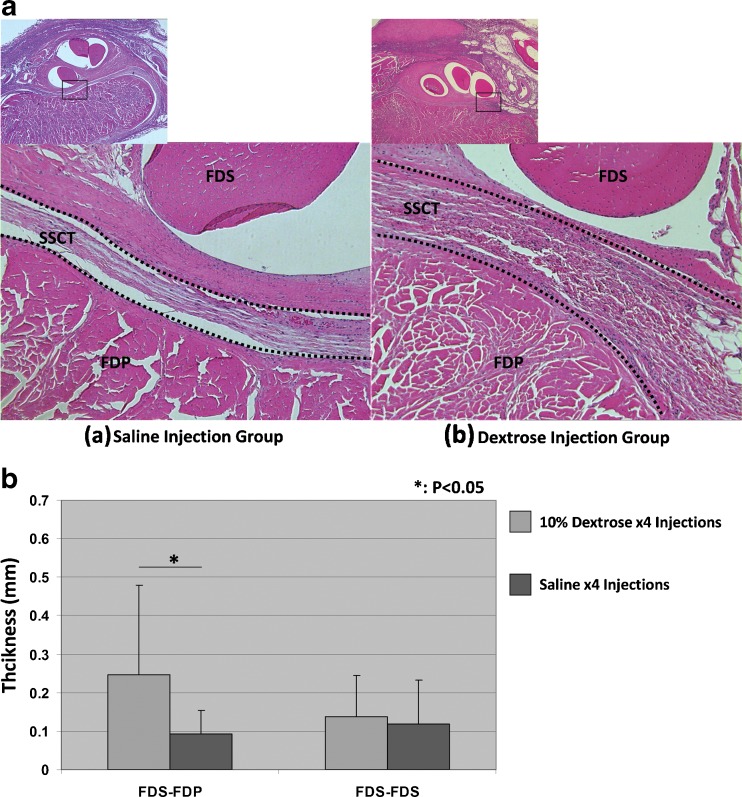
In the saline injection group, the collagen fibers of the SSCT were distributed evenly. In the dextrose injection group, the SSCT structure was irregular and thickened (Fig. 2a). The vascular proliferation or vascular hypertrophy was not obvious. The thickness of the SSCT between the FDS and FDP tendons was significantly larger (P < 0.05) in the dextrose-injected paws compared to the saline-injected paws (Fig. 2b).
Fig. 2.
Histological change of SSCT. a SSCT histology: (a) saline injection group and (b) dextrose injection group. b SSCT thickness: 1 thickness of SSCT between FDS and FDP; 2 thickness of SSCT between FDS and FDS. Asterisk shows a significant difference between the dextrose injection group and the saline injection group (P < 0.05)
Evaluation of the Median Nerve
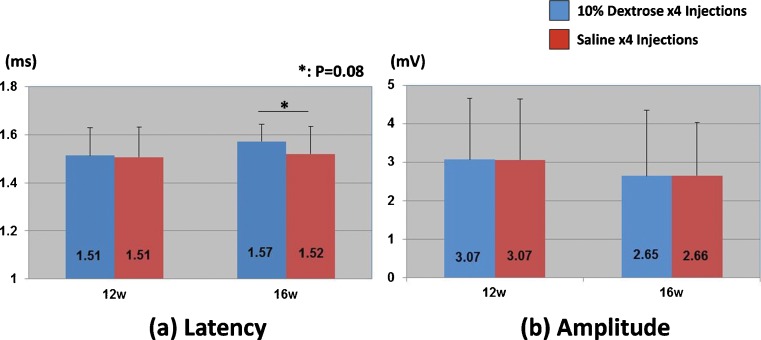
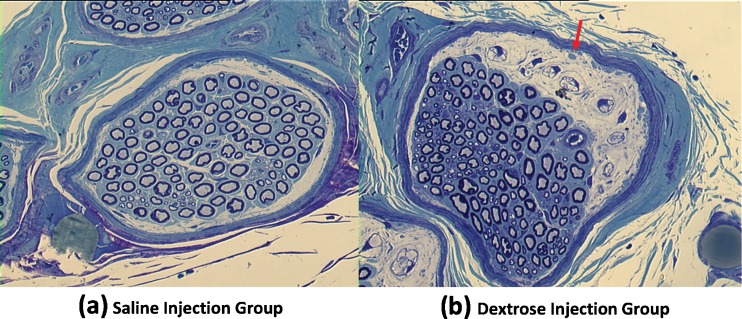
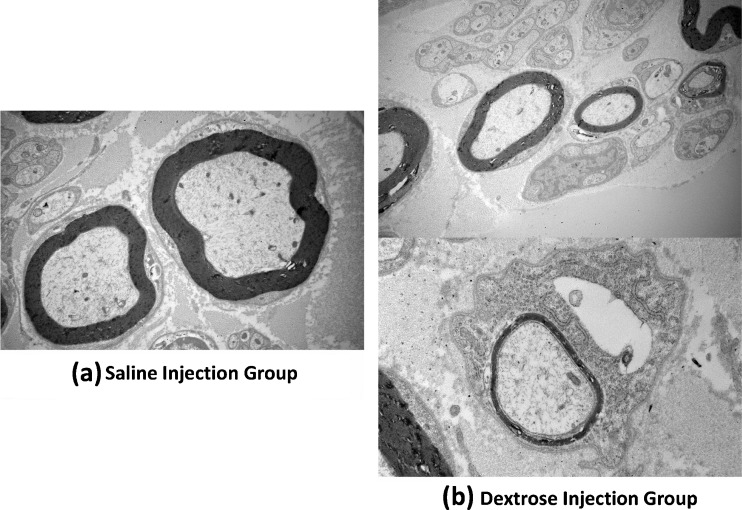
In the electrophysiological testing, there was a relatively longer distal motor latency in the dextrose injection group 16 weeks after injection, but this difference was not significant (P = 0.08) (Fig. 3). There was no obvious difference in the amplitude. Light microscopy showed edema in the median nerve bundles. The myelin sheath was thinner in the dextrose injection group compared to the saline injection group (Fig. 4). Under TEM, poorly myelinated nerve fibers were observed, with an increase in the axon/myelin ratio (Fig. 5). There was evidence of Wallerian degeneration as noted by the presence of stacked Schwann cells.
Fig. 3.
Results of electrophysiological test. a Latency. b Amplitude. Asterisk shows the difference between the dextrose injection group and the saline injection group (P = 0.08)
Fig. 4.
Nerve histology (light microscopy, toluidine blue stain). a Saline injection group. b Dextrose injection group
Fig. 5.
Nerve histology (TEM). a Saline injection group. b Dextrose injection group
Discussion
In this study, we assessed the effect of a series of four hypertonic dextrose injections on the rabbit carpal tunnel SSCT. Multiple injections of dextrose intensified the mechanical property changes and SSCT fibrosis compared with previous studies, in which only one or two injections were given [24, 25]. We did not observe any edema or thickening of the tendons themselves.
The previous studies had also shown that injections without dextrose do not induce any change in either the nerve or SSCT, establishing that it is the dextrose, not the trauma of an injection, is responsible for the changes seen [24, 25]. In addition, the series of four injections created histologic changes in the median nerve, showing that a larger dose of dextrose induces a more severe neuropathy.
Hypertonic dextrose has been suggested as a stimulant to initiate proliferation of new cells or stimulate repair in existing cells. Many in vitro studies have shown that human cells produce various growth factors after exposure to hypertonic dextrose [12]. These growth factors include platelet-derived growth factor [2], transforming growth factor beta [10], epidermal growth factor [5], basic fibroblast growth factor [14], insulin-like growth factor [17], and connective tissue growth factor [10]. In addition, these growth factors have been previously identified as key for stimulating repair and growth of tendon, ligament, and cartilage tissues [22]. Recent studies have confirmed the clinical benefit of 10 % dextrose injections in the treatment of lateral epicondylitis [18], low back pain, and knee osteoarthritis [1, 19, 20]. In addition to the effect of dextrose on growth factors observed, injection of dextrose in concentrations greater than 10 % can briefly produce an inflammatory response. Because progressive fibrosis and overexpression of transforming growth factor and connective tissue growth factor have been observed in patients with CTS [4, 6, 15, 16], we thought that hypertonic dextrose might be a useful treatment to induce a similar phenomenon in an experimental animal [11, 24, 25].
As noted above, previous studies have shown that two injections of 10 % dextrose had a more pronounced impact on SSCT fibrosis than single injections [25]. The mechanical testing showed that both the ultimate load and energy absorption of the dextrose injection group were significantly greater than the saline injection group, whether for one or two injections (including previous results with one or two injections) [25]. In comparing our data for four injections, again, there was no significant difference when comparing one or two saline injections with four saline injections, suggesting again that it is not the number of injections, but rather the dextrose which is effecting the changes that we observed in the dextrose-injected animals.
While the single or double injections of 10 and 20 % dextrose were demonstrated to affect the SSCT, they had a lesser effect on nerve function in the previous studies. In contrast, in the four dextrose injection group of this study, edema was observed in the median nerve bundles with a thinner myelin sheath. These histologic changes are consistent with a more severe neuropathy in the four-injection group, suggesting a dose effect, with lower doses causing SSCT fibrosis and higher doses resulting in more neuropathy. This is consistent with a hypothesis for carpal tunnel syndrome etiology, in which progressive SSCT fibrosis results in eventual neuropathy [4, 13].
We believe that the animal model described in this work may be applied for several potential applications. As has already been noted in previous studies [24, 25], it can serve as a useful model of CTS. The effect of stimulating dose can be studied here, as well as the effect of drugs or other agents that might block the progression of fibrosis in the face of a known stimulatory dose.
There are several limitations in this study. First, we do not know if the nerve changes we observed were caused by a dextrose-induced chronic compression or by the dextrose itself. In addition, short-term effects were not investigated. However, since other data have shown that dextrose has a longer-term effect at 16 weeks, studying the short-term effects on nerve histology, as well as the mechanism of action, including cytokine expression, would be appropriate in this model. Second, the local concentrations of dextrose after the injections were not measured. An in vitro study of hyperglycemic effect revealed that pericellular glucose concentrations above 35 mM induced hyperglycemic stress and cell injury [21]. While the dose used in this study is larger, we cannot estimate the local pericellular concentration and results may have some variability due to the differences in local concentration of dextrose in vivo. Third, since only a single time point was included in this study, we have no data on the time course of the observed changes. EP showed slight changes from 12 to 16 weeks after injection. With the long-term effect confirmed, it will be important to consider shorter time points.
Conclusion
In conclusion, we observed that a series of four injections of hypertonic dextrose intensified fibrosis and material property changes of the rabbit carpal tunnel SSCT and induced significant changes in nerve histology. This may be a helpful model for studying the effects of synovial fibrosis on carpal tunnel syndrome.
Acknowledgments
This study was funded by a grant from NIH (NIAMS AR49823).
Conflict of Interest
Yuichi Yoshii, Chunfeng Zhao, James D. Schmelzer, Phillip A. Low, Kai-Nan An, and Peter C. Amadio declare they have no conflict of interest.
Statement of Animal and Human Rights
All procedures were reviewed and approved by our Institutional Animal Care and Use Committee. All institutional and national guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent
Informed consent was not needed.
References
- 1.Dagenais S, Haldeman S, Wooley JR. Intraligamentous injection of sclerosing solutions (prolotherapy) for spinal pain: a critical review of the literature. Spine J. 2005;5:310–28. doi: 10.1016/j.spinee.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Di Paolo S, Gesualdo L, Ranieri E, et al. High glucose concentration induces the overexpression of transforming growth factor-beta through the activation of a platelet-derived growth factor loop in human mesangial cells. Am J Pathol. 1996;149:2095–106. [PMC free article] [PubMed] [Google Scholar]
- 3.Diao E, Shao F, Liebenberg E, et al. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res. 2005;23:218–23. doi: 10.1016/j.orthres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86:1458–66. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda K, Kawata S, Inui Y, et al. High concentration of glucose increases mitogenic responsiveness to heparin-binding epidermal growth factor-like growth factor in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:1962–8. doi: 10.1161/01.ATV.17.10.1962. [DOI] [PubMed] [Google Scholar]
- 6.Kim JK, Koh YD, Kim JS, et al. Oxidative stress in subsynovial connective tissue of idiopathic carpal tunnel syndrome. J Orthop Res. 2010;28:1463–8. doi: 10.1002/jor.21163. [DOI] [PubMed] [Google Scholar]
- 7.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg (Br) 1992;17:209–12. doi: 10.1016/0266-7681(92)90091-F. [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon SE, Dellon AL, Hudson AR, et al. Chronic nerve compression–an experimental model in the rat. Ann Plast Surg. 1984;13:112–20. doi: 10.1097/00000637-198408000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Mackinnon SE, Dellon AL, Hudson AR, et al. A primate model for chronic nerve compression. J Reconstr Microsurg. 1985;1:185–95. doi: 10.1055/s-2007-1007073. [DOI] [PubMed] [Google Scholar]
- 10.Murphy M, Godson C, Cannon S, et al. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem. 1999;274:5830–4. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- 11.Oh S, Ettema AM, Zhao C, et al. Dextrose-induced subsynovial connective tissue fibrosis in the rabbit carpal tunnel: a potential model to study carpal tunnel syndrome? Hand (N Y). 2008;3. [DOI] [PMC free article] [PubMed]
- 12.Oh JH, Ha H, Yu MR, et al. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998;54:1872–8. doi: 10.1046/j.1523-1755.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 13.Oh J, Zhao C, Zobitz ME, et al. Morphological changes of collagen fibrils in the subsynovial connective tissue in carpal tunnel syndrome. J Bone Joint Surg Am. 2006;88:824–31. doi: 10.2106/JBJS.E.00377. [DOI] [PubMed] [Google Scholar]
- 14.Ohgi S, Johnson PW. Glucose modulates growth of gingival fibroblasts and periodontal ligament cells: correlation with expression of basic fibroblast growth factor. J Periodontal Res. 1996;31:579–88. doi: 10.1111/j.1600-0765.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 15.Pierce CW, Tucci MA, Benghuzzi HA. The time course of transcriptional regulation in anoxic fibroblasts: implications for carpal tunnel syndrome–biomed 2010. Biomed Sci Instrum. 2010;46:299–304. [PubMed] [Google Scholar]
- 16.Pierce CW, Tucci MA, Lindley S, et al. Connective tissue growth factor (ctgf) expression in the tenosynovium of patients with carpal tunnel syndrome–biomed 2009. Biomed Sci Instrum. 2009;45:30–5. [PubMed] [Google Scholar]
- 17.Pugliese G, Pricci F, Locuratolo N, et al. Increased activity of the insulin-like growth factor system in mesangial cells cultured in high glucose conditions. Relation to glucose-enhanced extracellular matrix production. Diabetologia. 1996;39:775–84. doi: 10.1007/s001250050510. [DOI] [PubMed] [Google Scholar]
- 18.Rabago D, Lee KS, Ryan M, et al. Hypertonic dextrose and morrhuate sodium injections (prolotherapy) for lateral epicondylosis (tennis elbow): results of a single-blind, pilot-level, randomized controlled trial. Am J Phys Med Rehabil. 2013;92:587–96. doi: 10.1097/PHM.0b013e31827d695f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74. [PubMed] [Google Scholar]
- 20.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med. 2000;6:311–20. doi: 10.1089/10755530050120673. [DOI] [PubMed] [Google Scholar]
- 21.Russell JW, Golovoy D, Vincent AM, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–48. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 22.Woo SL, Hildebrand K, Watanabe N, et al. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999;367(Suppl):S312–23. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Osamura N, Zhao C, et al. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2008;41:3519–22. doi: 10.1016/j.jbiomech.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshii Y, Zhao C, Schmelzer JD, et al. The effects of hypertonic dextrose injection on connective tissue and nerve conduction through the rabbit carpal tunnel. Arch Phys Med Rehabil. 2009;90:333–9. doi: 10.1016/j.apmr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshii Y, Zhao C, Schmelzer JD, et al. Effects of hypertonic dextrose injections in the rabbit carpal tunnel. J Orthop Res. 2011;29:1022–7. doi: 10.1002/jor.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]