Abstract
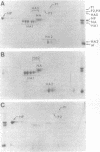
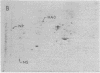
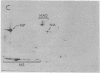
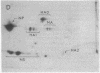
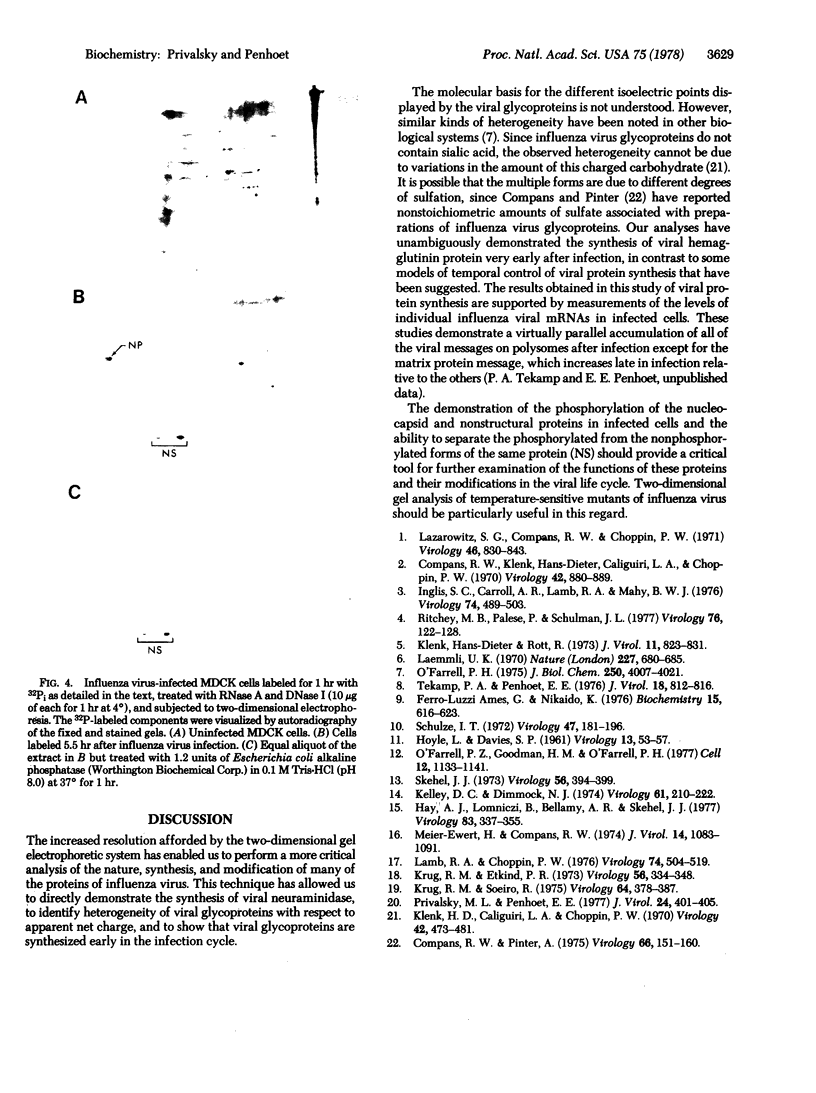
A modification of the two-dimensional protein electrophoresis system of O'Farrell was used to resolve influenza A virus proteins from each other and from host proteins in infected cells. Viral protein spots corresponding to the hemagglutinin proteins, neuraminidase, nucleocapsid protein, and nonstructural protein, were identified on the two-dimensional electrophoretogram. Use of the two-dimensional separation has allowed us to identify glycoprotein heterogeneity, to demonstrate directly the synthesis of neuraminidase, to analyze some viral proteins early after infection, and to demonstrate that the influenza virus NP and NS proteins are phosphorylated in infected MDCK cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Pinter A. Incorporation of sulfate into influenza virus glycoproteins. Virology. 1975 Jul;66(1):151–160. doi: 10.1016/0042-6822(75)90186-5. [DOI] [PubMed] [Google Scholar]
- HOYLE L., DAVIES S. P. Amino acid composition of the protein components of influenza virus A. Virology. 1961 Jan;13:53–57. doi: 10.1016/0042-6822(61)90031-9. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Dimmock N. J. Fowl plaque virus replication in mammalian cell-avian erythrocyte heterokaryons: studies concerning the actinomycin D and ultra-violet light sensitive phase in influenza virus replication. Virology. 1974 Sep;61(1):210–222. doi: 10.1016/0042-6822(74)90255-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Caliguiri L. A., Choppin P. W. The proteins of the parainfluenza virus SV5. II. The carbohydrate content and glycoproteins of the virion. Virology. 1970 Oct;42(2):473–481. doi: 10.1016/0042-6822(70)90290-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. Formation of influenza virus proteins. J Virol. 1973 Jun;11(6):823–831. doi: 10.1128/jvi.11.6.823-831.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Soeiro R. Studies on the intranuclear localization of influenza virus-specific proteins. Virology. 1975 Apr;64(2):378–387. doi: 10.1016/0042-6822(75)90114-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert H., Compans R. W. Time course of synthesis and assembly of influenza virus proteins. J Virol. 1974 Nov;14(5):1083–1091. doi: 10.1128/jvi.14.5.1083-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Penhoet E. E. Phosphorylated protein component present in influenza virions. J Virol. 1977 Oct;24(1):401–405. doi: 10.1128/jvi.24.1.401-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Difference in protein patterns of influenza A viruses. Virology. 1977 Jan;76(1):122–128. doi: 10.1016/0042-6822(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972 Jan;47(1):181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. Early polypeptide synthesis in influenza virus-infected cells. Virology. 1973 Nov;56(1):394–399. doi: 10.1016/0042-6822(73)90320-6. [DOI] [PubMed] [Google Scholar]
- Tekamp P., Penhoet E. E. Message activity of influenza viral RNA. J Virol. 1976 May;18(2):812–816. doi: 10.1128/jvi.18.2.812-816.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]