Abstract
Purpose
The World Health Organization (WHO) concluded that poor adherence to treatment is the most important cause of uncontrolled high blood pressure, with approximately 75% of patients not achieving optimum blood pressure control. The WHO estimates that between 20% and 80% of patients receiving treatment for hypertension are adherent. As such, the first objective of our study was to quantify the proportion of nonadherence to antihypertensive therapy in real-world observational study settings. The second objective was to provide estimates of independent risk indicators associated with nonadherence to antihypertensive therapy.
Materials and methods
We performed a systematic literature review and meta-analysis of all studies published between database inception and December 31, 2011 that reviewed adherence, and risk indicators associated with nonadherence, to antihypertensive medications.
Results
In the end, 26 studies met our inclusion and exclusion criteria and passed our methodological quality evaluation. Of the 26 studies, 48.5% (95% confidence interval 47.7%–49.2%) of patients were adherent to antihypertensive medications at 1 year of follow-up. The associations between 114 variables and nonadherence to antihypertensive medications were reviewed. After meta-analysis, nine variables were associated with nonadherence to antihypertensive medications: diuretics in comparison to angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs), ACE inhibitors in comparison to ARBs, CCBs in comparison to ARBs, those with depression or using antidepressants, not having diabetes, lower income status, and minority cultural status.
Conclusion
This study clarifies the extent of adherence along with determining nine independent risk indicators associated with nonadherence to antihypertensive medications.
Keywords: meta-analysis, antihypertensives, adherence
Introduction
In a comprehensive report on nonadherence to long term therapies, the World Health Organization (WHO) stated that treatment of hypertension can reduce the risk of stroke by 30%–43% and myocardial infarction by 15%, along with reducing the risk of a number of other chronic conditions.1 Similarly, in a meta-analysis of 147 randomized trials, the authors found that patients who received treatment with antihypertensive medications observed a 22% reduction in coronary heart disease events and a 41% reduction in stroke.2 However, the WHO also concluded that poor adherence to treatment is the most important cause of uncontrolled high blood pressure, with approximately 75% of patients not achieving optimum blood pressure control.1 The implications are vast. A meta-analysis on interventions for enhancing medication adherence completed by the Cochrane Collaboration concluded that effective ways to help patients follow medical treatments could have far larger effects on health outcomes than any individual treatment by itself.3 For example, one review estimated that better adherence to antihypertensive therapy could prevent 89,000 premature deaths in the US alone on an annual basis.4
In a meta-analysis on proportion and risk indicators for statin therapy, the authors found that only 49% of patients are adherent in real-world observational studies. After reviewing 147 variables, only six covariates were found to be independently associated with nonadherence to statin therapy: primary prevention, new statin users, copayment, lower income status, fewer than two lipid tests performed, and not having a comorbidity of hypertension.5
To date, a systematic literature review and meta-analysis has not been performed on the extent of nonadherence to antihypertensive therapy. As such, the WHO had to estimate that between 20%–80% of patients receiving treatment for hypertension are adherent.1 In other words, the true extent of nonadherence is not known and is a justification for this review.
The WHO also concluded that there is an urgent need for research to fill gaps in knowledge on the determinants of adherence.1 Two systematic reviews have been published on variables associated with nonadherence to antihypertensive therapy. The first reviewed the impact of depression, but included self-report adherence information.6 The second reviewed differences in adherence between angiotensin receptor blockers (ARBs) and other antihypertensive drug classes. However, the authors used filters to limit the search strategy and excluded studies that used a single point in time (ie, 1 year) to define adherence.7 As well, both studies modified established quality-assessment rating checklists for their quality review.6,7
The first objective of our study was to quantify the proportion of adherence to antihypertensive therapy in real-world observational study settings. The second objective was to provide estimates of independent risk indicators associated with nonadherence to antihypertensive therapy.
Materials and methods
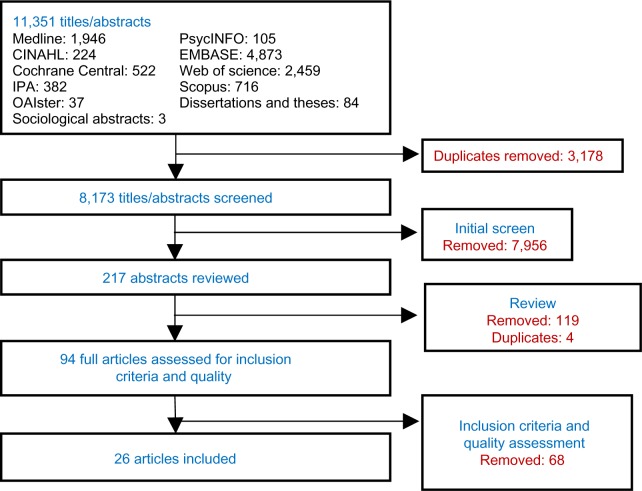
We performed a systematic literature review of electronic databases: Medline (Ovid), Embase (Ovid), International Pharmaceutical Abstracts (Ovid), the Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, Sociological Abstracts (ProQuest), ProQuest Dissertations and Theses, Theses Canada, and OAIster from inception to December 31, 2011. Multiple combinations of search terms and keywords were used to maximize the ability to capture relevant articles. Papers that were not published electronically were not included in our search. Reference sections of each article were reviewed for additional papers (Figure 1).
Figure 1.
Systematic literature-review process.
Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health; IPA, International Pharmaceutical Abstracts; OAIster, Open Archives Initiative.
Studies were included if they satisfied the following criteria: 1) nonadherence to antihypertensive medications as an outcome, 2) antihypertensive medications included angiotensin-converting enzyme inhibitors (ACEIs), ARBs, beta blockers, calcium channel blockers (CCBs), or thiazide diuretics for any indication, 3) dispensation records as the source of objective nonadherence information, 4) conducted multivariable modeling to determine the independent effect of covariates on the outcome of nonadherence, and 5) published in the English language.
Abstracts and full-text articles were assessed by two reviewers for relevance (MWA and ML). Study quality was reviewed with a checklist developed by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) for database studies. The checklist was developed for the specific purpose of critically evaluating the unique complexity of administrative datasets analyzing medication adherence.8,9 This checklist has been used in other systematic reviews.10 It consists of 27 quality-review questions related to data source, research design, study population, variable definitions, statistics, and discussion. We established a rule a priori that studies scoring 14/27 or higher (greater than 50%) on the quality checklist would be included in the meta-analysis, unless there was a major methodological violation.
Disagreement between the two reviewers was resolved by additional review and discussion, and then, if required, with the senior author (ML) making a final decision. We assessed agreement between reviewers with κ-statistics.11
We conducted a random-effects model meta-analysis using the inverse-variance method to estimate the effect of a risk indicator on medication adherence. A random-effects model accounts for potential heterogeneity between the populations and unmeasured confounding.12–15 Given that rates of adherence were being compared, rate ratios (RRs) were computed instead of odds ratios. Four or more articles were required for meta-analysis.
Sensitivity analysis reviewed the individual influence of a study by repeating the meta-analysis without studies with the largest and smallest relative weights. If this produced little change in inference (less than 15% change in rate ratio), inclusion of the study would not warrant caution in the interpretation. Sensitivity analysis also included country of origin and measures used to calculate adherence. We used the Z-test for overall effects and the χ2 statistic to test for differences in between-group effects. We included every risk indicator tested in other studies, but we obviously have no information on indicators that were not tested.
Results
A total of 11,351 titles and abstracts were identified. After review, and after removing duplicates, 217 abstracts were considered relevant to our study. From these, 94 articles were relevant, reviewed in full and evaluated for quality with the ISPOR checklist.8,9 Of these 94 articles, 26 passed the methodological quality review16–41 (Figure 1). The κ-statistic for agreement between the reviewers for the final articles was 0.73. Even among approved studies, the methodological quality was low, with an average score of 16.8 out of a maximum of 27.
Of the 26 studies included for analysis, the most common follow-up duration was 12 months, for which 17 studies and 20 cohorts had data. Among these, with a total sample size of 1,522,203, 48.5% (95% confidence interval [CI] 47.7%–49.2%) of patients were adherent to antihypertensive medications at 1 year of follow-up, with a range from 20% to 88%. One study had a sample size of 725,220, which contributed 47.6% of the total sample. Removal of this one large study resulted in an adherence level of 56.9% (95% CI 56.7%–57.1%). This paper was also the only study conducted outside of North America and Europe with follow-up duration of 12 months. The two most common adherence measures were proportion of days covered and discontinuation, with adherence levels of 62.2% (95% CI 61.9%–62.5%) for proportion of days covered and 55.1% (95% CI 55.0%–55.2%) for discontinuation.
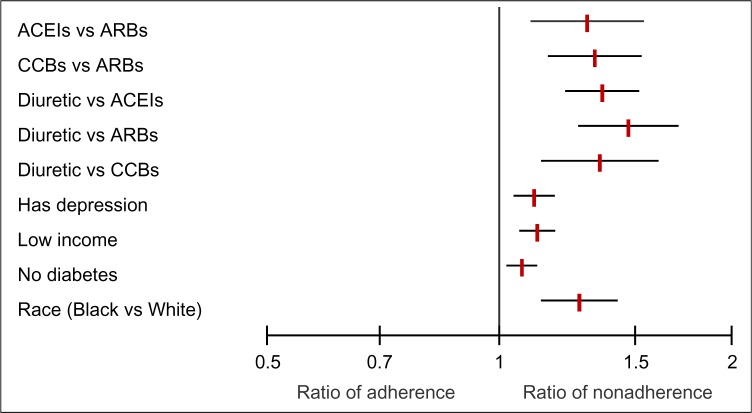
The second objective was to provide estimates of risk indicators associated with nonadherence to antihypertensive therapy. In total, the associations between 114 variables and nonadherence to antihypertensive medications were reviewed. In the end, nine variables were associated with nonadherence to antihypertensive medications, with five representing drug class and four from variables other than drug class (Table 1 and Figure 2).
Table 1.
Results of individual studies
| Rate ratio | 95% CI | Relative weight | Homogeneity | |
|---|---|---|---|---|
| ACEIs versus ARBs | ||||
| Bloom19 | 1.19 | 1.03–1.32 | 0.26 | |
| Elliott et al23 | 1.14 | 1.09–1.20 | 0.28 | |
| Wogen et al33 | 1.45 | 1.43–1.47 | 0.29 | |
| Degli Esposti et al36 | 1.58 | 1.12–2.08 | 0.16 | |
| Pooled | 1.30 | 1.10–1.54 | 1.00 | 0.000 |
| CCBs versus ARBs | ||||
| Sung et al38 | 1.10 | 1.09–1.11 | 0.29 | |
| Elliott et al23 | 1.32 | 1.26–1.39 | 0.28 | |
| Wogen et al33 | 1.33 | 1.31–1.35 | 0.29 | |
| Degli Esposti et al36 | 2.07 | 1.57–2.73 | 0.14 | |
| Pooled | 1.33 | 1.16–1.54 | 1.00 | 0.000 |
| Diuretics versus ACEIs | ||||
| Elliott et al23 | 1.53 | 1.47–1.61 | 0.14 | |
| Taira et al25 | 1.49 | 1.40–1.60 | 0.14 | |
| Van Wijk et al26 | 1.52 | 1.46–1.57 | 0.14 | |
| Van Wijk et al26 | 1.32 | 1.28–1.36 | 0.15 | |
| Van Wijk et al26 | 1.38 | 1.33–1.43 | 0.14 | |
| Friedman et al30 | 1.17 | 1.10–1.25 | 0.14 | |
| Sung et al38 | 1.16 | 1.14–1.17 | 0.15 | |
| Pooled | 1.36 | 1.22–1.51 | 1.00 | 0.000 |
| Diuretics versus ARBs | ||||
| Degli Esposti et al36 | 2.62 | 1.99–3.46 | 0.10 | |
| Taira et al25 | 1.61 | 1.50–1.70 | 0.15 | |
| Van Wijk et al26 | 1.50 | 1.42–1.57 | 0.15 | |
| Van Wijk et al26 | 1.45 | 1.31–1.56 | 0.15 | |
| Van Wijk et al26 | 1.52 | 1.47–1.56 | 0.15 | |
| Friedman et al30 | 1.07 | 0.93–1.22 | 0.14 | |
| Sung et al38 | 1.16 | 1.14–1.17 | 0.15 | |
| Pooled | 1.47 | 1.27–1.70 | 1.00 | 0.000 |
| Diuretics versus CCBs | ||||
| Friedman et al30 | 1.08 | 0.99–1.19 | 0.19 | |
| Taira et al25 | 2.01 | 1.90–2.20 | 0.20 | |
| Van Wijk et al26 | 1.46 | 1.35–1.53 | 0.20 | |
| Van Wijk et al26 | 1.15 | 1.06–1.22 | 0.20 | |
| Van Wijk et al26 | 1.23 | 1.16–1.29 | 0.20 | |
| Pooled | 1.35 | 1.14–1.60 | 1.00 | 0.000 |
| Depression versus no depression | ||||
| Rasmussen et al16 | 2.13 | 1.44–3.14 | 0.02 | |
| Rasmussen et al16 | 1.81 | 1.07–3.07 | 0.01 | |
| Perreault et al24 | 0.93 | 0.85–1.02 | 0.14 | |
| Van Wijk et al26 | 1.10 | 0.99–1.14 | 0.19 | |
| Van Wijk et al26 | 1.14 | 1.05–1.25 | 0.14 | |
| Van Wijk et al26 | 1.03 | 0.94–1.12 | 0.15 | |
| Siegel et al29 | 1.16 | 1.14–1.18 | 0.20 | |
| Evans et al39 | 1.17 | 1.09–1.26 | 0.16 | |
| Pooled | 1.11 | 1.05–1.18 | 1.00 | 0.000 |
| Low income versus higher | ||||
| Rasmussen et al16 | 1.05 | 0.91–1.11 | 0.09 | |
| Rasmussen et al16 | 1.07 | 0.94–1.23 | 0.06 | |
| Perreault et al24 | 1.34 | 1.26–1.42 | 0.09 | |
| Van Wijk et al26 | 0.94 | 0.86–1.02 | 0.08 | |
| Van Wijk et al26 | 1.19 | 1.15–1.23 | 0.09 | |
| Van Wijk et al26 | 1.05 | 1.01–1.10 | 0.09 | |
| Gogovor et al28 | 1.02 | 0.83–1.26 | 0.04 | |
| Gogovor et al28 | 1.26 | 1.11–1.42 | 0.06 | |
| Friedman et al30 | 1.10 | 1.08–1.12 | 0.10 | |
| Corrao et al31 | 1.02 | 0.98–1.05 | 0.09 | |
| Wong et al37 | 1.22 | 1.15–1.29 | 0.09 | |
| Evans et al39 | 1.60 | 1.31–1.96 | 0.04 | |
| Rasmussen et al40 | 0.98 | 0.90–1.14 | 0.09 | |
| Pooled | 1.12 | 1.06–1.18 | 1.00 | 0.000 |
| Diabetics versus nondiabetics | ||||
| Rasmussen et al16 | 0.98 | 0.68–1.22 | 0.03 | |
| Rasmussen et al16 | 1.16 | 0.85–1.38 | 0.02 | |
| Setoguchi et al17 | 1.03 | 1.00–1.06 | 0.12 | |
| Setoguchi et al17 | 1.03 | 0.99–1.06 | 0.11 | |
| Degli Esposti et al20 | 0.50 | 0.17–0.76 | 0.04 | |
| Perreault et al24 | 1.14 | 1.06–1.21 | 0.09 | |
| Taira et al25 | 1.13 | 1.10–1.20 | 0.12 | |
| Van Wijk et al26 | 1.01 | 0.92–1.09 | 0.09 | |
| Van Wijk et al26 | 1.03 | 1.00–1.14 | 0.09 | |
| Van Wijk et al26 | 1.17 | 1.10–1.24 | 0.09 | |
| Gogovor et al28 | 1.11 | 0.92–1.27 | 0.04 | |
| Gogovor et al28 | 1.19 | 1.07–1.29 | 0.06 | |
| Siegel et al29 | 1.02 | 0.99–1.04 | 0.12 | |
| Pooled | 1.07 | 1.02–1.11 | 1.00 | 0.000 |
| Black versus White | ||||
| Setoguchi et al17 | 1.23 | 1.19–1.27 | 0.15 | |
| Setoguchi et al17 | 1.09 | 1.06–1.13 | 0.15 | |
| Yang et al21 | 1.38 | 1.36–1.39 | 0.15 | |
| Shaya et al22 | 1.55 | 1.30–1.71 | 0.13 | |
| Van Wijk et al26 | 1.42 | 1.19–1.66 | 0.11 | |
| Siegel et al29 | 1.08 | 1.07–1.09 | 0.15 | |
| Yang et al21 | 1.28 | 1.27–1.30 | 0.15 | |
| Pooled | 1.27 | 1.14–1.42 | 1.00 | 0.000 |
Abbreviations: CI, confidence interval; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers.
Figure 2.
Covariates independently associated with nonadherence to antihypertensives.
Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers.
For five studies and seven cohorts with a total sample size of 1,082,417, patients who were dispensed diuretics, in comparison to ACEIs, were 36% more likely to be nonadherent (RR 1.36, 95% CI 1.22–1.51). Among five studies and seven cohorts with a total sample size of 1,028,044, patients who were dispensed diuretics, in comparison to ARBs, were 47% more likely to be nonadherent (RR 1.47, 95% CI 1.27–1.70). For three studies and five cohorts with a total sample size of 295,512, patients who were dispensed diuretics, in comparison to CCBs, were 35% more likely to be nonadherent (RR 1.35, 95% CI 1.14–1.60). Pooling of four studies with a total sample size of 232,665 revealed that patients who were dispensed ACEIs, in comparison to ARBs, were 30% more likely to be nonadherent (RR 1.30, 95% CI 1.10–1.54). Analysis of four studies with a total sample size of 936,162 showed that patients who were dispensed CCBs, in comparison to ARBs, were 33% more likely to be nonadherent (RR 1.33, 95% CI 1.16–1.54).
There were five studies and eight cohorts that reviewed the association between depression or the use of antidepressants and nonadherence. With a total sample size of 206,673, patients who were either depressed or dispensed antidepressants were 11% more likely to be nonadherent to antihypertensive medications (RR 1.11, 95% CI 1.05–1.18).
Comparing lowest-income to highest-income groups among nine studies and 13 cohorts with a sample size of 623,444, we found that lower-income groups were 12% more likely to be nonadherent (RR 1.12, 95% CI 1.06–1.18). Some studies reviewed race. Analysis of five studies and seven cohorts with a sample size of 1,771,428 revealed that residents who were of minority cultural status (African American race in American studies) in comparison to Caucasian (White) were 27% more likely to be nonadherent (RR 1.27, 95% CI 1.14–1.42).
There were eight studies and 13 cohorts that reviewed the association between having diabetes and nonadherence. With a total sample size of 252,306, patients without diabetes were 7% more likely to be nonadherent to antihypertensive medications (RR 1.07, 95% CI 1.02–1.11).
There was no known association between the other 105 variables and nonadherence to antihypertensive medications. For example, patients with ischemic heart disease were only 3% more likely to be nonadherent to antihypertensive medications (RR 1.03, 95% CI 0.90–1.18). As another example, patients with heart failure were 5% more likely to be nonadherent to antihypertensive medications (RR 1.05, 95% CI 0.98–1.12).42
Tests of homogeneity of variance for the nine variables associated with nonadherence were all P<0.001. This suggests highly significant heterogeneity between the studies. Stratification by country of origin and measure of adherence did not alter estimates to a significant degree. As well, sensitivity analysis of removing studies with the largest and smallest relative weights did not alter estimates to a significant degree. For example, removing non-North American and -European studies and removing the study with the largest sample size modified the odds ratio between diuretics and ACEIs from 1.36 (95% CI 1.22–1.51) to 1.40 (95% CI 1.24–1.56).
Only three variables had negative estimates or results contrary to the other studies in the meta-analysis. Among eight cohorts that reviewed the association between depression or the use of antidepressants and nonadherence, one estimate was inconsistent with the other seven. For the thirteen cohorts that reviewed income, two estimates were inconsistent with the other eleven estimates. Among thirteen cohorts that reviewed the association between diabetes and nonadherence, two estimates were inconsistent with the other eleven.
All of the included papers are summarized in detail in Table S1.
Discussion
Of the 26 studies that were included in our study and passed methodological quality review, 48.5% (95% CI 47.7%–49.2%) of patients were adherent to antihypertensive medications at 1 year of follow-up. This clarifies the original WHO estimate that between 20% and 80% of patients receiving treatment for hypertension are adherent.1 This is simply a range from the lowest to the highest rates found during their review. Policy makers and health practitioners could assume that nonadherence is as low as 20% and therefore not a major problem. We quantified the value through meta-analysis in order to clarify the need for action.
Our study also provides estimates of known risk indicators associated with nonadherence to antihypertensive therapy. In total, the associations between 114 variables and nonadherence to antihypertensive medications were reviewed. In the end, nine variables were associated with nonadherence to antihypertensive medications: diuretics in comparison to ACEIs and ARBs and CCBs, ACEIs in comparison to ARBs, CCBs in comparison to ARBs, those with depression or using antidepressants, not having diabetes, lower income status, and minority cultural status (African American race).
Although drug class has been shown to impact adherence, it is not clear if this is due to the properties of the drug class itself. One review suggests that differences may be due to patient selection or that out-of-pocket cost predicts drug choice.7 Also, the reality is that many patients are now started on more than one antihypertensive medication and combinations of drug classes.7 A recent paper found that differences in adherence by drug class were associated with their market availability, and that combination therapy of ARBs with diuretics had the highest proportion of adherence.43 Others would suggest that there are clinical differences like increased urination associated with diuretics.44 Regardless, our results were consistent with a meta-analysis that reviewed adherence of ARBs in comparison to other antihypertensive medications, although our study reviewed all drug classes.7
Depression is known to be associated with a threefold-greater risk of nonadherence to medical treatment in general.45 A previous systematic literature review found that definitive conclusions could not be drawn between depression and nonadherence to antihypertensive therapy, possibly because five of the eight accepted studies included nonobjective and self-report measures to determine nonadherence.6 Our review addressed this limitation.
Not having diabetes was also associated with nonadherence, although the effect was small. Patients who have a low perceived risk of disease have been found less likely to adhere to their medications.5 Lower-income status might also increase nonadherence due to affordability, although low-income status has also been associated with nonadherence to other health-seeking behaviors.46–49 The association between cultural status and adherence is likely to be due to confounding with income.50 In the US, African American cultural status is well known to be more likely associated with lower-income status at the population level.51
The main limitation of our meta-analysis was the inclusion of studies with wide variability and heterogeneity in measuring and defining adherence. Second, although our meta-analysis identified nine variables associated with nonadherence to antihypertensive medications, it remains unclear as to what extent adherence would be improved if these variables were addressed. Third, our interpretation is limited to variables reviewed. It is likely that other untested associations exist.
Conclusion
The known effectiveness of interventions for enhancing chronic disease medication adherence remains surprisingly weak.3 It is possible that this lack of effectiveness is due to not really knowing the risk indicators of nonadherence. This study provides some insight into factors associated, and not associated, with nonadherence to antihypertensive medications.
In summary, it is likely that effective ways to help patients follow medical treatments could have far larger effects on health outcomes than any individual treatment. The first step is to know the extent of nonadherence in patients and populations. The second is to have some idea as to what variables are associated with nonadherence.
Acknowledgments
Thank you to Dr David Blackburn from the College of Pharmacy and Nutrition at the University of Saskatchewan.
Footnotes
Disclosure
MWA’s student position at the University of Saskatchewan is funded through unrestricted financial support from the Government of Saskatchewan’s Ministry of Health, which in turn obtained unrestricted financial support from AstraZeneca Canada, Merck Frosst/Schering, and Pfizer Canada. None of these sponsors had input into the drafting or submission of this manuscript. ML reports no conflicts of interest in this work.
References
- 1.World Health Organization . Adherence to Long-Term Therapies: Evidence for Action. Geneva: WHO; 2003. [Google Scholar]
- 2.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2005:CD000011. doi: 10.1002/14651858.CD000011.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Cutler DM, Everett W. Thinking outside the pillbox – medication adherence as a priority for health care reform. N Engl J Med. 2010;362:1553–1555. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 5.Lemstra M, Blackburn DF, Crawley A, Fung R. Proportion and risk indicators of non-adherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Eze-Niliam CM, Thombs BD, Lima BB, Smith CG, Ziegelstein RC. The association of depression with adherence to antihypertensive medications: a systematic review. J Hypertension. 2010;28:1785–1795. doi: 10.1097/HJH.0b013e32833b4a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123:1611–1621. doi: 10.1161/CIRCULATIONAHA.110.983874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 9.Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies – report of the ISPOR task force on retrospective databases. Value Health. 2003;6:90–97. doi: 10.1046/j.1524-4733.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Alsabbagh W, Lemstra M, Eurich D, et al. Socioeconomic Status and Non Adherence to Anti-Hypertensive Drugs: A Systematic Review and Meta-Analysis. Value in Health. 2014. [Accessed February 4, 2014]. Available from: http://www.ispor.org/publications/value/articlesinpress.asp. [DOI] [PubMed]
- 11.Douglas GA. Practical Statistics for Medical Research. 1st ed. New York: Chapman and Hall; 1991. [Google Scholar]
- 12.Fleiss Jl. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 13.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2006. [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 17.Setoguchi S, Choudhry NK, Levin R, Shrank WH, Winkelmayer WC. Temporal trends in adherence to cardiovascular medications in elderly patients after hospitalization for heart failure. Clin Pharmacol Ther. 2010;88:548–554. doi: 10.1038/clpt.2010.139. [DOI] [PubMed] [Google Scholar]
- 18.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population-based study in the elderly. Am J Hypertens. 1997;10:697–704. doi: 10.1016/s0895-7061(97)00056-3. [DOI] [PubMed] [Google Scholar]
- 19.Bloom BS. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther. 1998;20:671–681. doi: 10.1016/s0149-2918(98)80130-6. [DOI] [PubMed] [Google Scholar]
- 20.Degli Esposti L, Degli Esposti E, Valpiani G, et al. A retrospective, population-based analysis of persistence with antihypertensive drug therapy in primary care practice in Italy. Clin Ther. 2002;24:1347–1357. doi: 10.1016/s0149-2918(02)80039-x. discussion 1346. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Thumula V, Pace PF, Banahan BF, 3rd, Wilkin NE, Lobb WB. Predictors of medication nonadherence among patients with diabetes in Medicare part D programs: a retrospective cohort study. Clin Ther. 2009;31:2178–2188. doi: 10.1016/j.clinthera.2009.10.002. discussion 2150–2151. [DOI] [PubMed] [Google Scholar]
- 22.Shaya FT, Du D, Gbarayor CM, Frech-Tamas F, Lau H, Weir MR. Predictors of compliance with antihypertensive therapy in a high-risk Medicaid population. J Natl Med Assoc. 2009;101:34–39. doi: 10.1016/s0027-9684(15)30808-7. [DOI] [PubMed] [Google Scholar]
- 23.Elliott WJ, Plauschinat CA, Skrepnek GH, Gause D. Persistence, adherence, and risk of discontinuation associated with commonly prescribed antihypertensive drug monotherapies. J Am Board Fam Med. 2007;20:72–80. doi: 10.3122/jabfm.2007.01.060094. [DOI] [PubMed] [Google Scholar]
- 24.Perreault S, Lamarre D, Blais L, et al. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39:1401–1408. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- 25.Taira DA, Gelber RP, Davis J, Gronley K, Chung RS, Seto TB. Antihypertensive adherence and drug class among Asian Pacific Americans. Ethn Health. 2007;12:265–281. doi: 10.1080/13557850701234955. [DOI] [PubMed] [Google Scholar]
- 26.van Wijk BL, Shrank WH, Klungel OH, Schneeweiss S, Brookhart MA, Avorn J. A cross-national study of the persistence of antihypertensive medication use in the elderly. J Hypertens. 2008;26:145–153. doi: 10.1097/HJH.0b013e32826308b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb D, Eurich D, McAlister F, et al. Changes in adherence to evidence-based medications in the first year after initial hospitalization for heart failure: observational cohort study from 1994 to 2003. Circ Cardiovasc Qual Outcomes. 2009;2:228–235. doi: 10.1161/CIRCOUTCOMES.108.813600. [DOI] [PubMed] [Google Scholar]
- 28.Gogovor A, Dragomir A, Savoie M, Perreault S. Comparison of persistence rates with angiotensin-converting enzyme inhibitors used in secondary and primary prevention of cardiovascular disease. Value Health. 2007;10:431–441. doi: 10.1111/j.1524-4733.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 29.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120:26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Friedman O, McAlister FA, Yun L, Campbell NR, Tu K. Antihypertensive drug persistence and compliance among newly treated elderly hypertensives in Ontario. Am J Med. 2010;123:173–181. doi: 10.1016/j.amjmed.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Corrao G, Zambon A, Parodi A, et al. Do socioeconomic disparities affect accessing and keeping antihypertensive drug therapy? Evidence from an Italian population-based study. J Hum Hypertens. 2009;23:238–244. doi: 10.1038/jhh.2008.84. [DOI] [PubMed] [Google Scholar]
- 32.Eagle KA, Kline-Rogers E, Goodman SG, et al. Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004;117:73–81. doi: 10.1016/j.amjmed.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Wogen J, Kreilick CA, Livornese RC, Yokoyama K, Frech F. Patient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care setting. J Manag Care Pharm. 2009;9:424–429. doi: 10.18553/jmcp.2003.9.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Thumula V, Pace PF, Banahan BF, 3rd, Wilkin NE, Lobb WB. Nonadherence to angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers among high-risk patients with diabetes in Medicare part D programs. J Am Pharm Assoc. 2010;50:527–531. doi: 10.1331/JAPhA.2010.09071. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454e1–e8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Degli Esposti E, Sturani A, Di Martino M, et al. Long-term persistence with antihypertensive drugs in new patients. J Hum Hypertens. 2002;16:439–444. doi: 10.1038/sj.jhh.1001418. [DOI] [PubMed] [Google Scholar]
- 37.Wong MC, Jiang JY, Su X, Wang H, Tang JL, Griffiths SM. Individuals at risk of beta-blocker discontinuation: a cohort study in 19,177 Chinese patients. Clin Res Cardiol. 2010;99:277–284. doi: 10.1007/s00392-010-0114-1. [DOI] [PubMed] [Google Scholar]
- 38.Sung SK, Lee SG, Lee KS, Kim DS, Kim KH, Kim KY. First-year treatment adherence among outpatients initiating antihypertensive medication in Korea: results of a retrospective claims review. Clin Ther. 2009;31:1309–1320. doi: 10.1016/j.clinthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Evans CD, Eurich DT, Remillard AJ, Shevchuk YM, Blackburn D. First-fill medication discontinuations and nonadherence to antihypertensive therapy: an observational study. Am J Hypertens. 2012;25:195–203. doi: 10.1038/ajh.2011.198. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen JN, Gislason GH, Rasmussen S, et al. Use of statins and beta-blockers after acute myocardial infarction according to income and education. J Epidemiol Community Health. 2007;61:1091–1097. doi: 10.1136/jech.2006.055525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrao G, Parodi A, Zambon A, et al. Reduced discontinuation of anti-hypertensive therapy by two-drug combination as first step. Evidence from daily life practice. J Hypertens. 2010;28:1584–1590. doi: 10.1097/HJH.0b013e328339f9fa. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . ICD-10: International Statistical Classification of Diseases and Related Health Problems. New York: WHO; 2008. [Google Scholar]
- 43.Evans CD, Eurich DT, Lu X, Remillard AJ, Shevchuk YM, Blackburn D. The association between market availability and adherence to antihypertensive medications: an observational study. Am J Hypertens. 2013;26:180–190. doi: 10.1093/ajh/hps017. [DOI] [PubMed] [Google Scholar]
- 44.Canadian Pharmacists Association . Compendium of Pharmaceuticals and Specialties: Thiazide Diuretics – Product Monograph. Ottawa: Canadian Pharmacists Association; 2012. [Google Scholar]
- 45.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: a meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 46.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 47.O’Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med. 2002;17:144–154. doi: 10.1046/j.1525-1497.2002.10431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Celano M, Geller RJ, Phillips KM, Ziman R. Treatment adherence among low-income children with asthma. J Pediatr Psychol. 1998;23:345–349. doi: 10.1093/jpepsy/23.6.345. [DOI] [PubMed] [Google Scholar]
- 49.Orlandi MA. Promoting health and preventing disease in health care settings: an analysis of barriers. Prev Med. 1987;16:119–130. doi: 10.1016/0091-7435(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 50.Diamond J. Guns, Germs, and Steel: The Fates of Human Societies. New York: WW Norton; 1999. [Google Scholar]
- 51.US Census Bureau People below poverty level and below 125 percent of poverty level by race and Hispanic origin: 1980 to 2009–2012. [Accessed January 24, 2013]. Available from: http://www.census.gov/compendia/statab/2012/tables/12s0711.pdf.