Abstract
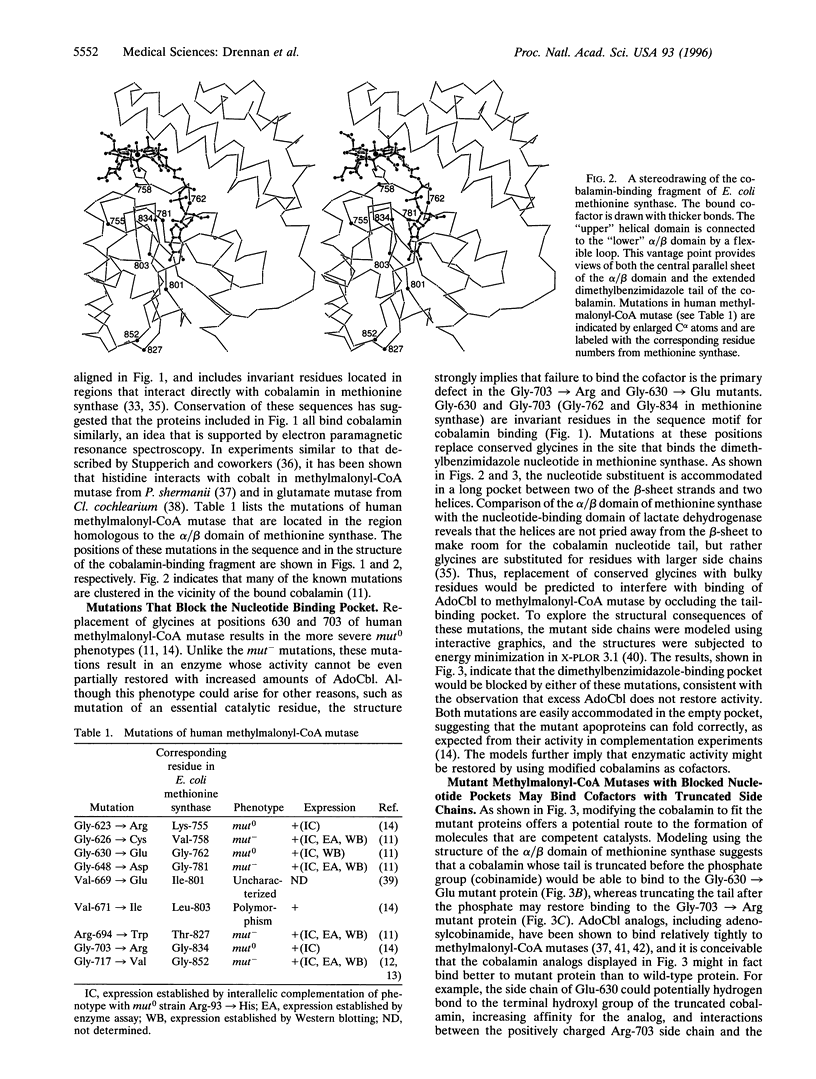
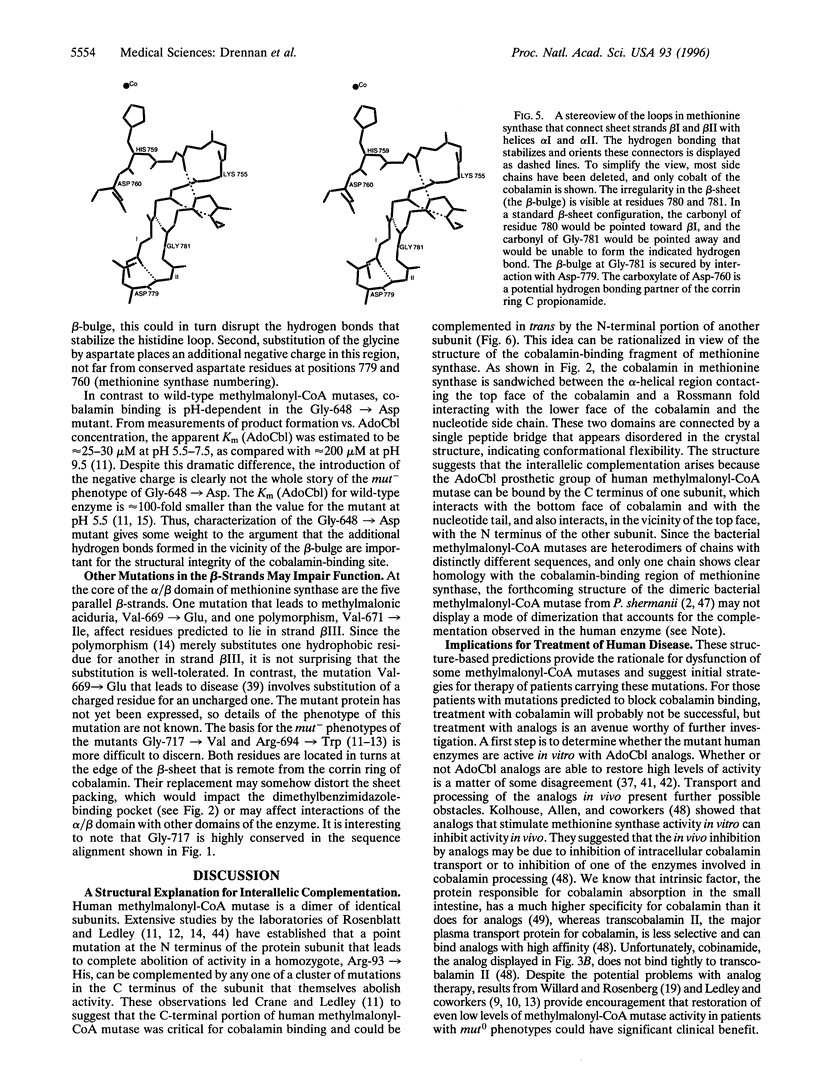
Inherited defects in the gene for methylmalonyl-CoA mutase (EC 5.4.99.2) result in the mut forms of methylmalonic aciduria. mut- mutations lead to the absence of detectable mutase activity and are not corrected by excess cobalamin, whereas mut- mutations exhibit residual activity when exposed to excess cobalamin. Many of the mutations that cause methylmalonic aciduria in humans affect residues in the C-terminal region of the methylmalonyl-CoA mutase. This portion of the methylmalonyl-CoA mutase sequence can be aligned with regions in other B12 (cobalamin)-dependent enzymes, including the C-terminal portion of the cobalamin-binding region of methionine synthase. The alignments allow the mutations of human methylmalonyl-CoA mutase to be mapped onto the structure of the cobalamin-binding fragment of methionine synthase from Escherichia coli (EC 2.1.1.13), which has recently been determined by x-ray crystallography. In this structure, the dimethylbenzimidazole ligand to the cobalt in free cobalamin has been displaced by a histidine ligand, and the dimethylbenzimidazole nucleotide "tail" is thrust into a deep hydrophobic pocket in the protein. Previously identified mut0 and mut- mutations (Gly-623 --> Arg, Gly-626 --> Cys, and Gly-648 --> Asp) of the mutase are predicted to interfere with the structure and/or stability of the loop that carries His-627, the presumed lower axial ligand to the cobalt of adenosylcobalamin. Two mutants that lead to severe impairment (mut0) are Gly-630 --> Glu and Gly-703 --> Arg, which map to the binding site for the dimethylbenzimidazole nucleotide substituent of adenosylcobalamin. The substitution of larger residues for glycine is predicted to block the binding of adenosylcobalamin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews E., Jansen R., Crane A. M., Cholin S., McDonnell D., Ledley F. D. Expression of recombinant human methylmalonyl-CoA mutase: in primary mut fibroblasts and Saccharomyces cerevisiae. Biochem Med Metab Biol. 1993 Oct;50(2):135–144. doi: 10.1006/bmmb.1993.1055. [DOI] [PubMed] [Google Scholar]
- Banerjee R. V., Johnston N. L., Sobeski J. K., Datta P., Matthews R. G. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. J Biol Chem. 1989 Aug 15;264(23):13888–13895. [PubMed] [Google Scholar]
- Beatrix B., Zelder O., Linder D., Buckel W. Cloning, sequencing and expression of the gene encoding the coenzyme B12-dependent 2-methyleneglutarate mutase from Clostridium barkeri in Escherichia coli. Eur J Biochem. 1994 Apr 1;221(1):101–109. doi: 10.1111/j.1432-1033.1994.tb18718.x. [DOI] [PubMed] [Google Scholar]
- Birch A., Leiser A., Robinson J. A. Cloning, sequencing, and expression of the gene encoding methylmalonyl-coenzyme A mutase from Streptomyces cinnamonensis. J Bacteriol. 1993 Jun;175(11):3511–3519. doi: 10.1128/jb.175.11.3511-3519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe J. T., Shih V. E., Levy H. L. Massachusetts Metabolic Disorders Screening Program. II. Methylmalonic aciduria. Pediatrics. 1981 Jan;67(1):26–31. [PubMed] [Google Scholar]
- Crane A. M., Jansen R., Andrews E. R., Ledley F. D. Cloning and expression of a mutant methylmalonyl coenzyme A mutase with altered cobalamin affinity that causes mut- methylmalonic aciduria. J Clin Invest. 1992 Feb;89(2):385–391. doi: 10.1172/JCI115597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane A. M., Ledley F. D. Clustering of mutations in methylmalonyl CoA mutase associated with mut- methylmalonic acidemia. Am J Hum Genet. 1994 Jul;55(1):42–50. [PMC free article] [PubMed] [Google Scholar]
- Crane A. M., Martin L. S., Valle D., Ledley F. D. Phenotype of disease in three patients with identical mutations in methylmalonyl CoA mutase. Hum Genet. 1992 May;89(3):259–264. doi: 10.1007/BF00220536. [DOI] [PubMed] [Google Scholar]
- Distefano M. D., Moore M. J., Walsh C. T. Active site of mercuric reductase resides at the subunit interface and requires Cys135 and Cys140 from one subunit and Cys558 and Cys559 from the adjacent subunit: evidence from in vivo and in vitro heterodimer formation. Biochemistry. 1990 Mar 20;29(11):2703–2713. doi: 10.1021/bi00463a013. [DOI] [PubMed] [Google Scholar]
- Drennan C. L., Huang S., Drummond J. T., Matthews R. G., Lidwig M. L. How a protein binds B12: A 3.0 A X-ray structure of B12-binding domains of methionine synthase. Science. 1994 Dec 9;266(5191):1669–1674. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- Drennan C. L., Matthews R. G., Ludwig M. L. Cobalamin-dependent methionine synthase: the structure of a methylcobalamin-binding fragment and implications for other B12-dependent enzymes. Curr Opin Struct Biol. 1994 Dec;4(6):919–929. doi: 10.1016/0959-440x(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Drummond J. T., Loo R. R., Matthews R. G. Electrospray mass spectrometric analysis of the domains of a large enzyme: observation of the occupied cobalamin-binding domain and redefinition of the carboxyl terminus of methionine synthase. Biochemistry. 1993 Sep 14;32(36):9282–9289. doi: 10.1021/bi00087a004. [DOI] [PubMed] [Google Scholar]
- Eberhard G., Schlayer H., Joseph H., Fridrich E., Utz B., Müller O. Untersuchungen zur biologischen Funktion der Nucleotidbase von Vitamin B12. Biol Chem Hoppe Seyler. 1988 Oct;369(10):1091–1098. [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Helfgott D., Rosenberg L. E. Biogenesis of the mitochondrial enzyme methylmalonyl-CoA mutase. Synthesis and processing of a precursor in a cell-free system and in cultured cells. J Biol Chem. 1984 May 25;259(10):6616–6621. [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Willard H. F., Gertler A., Rosenberg L. E. Purification and properties of methylmalonyl coenzyme A mutase from human liver. Arch Biochem Biophys. 1982 Apr 1;214(2):815–823. doi: 10.1016/0003-9861(82)90088-1. [DOI] [PubMed] [Google Scholar]
- Fleischmann R. D., Adams M. D., White O., Clayton R. A., Kirkness E. F., Kerlavage A. R., Bult C. J., Tomb J. F., Dougherty B. A., Merrick J. M. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995 Jul 28;269(5223):496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Holloway D. E., Marsh E. N. Adenosylcobalamin-dependent glutamate mutase from Clostridium tetanomorphum. Overexpression in Escherichia coli, purification, and characterization of the recombinant enzyme. J Biol Chem. 1994 Aug 12;269(32):20425–20430. [PubMed] [Google Scholar]
- Jansen R., Kalousek F., Fenton W. A., Rosenberg L. E., Ledley F. D. Cloning of full-length methylmalonyl-CoA mutase from a cDNA library using the polymerase chain reaction. Genomics. 1989 Feb;4(2):198–205. doi: 10.1016/0888-7543(89)90300-5. [DOI] [PubMed] [Google Scholar]
- Jansen R., Ledley F. D. Heterozygous mutations at the mut locus in fibroblasts with mut0 methylmalonic acidemia identified by polymerase-chain-reaction cDNA cloning. Am J Hum Genet. 1990 Nov;47(5):808–814. [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Allen R. H. Absorption, plasma transport, and cellular retention of cobalamin analogues in the rabbit. Evidence for the existence of multiple mechanisms that prevent the absorption and tissue dissemination of naturally occurring cobalamin analogues. J Clin Invest. 1977 Dec;60(6):1381–1392. doi: 10.1172/JCI108899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Utley C., Stabler S. P., Allen R. H. Mechanism of conversion of human apo- to holomethionine synthase by various forms of cobalamin. J Biol Chem. 1991 Dec 5;266(34):23010–23015. [PubMed] [Google Scholar]
- Ledley F. D., Crane A. M., Lumetta M. Heterogeneous alleles and expression of methylmalonyl CoA mutase in mut methylmalonic acidemia. Am J Hum Genet. 1990 Mar;46(3):539–547. [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Jansen R., Nham S. U., Fenton W. A., Rosenberg L. E. Mutation eliminating mitochondrial leader sequence of methylmalonyl-CoA mutase causes muto methylmalonic acidemia. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3147–3150. doi: 10.1073/pnas.87.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Levy H. L., Shih V. E., Benjamin R., Mahoney M. J. Benign methylmalonic aciduria. N Engl J Med. 1984 Oct 18;311(16):1015–1018. doi: 10.1056/NEJM198410183111604. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Lumetta M., Nguyen P. N., Kolhouse J. F., Allen R. H. Molecular cloning of L-methylmalonyl-CoA mutase: gene transfer and analysis of mut cell lines. Proc Natl Acad Sci U S A. 1988 May;85(10):3518–3521. doi: 10.1073/pnas.85.10.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D. Perspectives on methylmalonic acidemia resulting from molecular cloning of methylmalonyl CoA mutase. Bioessays. 1990 Jul;12(7):335–340. doi: 10.1002/bies.950120706. [DOI] [PubMed] [Google Scholar]
- Mancia F., Keep N. H., Nakagawa A., Leadlay P. F., McSweeney S., Rasmussen B., Bösecke P., Diat O., Evans P. R. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 A resolution. Structure. 1996 Mar 15;4(3):339–350. doi: 10.1016/s0969-2126(96)00037-8. [DOI] [PubMed] [Google Scholar]
- Marsh E. N., Holloway D. E. Cloning and sequencing of glutamate mutase component S from Clostridium tetanomorphum. Homologies with other cobalamin-dependent enzymes. FEBS Lett. 1992 Sep 28;310(2):167–170. doi: 10.1016/0014-5793(92)81321-c. [DOI] [PubMed] [Google Scholar]
- Marsh E. N., McKie N., Davis N. K., Leadlay P. F. Cloning and structural characterization of the genes coding for adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Biochem J. 1989 Jun 1;260(2):345–352. doi: 10.1042/bj2600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N., Leadlay P. F., Evans P. R. Crystallization and preliminary diffraction data for adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. J Mol Biol. 1988 Mar 20;200(2):421–422. doi: 10.1016/0022-2836(88)90252-5. [DOI] [PubMed] [Google Scholar]
- McKie N., Keep N. H., Patchett M. L., Leadlay P. F. Adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Active holoenzyme produced from Escherichia coli. Biochem J. 1990 Jul 15;269(2):293–298. doi: 10.1042/bj2690293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERATH P., STADTMAN E. R., KELLERMAN G. M., LYNEN F. [On the mechanism of the transformation of methylmalonyl-CoA into succinyl-CoA. III. Purification and properties of methylmalonyl-CoA-isomerase]. Biochem Z. 1962;336:77–98. [PubMed] [Google Scholar]
- Old I. G., Margarita D., Glass R. E., Saint Girons I. Nucleotide sequence of the metH gene of Escherichia coli K-12 and comparison with that of Salmonella typhimurium LT2. Gene. 1990 Mar 1;87(1):15–21. doi: 10.1016/0378-1119(90)90490-i. [DOI] [PubMed] [Google Scholar]
- Pookanjanatavip M., Yuthavong Y., Greene P. J., Santi D. V. Subunit complementation of thymidylate synthase. Biochemistry. 1992 Oct 27;31(42):10303–10309. doi: 10.1021/bi00157a018. [DOI] [PubMed] [Google Scholar]
- Qureshi A. A., Crane A. M., Matiaszuk N. V., Rezvani I., Ledley F. D., Rosenblatt D. S. Cloning and expression of mutations demonstrating intragenic complementation in mut0 methylmalonic aciduria. J Clin Invest. 1994 Apr;93(4):1812–1819. doi: 10.1172/JCI117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. L., Crane A. M., Jansen R., Ledley F. D., Rosenblatt D. S. Genetic characterization of a MUT locus mutation discriminating heterogeneity in mut0 and mut- methylmalonic aciduria by interallelic complementation. J Clin Invest. 1991 Jan;87(1):203–207. doi: 10.1172/JCI114972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Roy I., Leadlay P. F. Physical map location of the new Escherichia coli gene sbm. J Bacteriol. 1992 Sep;174(17):5763–5764. doi: 10.1128/jb.174.17.5763-5764.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell M. I., Matiaszuk N., Ledley F. D., Rosenblatt D. S. Varying neurological phenotypes among muto and mut- patients with methylmalonylCoA mutase deficiency. Am J Med Genet. 1993 Mar 1;45(5):619–624. doi: 10.1002/ajmg.1320450521. [DOI] [PubMed] [Google Scholar]
- Stupperich E., Eisinger H. J., Albracht S. P. Evidence for a super-reduced cobamide as the major corrinoid fraction in vivo and a histidine residue as a cobalt ligand of the p-cresolyl cobamide in the acetogenic bacterium Sporomusa ovata. Eur J Biochem. 1990 Oct 5;193(1):105–109. doi: 10.1111/j.1432-1033.1990.tb19310.x. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer M. F., Crane A. M., Ledley F. D. Primary structure and activity of mouse methylmalonyl-CoA mutase. Biochem J. 1990 Oct 15;271(2):449–455. doi: 10.1042/bj2710449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Rosenberg L. E. Inborn errors of cobalamin metabolism: effect of cobalamin supplementation in culture on methylmalonyl CoA mutase activity in normal and mutant human fibroblasts. Biochem Genet. 1979 Feb;17(1-2):57–75. doi: 10.1007/BF00484474. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Rosenberg L. E. Inherited deficiencies of human methylmalonyl CaA mutase activity: reduced affinity of mutant apoenzyme for adenosylcobalamin. Biochem Biophys Res Commun. 1977 Oct 10;78(3):927–934. doi: 10.1016/0006-291x(77)90511-3. [DOI] [PubMed] [Google Scholar]
- Zelder O., Beatrix B., Kroll F., Buckel W. Coordination of a histidine residue of the protein-component S to the cobalt atom in coenzyme B12-dependent glutamate mutase from Clostridium cochlearium. FEBS Lett. 1995 Aug 7;369(2-3):252–254. doi: 10.1016/0014-5793(95)00762-x. [DOI] [PubMed] [Google Scholar]
- Zelder O., Beatrix B., Leutbecher U., Buckel W. Characterization of the coenzyme-B12-dependent glutamate mutase from Clostridium cochlearium produced in Escherichia coli. Eur J Biochem. 1994 Dec 1;226(2):577–585. doi: 10.1111/j.1432-1033.1994.tb20083.x. [DOI] [PubMed] [Google Scholar]