Abstract
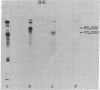
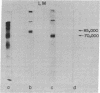
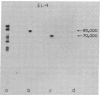
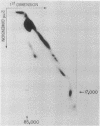
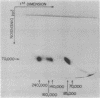
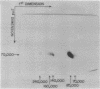
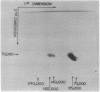
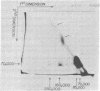
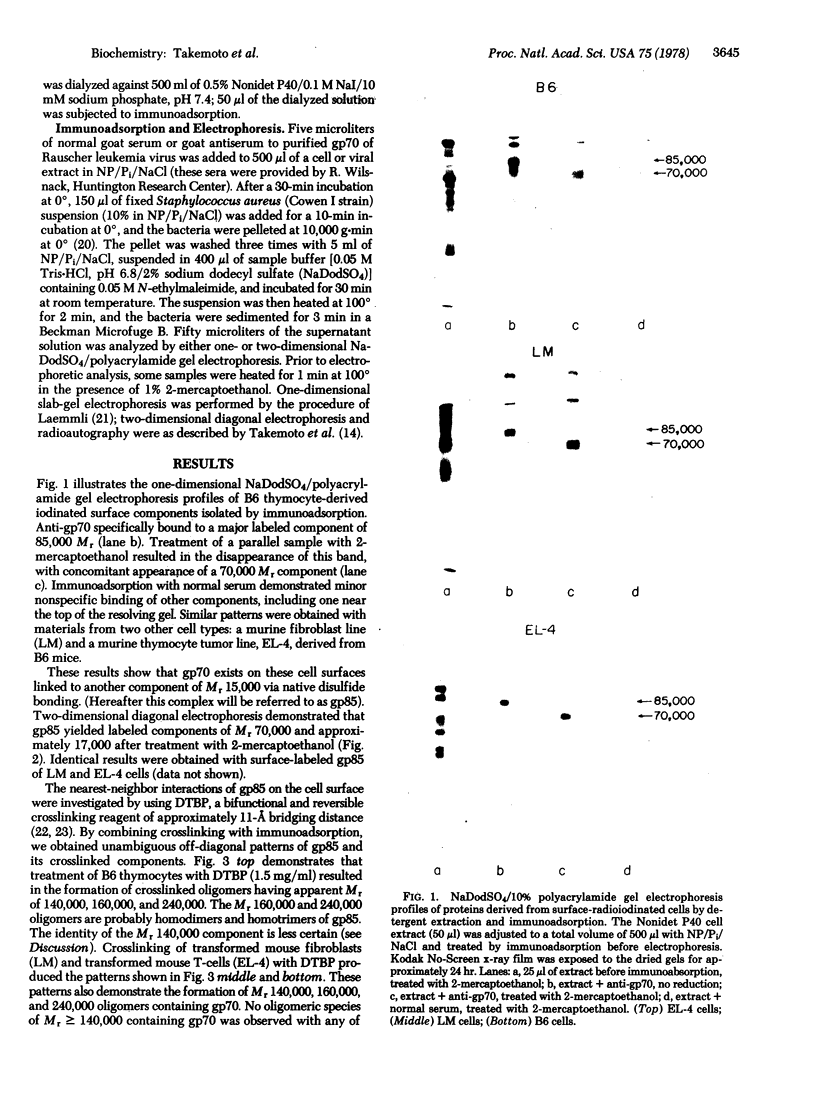
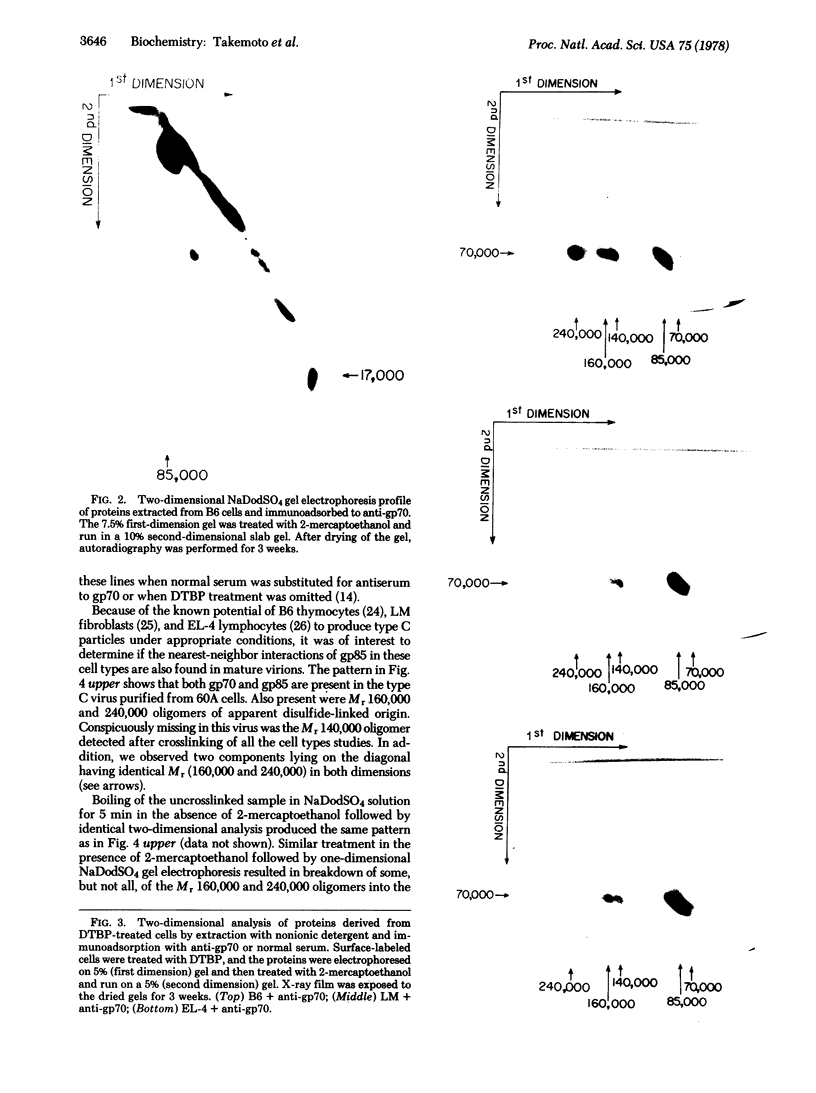
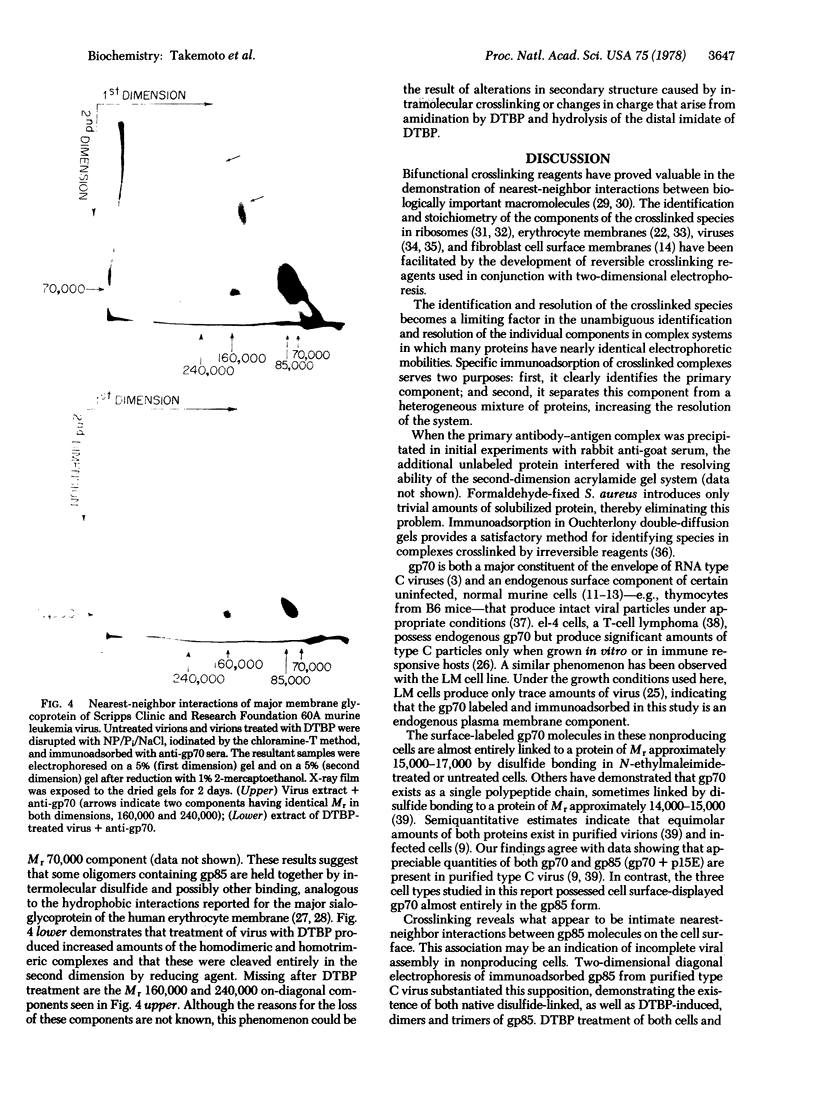
Formaldehyde-fixed Staphylococcus aureus and monospecific antiserum to gp70, the major envelope glycoprotein of murine leukemia virus, were used to immunoadsorb gp70 from Nonidet P40 extracts prepared from surface-radioiodinated murine cells. The labeled gp70 molecules in these cells were linked to a protein of approximately 15,000 daltons via native disulfide bonding. Prior treatment of cells with the reversible, bifunctional, crosslinking reagent dimethyl-3,3'-dithiobispropionimidate, followed by immunoadsorption and two-dimensional diagonal electrophoresis, revealed apparent homodimers and homotrimers of the 85,000-dalton complex. Identical treatment of purified type C RNA tumor virus from murine cells also revealed homodimeric and homotrimeric species, demonstrating similar self-associating tendencies of this glycoprotein in both intact virus and the plasma membrane of nonproducing murine cells. One cross-linked product consistently detected on the surfaces of murine cells was not present after crosslinking of a representative strain of murine leukemia virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Cloyd M. W., Bolognesi D. P., Bigner D. D. Immunofluorescent analysis of expression of the RNA tumor virus major glycoprotein, gp71, on the surfaces of normal murine cells. Cancer Res. 1977 Mar;37(3):931–938. [PubMed] [Google Scholar]
- Colnaghi M. I., Pierotti M. A., Torre G. D., Miotti S. Antigen fluctuation and virus activation in EL 4 cells transplanted in hybrid mice or cultured in vitro. J Natl Cancer Inst. 1977 Jul;59(1):123–130. doi: 10.1093/jnci/59.1.123. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Fine R. E., Bray D. Actin in growing nerve cells. Nat New Biol. 1971 Nov 24;234(47):115–118. doi: 10.1038/newbio234115a0. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976 Mar 9;15(5):1137–1144. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heininger D., Touton M., Chakravarty A. K., Clark W. R. Activation of cytotoxic function in T lymphocytes. J Immunol. 1976 Dec;117(6):2175–2180. [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Schäfer W. Properties of mouse leukemia viruses. IX. Active and passive immunization of mice against Friend leukemia with isolated viral GP71 glycoprotein and its corresponding antiserum. Virology. 1975 Jul;66(1):327–329. doi: 10.1016/0042-6822(75)90203-2. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Karshin W. L., Arcement L. J., Naso R. B., Arlinghaus R. B. Common precursor for Rauscher leukemia virus gp69/71, p15(E), and p12(E). J Virol. 1977 Sep;23(3):787–798. doi: 10.1128/jvi.23.3.787-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kindig D. A., Karp R., Kirsten W. H. Further characterization of L-cell virions. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1103–1109. doi: 10.1073/pnas.59.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Krantz M. J., Strand M., August J. T. Biochemical and immunological characterization of the major envelope glycoprotein gp69/71 and degradation fragments from Rauscher leukemia virus. J Virol. 1977 Jun;22(3):804–815. doi: 10.1128/jvi.22.3.804-815.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Halpern M. S. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976 Jun;18(3):956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamnson R. N., Shander M. H., Halpern M. S. A structural protein complex in Moloney leukemia virus. Virology. 1977 Jan;76(1):437–439. doi: 10.1016/0042-6822(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Jensen F., Kennel S. J., Dixon F. J., Des Roches G., Francke U. Karyotypic, virologic, and immunologic analyses of two continuous lymphocyte lines established from New Zealand black mice: possible relationship of chromosomal mosaicism to autoimmunity. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2965–2969. doi: 10.1073/pnas.69.10.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C., Ortanderl F., Fasold H. The use of a new series of cleavable protein-crosslinkers on the Escherichia coli ribosome. FEBS Lett. 1974 Nov 15;48(2):288–292. doi: 10.1016/0014-5793(74)80488-6. [DOI] [PubMed] [Google Scholar]
- Meier H., Taylor B. A., Cherry M., Buebner R. J. Host-gene control of type-C RNA tumor virus expression and tumorigenesis in inbred mice. Proc Natl Acad Sci U S A. 1973 May;70(5):1450–1455. doi: 10.1073/pnas.70.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Ruoho A., Bartlett P. A., Dutton A., Singer S. J. A disulfide-bridge bifunctional imidoester as a reversible cross-linking reagent. Biochem Biophys Res Commun. 1975 Mar 17;63(2):417–423. doi: 10.1016/0006-291x(75)90704-4. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. D., Kato K., Cullen S. E., Nathenson S. G. H-2 histocompatibility alloantigens. Some biochemical properties of the molecules solubilized by NP-40 detergent. Biochemistry. 1973 May 22;12(11):2157–2164. doi: 10.1021/bi00735a023. [DOI] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Diagonal polyacrylamide-dodecyl sulfate gel electrophoresis for the identification of ribosomal proteins crosslinked with methyl-4-mercaptobutyrimidate. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3946–3950. doi: 10.1073/pnas.71.10.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Boyse E. A., Obata Y., Ikeda H., Sarkar N. H., Hoffman H. A. New mutant and congenic mouse stocks expressing the murine leukemia virus-associated thymocyte surface antigen GIX. J Exp Med. 1975 Aug 1;142(2):512–517. doi: 10.1084/jem.142.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Traut R. R., Kahan L. Protein-protein proximity in the association of ribosomal subunits of Escherichia coli: crosslinking of 30 S protein S16 to 50 S proteins by glutaraldehyde or formaldehyde. J Mol Biol. 1974 Aug 15;87(3):509–522. doi: 10.1016/0022-2836(74)90101-6. [DOI] [PubMed] [Google Scholar]
- Tuech J. K., Morrison M. Human erythrocyte membrane sialoglycoproteins: a study of interconversion. Biochem Biophys Res Commun. 1974 Jul 10;59(1):352–360. doi: 10.1016/s0006-291x(74)80214-7. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J., Waterfield M. Evidence from studies with a cross-linking reagent that the haemagglutinin of influenza virus is a trimer. Virology. 1977 Jun 15;79(2):446–448. doi: 10.1016/0042-6822(77)90371-3. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Tsukamoto-Adey A., Weissman I. L. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membranes. Virology. 1977 Feb;76(2):539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Oncornavirus budding: kinetics of formation and utilization of viral membrane glycoprotein. Virology. 1976 Feb;69(2):464–473. doi: 10.1016/0042-6822(76)90477-3. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Polypeptides of Moloney sarcoma-leukemia virions: their resolution and incorporation into extracellular virions. Virology. 1974 Oct;61(2):575–587. doi: 10.1016/0042-6822(74)90291-8. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]