Abstract
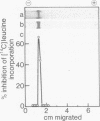
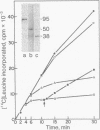
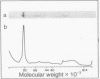
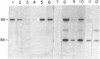
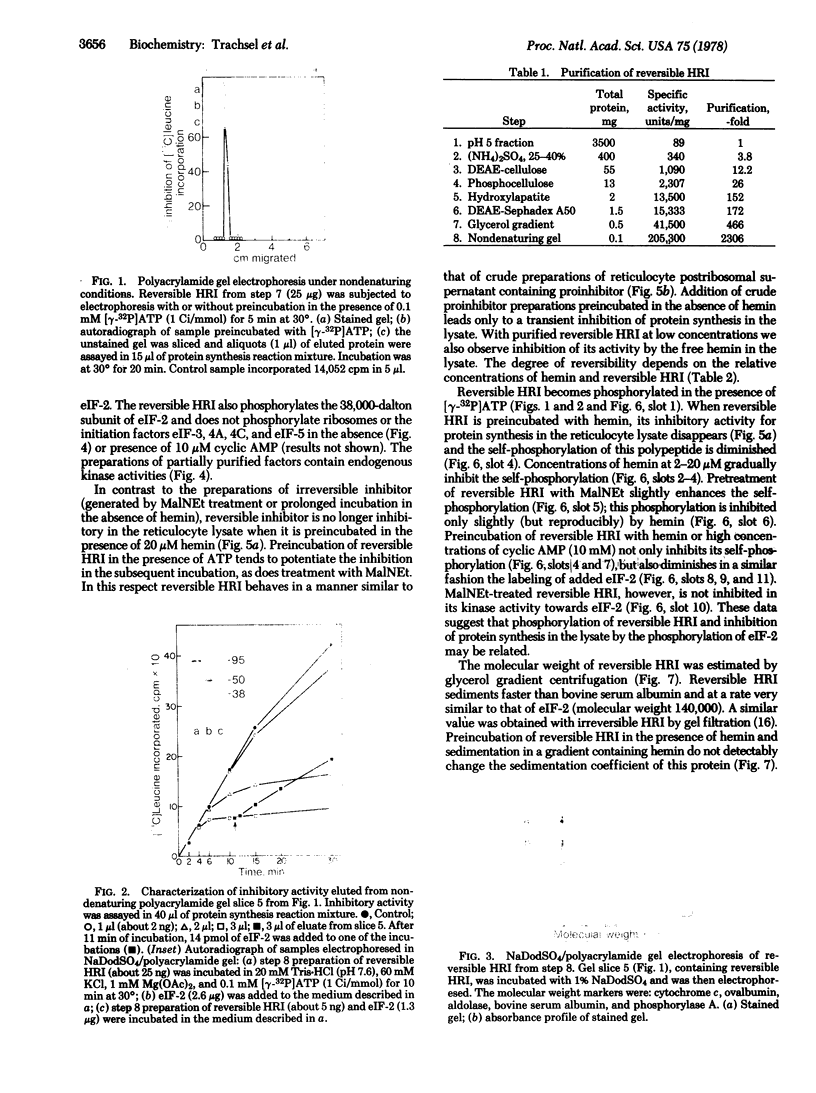
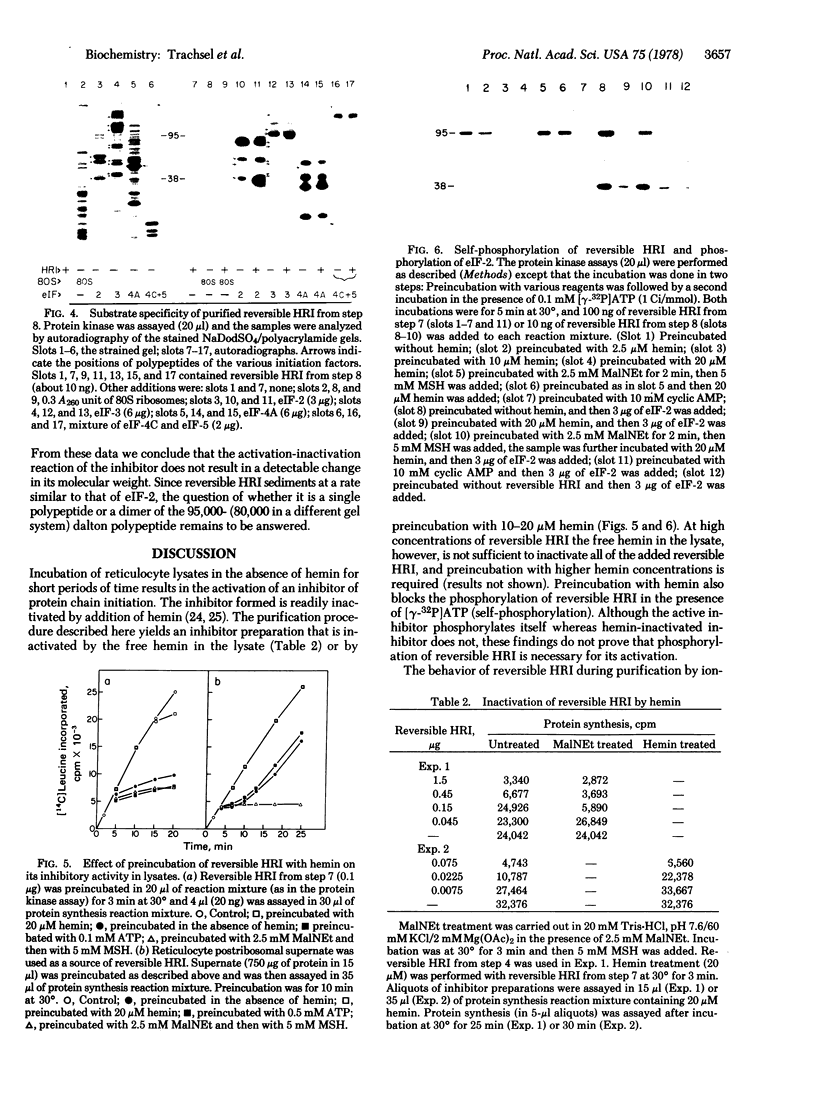
To define the mechanism of regulation of the protein kinase that is activated in heme deficiency and that inhibits initiation of protein synthesis, we have isolated and purified the heme-reversible form of the protein kinase from rabbit reticulocytes. The inhibitory activity is found in a single band after polyacrylamide gel electrophoresis under nondenaturing conditions. It migrates as a 95,000-dalton polypeptide in 15% sodium dodecyl sulfate/polyacrylamide gels. This purified inhibitor becomes self-phosphorylated in the presence of ATP; the phosphorylated protein and the inhibitory activity copurify. The inhibitor produces characteristic biphasic kinetics of inhibition in reticulocyte lysates and phosphorylates the 38,000-dalton subunit of eukaryotic initiation factor 2 (eIF-2); the inhibition is reversed by added eIF-2. In contrast to the heme-irreversible inhibitor, this heme-reversible inhibitor is no longer inhibitory after incubation with 20 micron hemin. Incubation with hemin also inhibits self-phosphorylation. Preincubation of the heme-reversible inhibitor in the presence of ATP potentiates the inhibition of protein synthesis in the subsequent incubation, as does treatment with N-ethylmaleimide. Phosphorylation of the heme-reversible inhibitor and inhibition of protein synthesis in the lysate due to phosphorylation of eIF-2 appear to be related. These findings suggest that hemin acts directly on the heme-reversible inhibitor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Kemp S. F. Effects of hemin and other porphyrins on protein synthesis in a reticulocyte lysate cell-free system. J Mol Biol. 1969 Jun 14;42(2):247–258. doi: 10.1016/0022-2836(69)90041-2. [DOI] [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Balkow K., Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: effect of a translational repressor on Met-tRNAf binding to the small ribosomal subunit and its relation to the activity and alailability of an initiation factor. Biochim Biophys Acta. 1973 Oct 26;324(3):397–409. doi: 10.1016/0005-2787(73)90284-0. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Hunt T., Jackson R. J. A complex between met-tRNA F and native 40S subunits in reticulocyte lysates and its disappearance during incubation with double-stranded RNA. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1556–1564. doi: 10.1016/0006-291x(72)90891-1. [DOI] [PubMed] [Google Scholar]
- Das A., Gupta N. K. Protein synthesis in rabbit reticulocytes XX: a supernatant factor (TDI) inhibits ternary complex (Met-tRNAf-EIF-1-GTP) dissociation and Met-tRNAf binding to 40S ribosomes. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1433–1441. doi: 10.1016/0006-291x(77)91453-x. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Majumdar A., George A. D., Gupta N. K. Protein synthesis in rabbit reticulocytes. XV. Isolation of a ribosomal protein factor (CO-EIE-1) which stimulates Met-tRNAfMet binding to EIF-1. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1234–1241. doi: 10.1016/0006-291x(76)90786-5. [DOI] [PubMed] [Google Scholar]
- Datta A., de Haro C., Sierra J. M., Ochoa S. Role of 3':5'-cyclic-AMP-dependent protein kinase in regulation of protein synthesis in reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1463–1467. doi: 10.1073/pnas.74.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Henderson A. B., Pinphanichakarn P., Wallis M. H., Hardesty B. Partial reaction of peptide initiation inhibited by phosphorylation of either initiation factor eIF-2 or 40S ribosomal proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1445–1449. doi: 10.1073/pnas.74.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Brayley A., Hunt T., Jackson R. J. The effect of cyclic AMP and related compounds on the control of protein synthesis in reticulocyte lysates. Biochem Biophys Res Commun. 1974 Feb 4;56(3):745–752. doi: 10.1016/0006-291x(74)90668-8. [DOI] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Roy R., Das A., Dasgupta A., Gupta N. K. Protein synthesis in rabbit reticulocytes XIX: EIF-2 promotes dissociation of Met-tRNAf-EIF-1-GTP complex and Met-tRNAf binding to 40S ribosomes. Biochem Biophys Res Commun. 1977 Sep 9;78(1):161–169. doi: 10.1016/0006-291x(77)91235-9. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: action of an inhibitor formed in the absence of hemin on the reticulocyte cell-free system and its reversal by a ribosomal factor. Biochim Biophys Acta. 1972 Jul 31;272(4):638–650. [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M., Das A., Dasgupta A., Majumdar A., Ralston R., Roy R., Gupta N. K. Regulation of protein synthesis in rabbit reticulocyte lysates by the heme-regulated protein kinase: inhibition of interaction of Met-tRNAfMet binding factor with another initiation factor in formation of Met-tRNAfMet.40S ribosomal subunit complexes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):745–749. doi: 10.1073/pnas.75.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Binding and release of eukaryotic initiation factor eIF-2 and GTP during protein synthesis initiation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):204–208. doi: 10.1073/pnas.75.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]