Abstract
Objective
While transforming growth factor β (TGFβ) signaling plays a critical role in chondrocyte metabolism, the TGFβ signaling pathways and target genes involved in cartilage homeostasis and the development of osteoarthritis (OA) remain unclear. Using an in vitro cell culture method and an in vivo mouse genetic approach, we undertook this study to investigate TGFβ signaling in chondrocytes and to determine whether Mmp13 and Adamts5 are critical downstream target genes of TGFβ signaling.
Methods
TGFβ receptor type II (TGFβRII)–conditional knockout (KO) (TGFβRIICol2ER) mice were generated by breeding TGFβRIIflox/flox mice with Col2-CreER–transgenic mice. Histologic, histomorphometric, and gene expression analyses were performed. In vitro TGFβ signaling studies were performed using chondro-genic rat chondrosarcoma cells. To determine whether Mmp13 and Adamts5 are critical downstream target genes of TGFβ signaling, TGFβRII/matrix metalloproteinase 13 (MMP-13)– and TGFβRII/ADAMTS-5–double-KO mice were generated and analyzed.
Results
Inhibition of TGFβ signaling (deletion of the Tgfbr2 gene in chondrocytes) resulted in up-regulation of Runx2, Mmp13, and Adamts5 expression in articular cartilage tissue and progressive OA development in TGFβRIICol2ER mice. Deletion of the Mmp13 or Adamts5 gene significantly ameliorated the OA-like phenotype induced by the loss of TGFβ signaling. Treatment of TGFβRIICol2ER mice with an MMP-13 inhibitor also slowed OA progression.
Conclusion
Mmp13 and Adamts5 are critical downstream target genes involved in the TGFβ signaling pathway during the development of OA.
Osteoarthritis (OA) is the most common degenerative joint disease, affecting >25% of the US population age >18 years. The major pathologic changes of OA include abnormal articular chondrocyte maturation, progressive loss and destruction of articular cartilage, osteophyte formation, and subchondral sclerosis. The etiology of OA is multifactorial, including joint injury, obesity, aging, and heredity (1–3). There are currently no interventions to restore degraded cartilage or decelerate the progression of OA, as the precise signaling pathways involved in initiation and progression of OA are still poorly understood.
Ex vivo studies with tissues obtained from OA patients and in vivo studies with mutant mouse models suggest that the factors involved in development of OA include growth factors, such as transforming growth factor β(TGFβ) and Indian hedgehog (IHH), and signaling molecules, such as Smads, β-catenin, and hypoxia-inducible factor 2α (HIF-2α) (4–8). TGFβ signaling strongly inhibits chondrocyte hypertrophy and maturation. In canonical TGFβ signaling, the TGFβ ligand binds to TGFβ receptor type II (TGFβRII), which then phosphorylates the type I transmembrane serine/threonine kinase receptor. The activated kinase subsequently phosphorylates Smad2 or Smad3 (receptor-activated Smad), which then forms a heteromeric complex with Smad4 (common-mediator Smad), translocates into the nucleus, and interacts with other DNA binding proteins to regulate target gene transcription (9).
Recent genetic manipulations suggest that dys-regulation of TGFβ signaling induces development of OA via the TGFβ/Smad3 signaling pathway in chondrocytes (4,10). Transgenic mice overexpressing a dominant-negative form of TGFβRII (dn-TGFβRII) in skeletal tissue were found to exhibit cartilage disorganization and progressive cartilage degradation resembling the features of OA in humans (10). Similar to the dn-TGFβRII–transgenic mice, Smad3-knockout (KO) mice were also found to display progressive articular cartilage degradation and osteophyte formation (4). A recent study showed that a single-nucleotide polymorphism in the Smad3 gene was correlated with the incidence of hip and knee OA in a cohort of 527 patients (11). More recently, different types of Smad3 mutations were identified in patients with a syndromic form of aortic aneurysms and early-onset OA (12,13). These observations strongly support the notion that the TGFβ/Smad3 signaling pathway in chondrocytes plays an essential role in the development of OA. However, the critical downstream target genes of TGFβ signaling involved in the development of OA remain unknown.
The progressive loss of articular cartilage is a fundamental feature of OA. Articular cartilage consists of a dense meshwork of interconnected collagen fibrils within which is embedded a rich matrix of negatively charged proteoglycans. The negative charge attracts ions and provides the tissue with a high osmotic pressure that resists compressive force and provides boundary lubrication (14). Human clinical and animal studies have shown that matrix metalloproteinase 13 (MMP-13) plays a pivotal role during cartilage degradation. MMP-13 is a primary collagenase that preferentially cleaves type II collagen in articular cartilage (15). Clinical investigations have revealed that MMP-13 expression is elevated in articular cartilage of OA patients (16). Expression of the constitutively active Mmp13 gene leads to an OA-like phenotype in mice (17). In addition to MMP-13, ADAMTS-5, the principal enzyme responsible for degradation of aggrecan in articular cartilage, plays a critical role during OA development. Studies have shown that expression levels of Adamts5 are significantly increased during OA development (18). Deletion of the Adamts5 gene prevented articular cartilage degradation in mouse models of surgically or chemically induced OA (19–21).
To determine the mechanism of inhibition of TGFβ signaling in the development of OA, we generated chondrocyte-specific Tgfbr2Col2ER mice by breeding Col2-CreER–transgenic mice (22,23) with Tgfbr2flox/flox mice. Gene deletion was induced by injection of tamox-ifen into 2-week-old mice, and alterations in the articular cartilage were analyzed at ages 3 and 6 months. Our studies demonstrated that deletion of the Tgfbr2 gene at the postnatal/adult stage led to a severe OA-like phenotype. Our in vitro studies demonstrated that inhibition of TGFβ signaling up-regulated Mmp13 and Adamts5 expression in articular cartilage tissue. MMP-13 is a collagenase that mainly degrades type II collagen. ADAMTS-5 is an aggrecanase that degrades aggrecan. Type II collagen and aggrecan are the principal matrix components present in articular cartilage. Because both MMP-13 and ADAMTS-5 play critical roles in the development of OA (12,21,24), we reasoned that Mmp13 and Adamts5 might be the key downstream target genes of TGFβ signaling in articular chondrocytes during OA development. In this study, we demonstrated that deletion of the Mmp13 or Adamts5 gene in mice of the Tgfbr2Col2ER background significantly prevented the OA-like phenotype observed in Tgfbr2Col2ER mice, which suggests that MMP-13 and ADAMTS-5 are critical downstream targets of TGFβ signaling during OA development.
MATERIALS AND METHODS
Generation of Tgfbr2Col2ER–conditional KO mice and Tgfbr2/Mmp13– and Tgfbr2/Adamts5–double-KO mice
Col2-CreER mice were generated in our laboratory (22,23). Tgfbr2flox/flox mice were obtained from the National Cancer Institute (25). To generate Tgfbr2–conditional KO mice, Tgfbr2flox/flox mice were crossed with Col2-CreER–transgenic mice. The resulting Col2-CreER/Tgfbr2flox/flox (Tgfbr2Col2ER) mice were injected intraperitoneally (IP) with tamoxifen (1 mg/10 gm body weight/day, for 5 days) at age 2 weeks and were killed at ages 3 and 6 months for histologic analysis. The Cre-negative littermates were used as controls. The primer names and sequences used for mouse genotyping are available online at http://www.rushu.rush.edu/biochem. To generate double-KO mice, Tgfbr2Col2ER mice were crossed with Mmp13flox/flox or Adamts5−/− (26) mice (obtained from The Jackson Laboratory).
Cre recombination efficiency
To determine whether the Col2-CreER transgene targets floxed genes specifically in articular cartilage, Col2-CreER–transgenic mice were bred with Rosa-tomato (mT/mG) and Rosa-lacZ (R26R) reporter mice. Tamoxifen (1 mg/10 gm body weight/day, for 5 days) was injected IP into 2-week-old mice. The mice were killed at age 1 month. Histologic sections were analyzed using a fluorescence microscope and X-Gal staining.
Treatment with MMP-13 inhibitor
An MMP-13 inhibitor, CL82198 (Tocris), was used for the in vivo experiment. The inhibitory effect of CL82198 on MMP-13 enzymatic activity was confirmed using an MMP-13 fluorometric drug discovery kit (Enzo Life Sciences). Both TGFβRIICol2ER mice and Cre-negative control mice were injected IP with tamoxifen (1 mg/10 gm body weight/day for 5 days) at age 2 weeks and then treated with the MMP-13 inhibitor (10 mg/kg body weight, IP, every other day until the mice were killed) or vehicle (phosphate buffered saline). At age 3 months, the mice were killed and their knee joint tissues were harvested for histologic and histomorphometric analyses.
Histology and immunohistochemistry
Knee joint tissues from 3- and 6-month-old mice were fixed in 4% parafor-maldehyde, decalcified, dehydrated, and embedded in paraffin. Serial midsagittal sections (3-μm thick) were cut and stained with Alcian blue/hematoxylin and eosin for morphologic analysis. Immunohistochemistry was performed on the 3-μm–thick tissue sections using antibodies to TGFβRII (Santa Cruz Biotechnology) and type X collagen (Quartett).
Real-time quantitative polymerase chain reaction (PCR) analysis
Total RNA was extracted from rat chondro-sarcoma cells or mouse articular cartilage tissue using TRIzol according to the protocol of the manufacturer (Invitrogen). DNase I–treated total RNA was reverse-transcribed using oligo(dT), and complementary DNA (cDNA) was amplified by PCR in a total volume of 20 μl. The solution contained 10 μl SYBR Green Master Mix (Thermo Scientific), 1 μl of the diluted (1:5) cDNA, and 10 pmoles of forward and reverse primers (a list of genes specified by these primers is available at http://www.rushu.rush.edu/biochem).
Cell culture and transfection
Rat chondrosarcoma cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum at 37°C in a 5% CO2 atmosphere. RUNX-2 and cyclin-dependent kinase 4 (CDK-4) plasmids or Tgfbr2, Runx2, and CDK4 small interfering RNAs (siRNAs) were transiently transfected into rat chondrosarcoma cells in 6-cm culture dishes using Lipofectamine 2000 (Invitrogen). Empty vector or scrambled siRNA was used as control. Western blotting and immunoprecipitation assays were performed 24 hours after transfection. The siRNAs siTgfbr2 (catalog no. L-091837), siRunx2 (catalog no. L-082676), and siCDK4 (catalog no. L-082676) (on-target plus rat smart pool) were purchased from Thermo Fisher Scientific.
Luciferase and chromatin immunoprecipitation (ChIP) assays
Plasmid containing the 3.4-kb human Mmp13 promoter was transfected into rat chondrosarcoma cells, and luciferase assay was performed to check the effect of TGFβ (2 ng/ml) on Mmp13 transcriptional activity. ChIP assay was performed as previously described (27) to determine whether RUNX-2 specifically binds to the proximal promoter region of the Mmp13 gene in rat chondrosarcoma cells. Three sets of primers were used in the ChIP assay (further information is available at http://www.rushu.rush.edu/biochem).
Western blot analysis and ubiquitination assay
Western blot analysis and ubiquitination assay were performed as described previously (28,29). For the RUNX-2 ubiquitination assay, the proteasome inhibitor MG132 (10 μM) was added to the cell culture 4 hours before cells were harvested. The rat anti–RUNX-2 monoclonal antibody was purchased from MBL. The rabbit anti–CDK-4 polyclonal antibody (C-22) was purchased from Santa Cruz Biotechnology. The UbiQapture kit was purchased from Enzo Life Sciences.
Statistical analysis
Results are presented as the mean ± SD. Statistical tests included Student’s unpaired t-test and two-way analysis of variance followed by Tukey’s test. P values less than 0.05 were considered significant.
RESULTS
Col2-CreER–targeted Cre recombination in articular chondrocytes
To specifically target articular chondrocytes at postnatal/adult stages, we generated Col2-CreER–transgenic mice (22,23). To evaluate the targeting efficiency and specificity of these mice, we bred them with Rosa-tomato (mT/mG) and Rosa-lacZ (R26R) reporter mice.
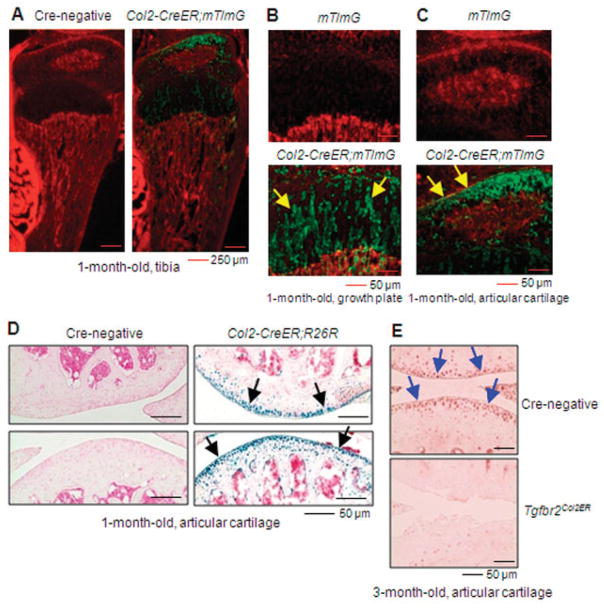
Analysis of histologic sections from 1-month-old mice using fluorescence microscopy or X-Gal staining showed efficient targeting (>85%) of growth plate and articular chondrocytes in transgenic mice following tamoxifen administration at age 2 weeks (Figures 1A–D). We then used these mice to generate TGFβRIICol2ER mice by breeding Col2-CreER mice with TGFβRIIflox/flox mice. Immunohistochemical analysis demonstrated that TGFβRII expression was undetectable in the majority of articular chondrocytes from 3-month-old TGFβRIICol2ER mice (Figure 1E).
Figure 1.
Directed Cre recombination in articular chondrocytes from Col2-CreER mice. Col2-CreER;mT/mG mice and Col2-CreER;R26R mice were generated by breeding Col2-CreER–transgenic mice with Rosa-Tomato (mT/mG) mice and Rosa-lacZ (R26R) mice, respectively. Tibia samples were harvested from 1-month-old mice after they were injected with tamoxifen at age 2 weeks. A–C, Analysis of histologic sections from mT/mG mice and Col2-CreER;mT/mG mice using fluorescence microscopy demonstrated the efficiency of Cre recombination in the growth plate and articular chondrocytes. Arrows in B indicate Col2-CreER–targeted growth plate chondrocytes; arrows in C indicate Col2-CreER–targeted articular chondrocytes. D, X-Gal staining showed that the efficiency of Col2-CreER–mediated Cre recombination was >85% in articular chondrocytes. Arrows indicate Col2-CreER–targeted articular chondrocytes. E, Immunohistochemical analysis showed that transforming growth factor β receptor type II (TGFβRII) expression was dramatically reduced in articular chondrocytes from 3-month-old TGFβRIICol2ER mice. Arrows indicate TGFβRII-positive articular chondrocytes.
Postnatal deletion of the Tgfbr2 gene induces an OA-like phenotype
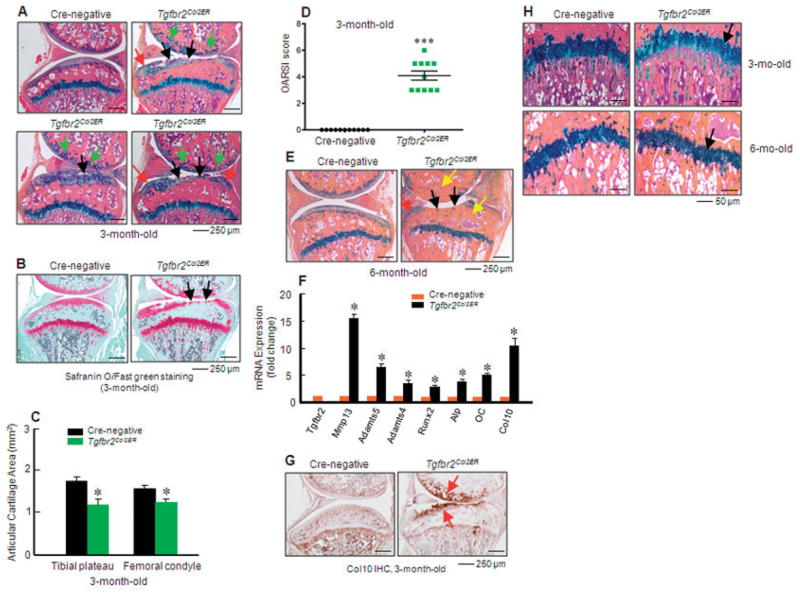
To determine the role of TGFβ signaling in OA development in postnatal and adult mice, we generated TGFβRIICol2ER mice. Mice were injected with tamoxifen at age 2 weeks. A severe and progressive OA-like phenotype was observed in 3- and 6-month-old TGFβRIICol2ER mice. Histologic examination revealed tears and clefts in the articular cartilage surface, localized damage of articular cartilage tissues, increased numbers of hypertrophic chondrocytes localized at the surface of articular cartilage, early osteophyte formation, and increases in subchondral bone mass (especially at the upper ends of tibiae) in 3-month-old TGFβRIICol2ER mice (Figures 2A and B). Histomorphometric analysis showed a significant reduction in articular cartilage area in 3-month-old TGFβRIICol2ER mice (Figure 2C). We also analyzed the data using the histologic scoring system recommended by the Osteoarthritis Research Society International (OARSI) (30), and we found that TGFβRIICol2ER mice had significantly higher scores for OA damage compared to Cre-negative control mice (Figure 2D). Loss of the entire articular cartilage, extensive formation of osteophytes, and substantially increased subchondral bone mass were observed in 6-month-old TGFβRIICol2ER mice (Figure 2E).
Figure 2.
Transforming growth factor β receptor type II (TGFβRII)–conditional knockout (TGFβRIICol2ER) mice show a progressive osteoarthritis (OA)–like phenotype. A and B, Knee joint samples were dissected from 3-month-old mice, and Alcian blue/hematoxylin and eosin and Safranin O–fast green staining were performed. TGFβRIICol2ER mice displayed early signs of an OA-like phenotype, including enhanced chondrocyte hypertrophy in the superficial zone (green arrows), tears and clefts in the articular surface (black arrows), and osteophyte formation (red arrows). C, Histomorphometric analysis showed that articular cartilage area in both the tibia and femur was significantly reduced in 3-month-old TGFβRIICol2ER mice (* = P < 0.05 versus Cre-negative mice, by Student’s unpaired t-test; n = 11 mice per group). D, Analysis using the histologic scoring system recommended by the Osteoarthritis Research Society International (OARSI) revealed articular cartilage destruction in 3-month-old TGFβRIICol2ER mice (** = P < 0.01 versus Cre-negative mice, by Student’s unpaired t-test). E, Severe loss of articular cartilage tissue (black arrows), osteophyte formation (red arrow), and subchondral sclerosis (yellow arrows) were observed in 6-month-old TGFβRIICol2ER mice. F, Total RNA was isolated from articular cartilage of 1-month-old TGFβRIICol2ER mice and their littermates, and real-time polymerase chain reaction was performed. Loss of TGFβ signaling resulted in increased expression of hypertrophic chondrocyte marker genes (* = P < 0.05 versus Cre-negative mice, by Student’s unpaired t-test; n = 4 mice per group). G, Immunohistochemical (IHC) analysis showed that type X collagen protein levels were increased in TGFβRIICol2ER mice, especially in the superficial zone (red arrows). H, Structure and morphology of growth plate cartilage (black arrows) were not significantly changed. Values in C, D, and F are the mean ± SD. In D, squares represent individual mice.
To determine changes in gene expression in articular cartilage tissue during OA development, we extracted RNA from articular cartilage tissue of 1-month-old TGFβRIICol2ER mice and found that expression of Mmp13, Adamts5, Adamts4, Runx2, Alp, Oc, and Col10 increased significantly (Figure 2F), which suggested that the chondrocyte differentiation process was accelerated in TGFβRIICol2ER mice. Consistent with these findings, increased type X collagen protein levels were also detected in the superficial zone of articular cartilage in 3-month-old TGFβRIICol2ER mice (Figure 2G). In contrast, no significant changes were found in the growth plate cartilage of 3- and 6-month-old TGFβRIICol2ER mice (Figure 2H). These results indicate that postnatal TGFβ signaling in articular chondrocytes plays a critical role in maintaining normal articular cartilage function. Endogenous TGFβ signaling in articular chondrocytes prevents chondrocyte hypertrophy. This unique feature distinguishes articular chondrocytes from growth plate chondrocytes.
Mmp13 and Adamts5 up-regulation is mediated by RUNX-2 in TGFβRIICol2ER mice
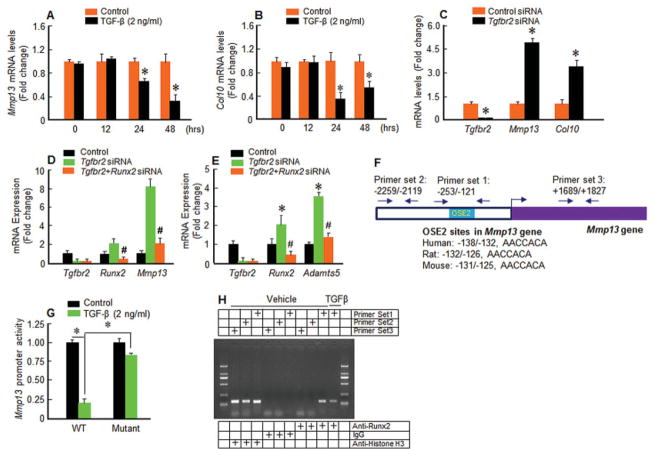
The mechanism of TGFβ regulation of Mmp13 expression was investigated using an in vitro cell culture system. Treatment with TGFβ significantly inhibited Mmp13 and Col10 expression in rat chondrosarcoma cells at the 24- and 48-hour time points (Figures 3A and B). In contrast, transfection of Tgfbr2 siRNA up-regulated Mmp13 and Col10 expression in rat chondrosarcoma cells (Figure 3C). Cotransfection of Runx2 siRNA significantly inhibited the stimulatory effects of Tgfbr2 siRNA on Mmp13 and Adamts5 expression (Figures 3D and E). We then cloned the 3.4-kb human Mmp13 promoter and identified a putative RUNX-2 binding site (osteoblast-specific element 2 [OSE-2]) located in the proximal region of the Mmp13 promoter; this binding site is conserved among humans, mice, and rats (Figure 3F). We found that treatment with TGFβ (2 ng/ml) significantly inhibited Mmp13 promoter activity. However, this inhibitory effect was largely prevented when the RUNX-2 binding site was mutated (Figure 3G). Using a ChIP assay, we further showed that RUNX-2 directly binds to its binding site (5′-PyGPyGGTPy-3′) at the rat Mmp13 promoter in rat chondrosarcoma cells, and addition of TGFβ suppressed RUNX-2 binding to this OSE-2 site (Figure 3H). These findings suggest that RUNX-2 may mediate the increase in Mmp13 expression that occurs with impaired TGFβ signaling.
Figure 3.
Mmp13 up-regulation is mediated by a RUNX-2–dependent mechanism in transforming growth factor β receptor type II (TGFβRII)–conditional knockout (TGFβRIICol2ER) mice. A and B, Treatment with TGFβ (2 ng/ml) inhibited Mmp13 (A) and Col10 (B) expression at 24 and 48 hours in rat chondrosarcoma cells (* = P < 0.05 versus control, by Student’s unpaired t-test; n = 3 wells per group). C, Transfection of Tgfbr2 small interfering RNA (siRNA) up-regulated Mmp13 and Col10 expression in rat chondrosarcoma cells (* = P < 0.05 versus control siRNA, by Student’s unpaired t-test; n = 3 wells per group). D and E, Transfection of Runx2 siRNA inhibited Tgfbr2 siRNA–induced Mmp13 (D) and Adamts5 (E) expression in rat chondrosarcoma cells (# = P < 0.05 versus Tgfbr2 siRNA alone; * = P < 0.05 versus control, by Student’s unpaired t-test). F, Shown are locations and sequences of osteoblast-specific element 2 (OSE-2) sites in the Mmp13 promoter. G, Rat chondrosarcoma cells were transfected with 3.4-kb wild-type (WT) or mutant (OSE-2 site mutation) human Mmp13 promoter and treated with TGFβ. TGFβ significantly inhibited Mmp13 promoter activity, and the inhibitory effect of TGFβ on the Mmp13 promoter was prevented when rat chondrosarcoma cells were transfected with the mutant Mmp13 promoter (* = P < 0.05 by Student’s unpaired t-test; n = 3 wells per group). H, Chromatin immunoprecipitation assay was performed using digested chromatin from rat chondrosarcoma cells that were either left untreated or treated with TGFβ (2 ng/ml). Purified DNA was subjected to polymerase chain reaction using Mmp13-specific primer set 1 (−253/−121), which amplifies the fragment spanning the RUNX-2 binding sequence, or the negative control primer set 2 (−2259/−2119) or the positive control primer set 3 (+1689/+1827). Values in A–E and G are the mean ± SD.
TGFβ regulates RUNX-2 degradation via up-regulation of CDK-4
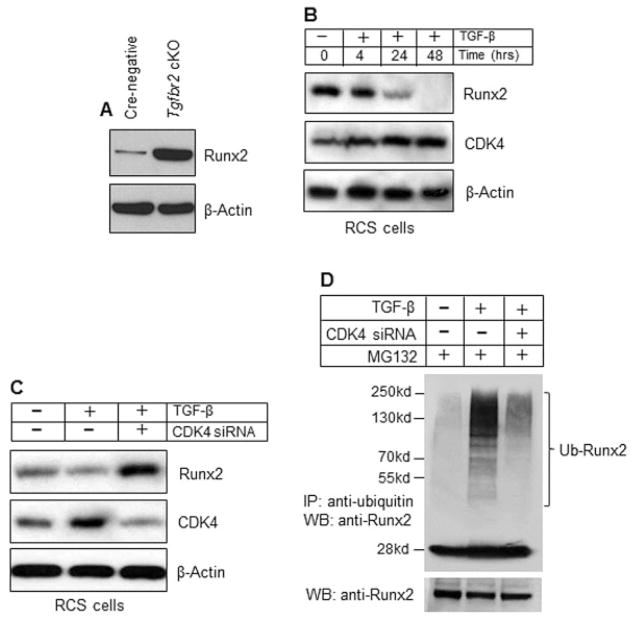
In chondrocytes of TGFβRIICol2ER mice, Runx2 messenger RNA (mRNA) levels were increased 2–3-fold (Figure 2F). However, RUNX-2 protein levels were increased >8-fold in articular chondrocytes of TGFβRIICol2ER mice (Figure 4A). These findings suggest that RUNX-2 protein stability was regulated by TGFβ signaling in articular chondrocytes. In previous studies we showed that TGFβ stimulated cyclin D1 expression in chondrocytes (31), and cyclin D1 and CDK-4 induce RUNX-2 phosphorylation and subsequent ubiquitination and proteasome degradation (28). In the present study, we found that TGFβ up-regulated CDK-4 expression while it down-regulated RUNX-2 protein levels (Figure 4B). TGFβ-mediated down-regulation of RUNX-2 protein levels could be prevented by transfection of CDK4 siRNA in rat chondrosarcoma cells (Figure 4C). Consistently, treatment with TGFβ enhanced RUNX-2 ubiquitination in rat chondrosarcoma cells, and this effect was significantly prevented by the transfection of CDK4 siRNA (Figure 4D). Based on these findings, we hypothesize that up-regulation of Mmp13 expression in TGFβRIICol2ER mice was mediated by increased RUNX-2 protein stability through a CDK-4–dependent mechanism. These findings suggest that TGFβ signaling may regulate downstream target genes such as Mmp13 and Adamts5 through activation of RUNX-2 in chondrocytes.
Figure 4.
Transforming growth factor β (TGFβ) enhances RUNX-2 degradation through cyclin-dependent kinase 4 (CDK-4)–mediated ubiquitination. A, Cell lysates were extracted from primary articular chondrocytes isolated from 1-month-old TGFβ receptor type II (TGFβRII)–conditional knockout (cKO) (TGFβRIICol2ER) mice and their Cre-negative littermates. Western blot (WB) analysis showed that RUNX-2 protein levels were significantly increased (>8-fold) in articular chondrocytes from TGFβRIICol2ER mice. B, Rat chondrosarcoma (RCS) cell cultures were treated with TGFβ (2 ng/ml) for 4, 24, and 48 hours, and Western blot analysis was performed. Treatment with TGFβ reduced RUNX-2 protein levels and increased CDK-4 expression in rat chondrosarcoma cells. C, Rat chondrosarcoma cells were transfected with CDK4 small interfering RNA (siRNA) and treated with TGFβ (2 ng/ml). Western blot analysis showed that inhibition of CDK-4 expression enhanced RUNX-2 protein levels in rat chondrosarcoma cells. D, RUNX-2 ubiquitination (Ub) assay was performed using rat chondrosarcoma cells transfected with CDK4 siRNA and treated with TGFβ. The proteasome inhibitor MG132 (10 μM) was added to the cell culture 4 hours before cell lysates were collected. Ubiquitinated proteins were immunoprecipitated (IP) using a UbiQapture kit, and polyubiquitinated RUNX-2 was detected using an anti–RUNX-2 antibody. Treatment with TGFβ significantly enhanced RUNX-2 ubiquitination, and knocking down CDK4 expression significantly prevented RUNX-2 ubiquitination induced by TGFβ.
Inhibition of MMP-13 prevents the OA-like phenotype observed in TGFβRIICol2ER mice
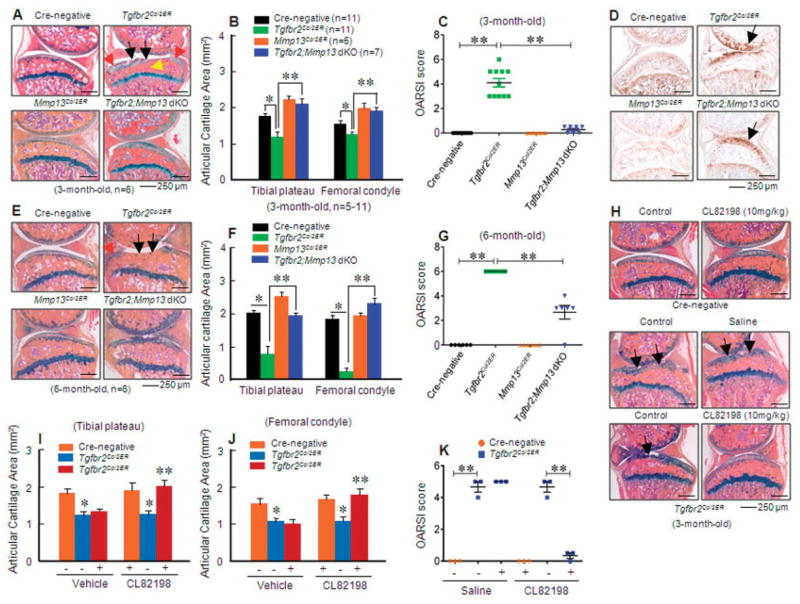
To determine the role of Mmp13 and Adamts5 in OA development in TGFβ βRIICol2ER mice, we bred TGFβRIICol2ER mice with MMP-13flox/flox mice (32) or ADAMTS-5−/− mice (26) and produced Col2-CreER/TGFβRIIflox/flox/MMP-13flox/flox– or Col2-CreER/TGFβRIIflox/flox/ADAMTS-5–double-KO mice. Histologic examination revealed that deletion of the Mmp13 gene in TGFβRIICol2ER mice significantly ameliorated articular cartilage defects observed in 3- and 6-month-old TGFβRIICol2ER mice. The findings included reduced loss of articular cartilage and a decrease in subchondral sclerosis and osteophyte formation (Figures 5A and E). Histomorphometric analysis further demonstrated a significant increase in pro-teoglycan levels and restoration of articular cartilage morphology when the Mmp13 gene was deleted in 3- and 6-month-old double-KO mice (Figures 5B and F). Deletion of the Mmp13 gene had no significant effect on type X collagen expression in 3-month-old TGFβRII/MMP-13–double-KO mice (Figure 5D). These results suggest that different features of the OA-like phenotype may be regulated by distinct molecular mechanisms.
Figure 5.
Mmp13 deletion prevents the osteoarthritis (OA)–like phenotype observed in transforming growth factor β receptor type II (TGFβRII)–conditional knockout (TGFβRIICol2ER) mice. A and E, Alcian blue/hematoxylin and eosin staining was performed in 3-month-old (A) and 6-month-old (E) mice. TGFβRIICol2ER mice displayed a phenotype like that of progressive OA. Deletion of the Mmp13 gene in TGFβRIICol2ER mice prevented their OA-like phenotype, including articular cartilage degradation (black arrows), osteophyte formation (red arrows), and subchondral sclerosis (yellow arrow). B, C, F, and G, Analysis using histomorphometry (B and F) and the histologic scoring system recommended by the Osteoarthritis Research Society International (OARSI) (C and G) showed that Mmp13 deletion in 3- and 6-month-old TGFβRIICol2ER mice protected against articular cartilage degradation induced by the loss of TGFβ signaling (* = P < 0.05; ** = P < 0.01 by two-way analysis of variance [ANOVA] followed by Tukey’s test). D, There were no significant changes in type X collagen protein levels (arrows) between TGFβRIICol2ER mice and TGFβRII/matrix metalloproteinase 13 (MMP-13)–double-knockout (dKO) mice. H–J, The MMP-13 inhibitor CL82198 (10 mg/kg) was injected into TGFβRIICol2ER mice. Histologic examination (H) and histomorphometric analyses (I and J) showed that the MMP-13 inhibitor protected against articular cartilage degradation observed in TGFβRIICol2ER mice (* = P < 0.05 versus Cre-negative control; ** = P < 0.05 versus TGFβRIICol2ER mice receiving vehicle, by two-way ANOVA followed by Tukey’s test). Arrows in H indicate loss of cartilage tissue. K, Analysis using the OARSI scoring system showed that the MMP-13 inhibitor slowed articular cartilage degeneration in 3-month-old TGFβRIICol2ER mice (** = P < 0.01 by two-way ANOVA followed by Tukey’s test). Values in B, C, F, G, and I–K are the mean ± SD. In C, G, and K, symbols represent individual mice. MMP-13Col2ER = MMP-13 knockout.
The articular cartilage areas in MMP-13Col2ER mice (Figures 5B and F) and double-KO mice (Figures 5B and F) were significantly increased compared to those in Cre-negative control mice, which suggests that under normal conditions there is cartilage tissue turnover induced by MMP-13, so that deletion of the Mmp13 gene could lead to higher articular cartilage volume. We also analyzed the data using the histologic scoring system recommended by the OARSI, and we found that deletion of the Mmp13 gene was significantly chondro-protective in 3- and 6-month-old TGFβRIICol2ER mice (Figures 5C and G).
To further determine the role of MMP-13 in OA development in TGFβRIICol2ER mice, we investigated the efficacy of the MMP-13 inhibitor CL82198 (33) in reversing the OA-like phenotype of these mice. Immediately following injection of tamoxifen, CL82198 (10 mg/kg) was injected IP every other day into TGFβRIICol2ER mice. Knee joint tissues were harvested at age 3 months, and the effect of CL82198 on the articular cartilage phenotype was examined histologically. The loss of articular cartilage tissues seen in TGFβRIICol2ER mice was significantly ameliorated by treatment with the MMP-13 inhibitor (Figure 5H). Consistent with the histologic findings, histomorphometric analysis confirmed that articular cartilage areas at both the tibial plateau and femoral condyle were significantly increased by treatment with the MMP-13 inhibitor compared to the vehicle control (Figures 5I and J). Analysis using the histologic scoring system recommended by the OARSI showed that the MMP-13 inhibitor effectively slowed histologic damage observed in TGFβRIICol2ER mice (Figure 5K). These results suggest that MMP-13 could be a potential target of drugs for the treatment of OA.
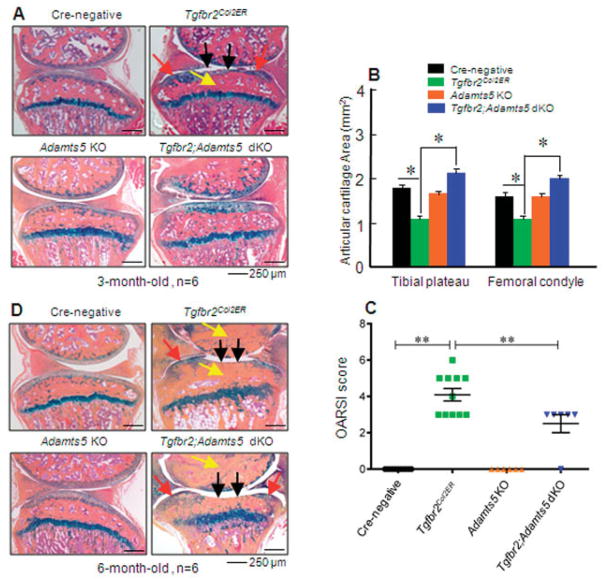
Deletion of the Adamts5 gene prevents early-stage OA development in TGFβRIICol2ER mice
The articular cartilage defects observed in TGFβRIICol2ER mice could also be prevented in 3-month-old TGFβRII/ADAMTS-5–double-KO mice following deletion of the Adamts5 gene (Figures 6A and B). Analysis using the histologic scoring system recommended by the OARSI yielded a similar result. Deletion of the Adamts5 gene was significantly chondroprotective in 3-month-old TGFβRIICol2ER mice (Figure 6C). However, deletion of the Adamts5 gene had no significant effect on articular cartilage defects observed in 6-month-old TGFβRIICol2ER mice (Figure 6D). These results suggest that although both Mmp13 and Adamts5 are downstream target genes of TGFβ signaling in articular chondrocytes, Mmp13 may have a more significant and prolonged effect on OA development in TGFβRIICol2ER mice. In this study, we found that deletion of the Mmp13 or Adamts5 gene not only inhibited articular cartilage degradation but also prevented defects of subchondral sclerosis and osteophyte formation observed in TGFβRIICol2ER mice.
Figure 6.
Deletion of the Adamts5 gene partially prevents the osteoarthritis (OA)–like phenotype observed in transforming growth factor β receptor type II (TGFβRII)–conditional knockout (KO) (TGFβRIICol2ER) mice. A and D, Histologic examination of knee joint tissues dissected from 3-month-old (A) and 6-month-old (D) mice was performed. Alcian blue/hematoxylin and eosin staining demonstrated that 3- and 6-month-old TGFβRIICol2ER mice displayed progressive development of OA. The OA-like phenotype induced by the loss of TGFβ signaling was completely prevented in 3-month-old TGFβRIICol2ER mice with deletion of the Adamts5 gene (TGFβRII/ADAMTS-5–double-KO [dKO] mice), but there was no effect in 6-month-old TGFβRII/ADAMTS-5–double-KO mice. Black arrows indicate loss of articular cartilage; red arrows indicate osteophyte formation; yellow arrows indicate subchondral sclerosis. B, Histomorphometric analysis showed that articular cartilage areas in the tibial plateau and femoral condyle were decreased in TGFβRIICol2ER mice compared to Cre-negative control mice (* = P < 0.05 by two-way analysis of variance [ANOVA] followed by Tukey’s test). In contrast, deletion of the Adamts5 gene in 3-month-old TGFβRIICol2ER mice completely protected against this articular cartilage degradation (* = P < 0.05 by two-way ANOVA followed by Tukey’s test). C, Analysis using the histologic scoring system recommended by the Osteoarthritis Research Society International (OARSI) showed that deletion of the Adamts5 gene in 3-month-old TGFβRIICol2ER mice protected against articular cartilage loss induced by the loss of TGFβ signaling (** = P < 0.01 by two-way ANOVA followed by Tukey’s test). Values in B and C are the mean ± SD. In C, symbols represent individual mice.
DISCUSSION
Although dn-TGFβRII–transgenic mice and Smad3-KO mice have already been shown to have an OA-like phenotype (10,29,34), it remains unclear whether TGFβ signaling plays a critical role in postnatal OA development, since in dn-TGFβRII–transgenic mice and Smad3-KO mice, TGF β signaling was inhibited at an early embryonic stage. The Col2-CreER–transgenic mouse model is a valuable tool, allowing chondrocyte-specific gene targeting in an inducible manner (22,23,35). In the present study, the Tgfbr2 gene was deleted specifically in chondrocytes at the postnatal stage. TGFβRIICol2ER mice exhibit a phenotype similar to that of human OA, including increased chondrocyte hypertrophy observed in the superficial zone of articular cartilage, tears and clefts in the articular surface, severe loss of articular cartilage tissue, osteophyte formation at the margins of cartilage tissue, especially at the late stage of disease (in 6-month-old mice), and subchondral sclerosis.
It is interesting to note that inhibition of TGFβ signaling at the postnatal stage leads to articular cartilage damage and a severe OA–like phenotype, while the morphology of growth plate cartilage is relatively normal. In the present study, we induced Tgfbr2 gene deletion at age 2 weeks to compare roles of TGFβ signaling in both articular and growth plate chondrocytes. We found that deletion of the Tgfbr2 gene in chondrocytes at the postnatal stage (in 2-week-old mice) led to a severe OA–like phenotype in articular cartilage but had no significant effect on growth plate cartilage, which suggests that TGFβ signaling plays a specific role in maintaining integrity of articular cartilage at the postnatal stage. To further determine changes in growth plate cartilage development, we also performed gene expression analysis using primary sternal chondrocytes isolated from Cre-negative and TGFβRIICol2ER mice. We found that expression of chondrocyte marker genes such as Runx2, Alp, Oc, Mmp13, and Col10 was not significantly changed (data not shown). We also found that there was no significant change in type X collagen protein expression in growth plate chondrocytes from TGFβRIICol2ER mice. This finding suggests that TGFβ signaling may play a specific role in maintaining integrity of articular cartilage at the postnatal and adult stages and in preventing abnormal differentiation of articular chondrocytes. This unique feature distinguishes articular chondrocytes from growth plate chondrocytes.
In the present study, we demonstrated that Mmp13 mRNA levels were significantly up-regulated in TGFβRIICol2ER mice. Our in vitro studies demonstrated that inactivation of TGFβ signaling stimulates Mmp13 gene transcription in a RUNX-2–dependent manner. Deletion of the Mmp13 gene significantly prevented the articular cartilage destruction observed in TGFβRIICol2ER mice. Treatment of TGFβRIICol2ER mice with an MMP-13 inhibitor also significantly inhibited articular cartilage degradation. While the MMP-13 inhibitor used in this study has nonspecific inhibitory effects on other MMPs, our studies suggest that MMP-13 inhibition is a possible therapeutic strategy for the treatment of OA. Although deletion of the Adamts5 gene did not protect against articular cartilage degradation and other features of OA observed in 6-month-old TGFβRIICol2ER mice, deletion of the Adamts5 gene fully prevented the articular cartilage defects observed in 3-month-old TGFβRIICol2ER mice. These results further suggest that up-regulation of Adamts5 may play an important role in the development of early-stage OA in TGFβRIICol2ER mice.
While deletion of the Mmp13 gene significantly prevented the degradation of articular cartilage observed in TGFβRIICol2ER mice, the rescue was incomplete. These findings are consistent with those in a study in which MMP-13Col2ER mice were subjected to meniscal injury (36). There are 2 possible reasons for these findings. First, the Cre recombination efficiency mediated by Col2-CreER is ~85% in articular chondrocytes; thus, MMP-13 activity in the other 15% of chondrocytes remains normal in TGFβRII/MMP-13–double-KO mice. Second, it is known that deletion of Adamts4 and Adamts5 genes protects against OA development (19–21), and deletion of the Adamts5 gene may be compensated for by ADAMTS-4, a member of the same family. In exploring the roles of both collagenase and aggrecanase in OA development in TGFβRIICol2ER mice, further investigation is required to determine if double deletion of the Mmp13 and Adamts5 genes could fully protect against the OA-like phenotype observed in TGFβRIICol2ER mice.
Deletion of the Mmp13 gene significantly prevented several features of OA observed in TGFβRIICol2ER mice, such as articular cartilage degradation, osteophyte formation, and subchondral sclerosis. However, Mmp13 deletion failed to inhibit chondrocyte hypertrophy, since type X collagen was still highly expressed in the superficial zone of articular cartilage in the TGFβRII/MMP-13–double-KO mice. This is probably because the Mmp13 gene is downstream of RUNX-2. Most hypertrophic chondrocyte marker genes are induced by the Runx2 gene, which is independent of Mmp13 gene expression. It would be interesting to determine whether deletion of the Runx2 gene completely prevents the OA-like phenotype observed in TGFβRIICol2ER mice.
The current study shows that TGFβ signaling regulates the expression of genes that are critical in the maintenance of the articular cartilage matrix. In the absence of TGFβ signaling, Mmp13 and Adamts5 are induced and lead to articular cartilage tissue degeneration and the development of OA. However, it is still not known how TGFβ signaling is reduced or inactivated during the development of OA in patients. Potential causes of TGFβ signaling inactivation in articular chondrocytes include a loss-of-function mutation in the Tgfbr2 gene or mutation of other genes responsible for mediating TGFβ signaling, such as Smad3. There are several lines of evidence that mechanical loading leads to inactivation of TGFβ signaling in bone cells (37). Using immunohistochemistry, we recently demonstrated that levels of TGFβRII and phosphorylated Smad3 were significantly down-regulated in a mouse model of OA induced by meniscus injury (results not shown), which suggests that 1 potential mechanism of meniscus injury–induced OA may be mediated by down-regulation of TGFβ signaling in articular chondrocytes. Finally, tumor necrosis factor α and interleukin-1β are 2 important proinflammatory cytokines involved in the development of OA. Recent studies demonstrate that these cytokines inhibit TGFβ/Smad signaling in other cell types (37–39). Since many other signaling pathways and transcription factors are involved in OA development, it would be interesting to investigate the interaction of TGFβ signaling with other signaling pathways during OA development, such as the Wnt/β-catenin, IHH, and HIF-2α pathways.
Previous studies demonstrate that TGFβ signaling has different effects on cells at various stages of differentiation. In mesenchymal progenitor cells, TGFβ promotes chondrogenesis (40,41). However, in chondrocytes, TGFβ inhibits differentiation and hypertrophy (42,43). It would be interesting to know whether inhibition of TGFβ signaling in mesenchymal progenitor cells leads to a phenotype similar to the one we observed in the current study. This question could be addressed by generating and analyzing Prx1-CreER/TGFβRIIflox/flox mice or Nestin-CreER/TGFβRIIflox/flox mice. It has been reported that exogenous application of TGFβ may cause fibrosis and osteophyte formation (36,44). In the present study, we found that deletion of the Tgfbr2 gene does not cause synovial fibrosis and does lead to slight osteophyte formation. A possible explanation for this discrepancy is that we have selectively deleted the Tgfbr2 gene in articular chondrocytes. However, exogenous application of TGFβ could nonspecifically target other tissues in the joint.
In the present study, we induced Tgfbr2 gene deletion in 2-week-old mice. Since mice are still growing at this stage, which may affect articular cartilage structure, further investigation is still required using deletion of the Tgfbr2 gene in mice 2 months of age or even older. Using Col2-CreER– and Aggrecan-CreER–transgenic mice, we are currently testing whether deletion of the Tgfbr2 gene at different ages of adult mice (2, 4, and 6 months) will also produce an OA-like phenotype. These studies will help us determine the effect of Tgfbr2 gene deletion on OA development and progression in adult mice.
Supplementary Material
Acknowledgments
Supported by the NIH (grants AR-055915 and AR-054465 to Dr. Chen). Dr. Jin’s work was supported in part by the National Natural Science Foundation of China (grant 81202710).
We gratefully acknowledge the technical expertise of Ryan Tierney and Sarah Mack of the Center for Musculo-skeletal Research Histology Core at the University of Rochester.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Im, O’Keefe, Chen.
Acquisition of data. Shen, Li, B. Wang, Jin, M. Wang, Im, Chen.
Analysis and interpretation of data. Shen, Zhang, Yang, Im, O’Keefe, Chen.
References
- 1.Felson DT. Osteoarthritis of the knee [published erratum appears in N Engl J Med 2006;354:2520] N Engl J Med. 2006;354:841–8. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 2007;65:222–8. [PubMed] [Google Scholar]
- 4.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. Modulating Hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–5. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678–86. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–93. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-β superfamily signalling. Genes Cells. 2002;7:1191–204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 10.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, et al. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–52. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM, et al. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum. 2010;62:2347–52. doi: 10.1002/art.27530. [DOI] [PubMed] [Google Scholar]
- 12.Van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 13.Van de Laar IM, van der Linde D, Oei EH, Bos PK, Bessems JH, Bierma-Zeinstra SM, et al. Phenotypic spectrum of the SMAD3-related aneurysms–osteoarthritis syndrome. J Med Genet. 2012;49:47–57. doi: 10.1136/jmedgenet-2011-100382. [DOI] [PubMed] [Google Scholar]
- 14.Han EH, Chen SS, Klisch SM, Sah RL. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J. 2011;101:916–24. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–45. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–24. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 17.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, et al. Expression and regulation of aggrecanase in arthritis: the role of TGF-β. J Immunol. 2002;168:1405–12. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- 19.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–4. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- 21.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45:44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreERT2 transgenic mice. Osteoarthritis Cartilage. 2008;16:129–30. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–8. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-β type II receptor using Cre:Lox. Genesis. 2002;32:73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 26.McCulloch DR, Le Goff C, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metallo-protease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009;9:314–23. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Wei XC, Zhu TH, Zhang M, Shen R, Xing LP, et al. Bone morphogenetic protein 2 activates Smad6 gene transcription through bone-specific transcription factor Runx2. J Biol Chem. 2007;282:10742–8. doi: 10.1074/jbc.M610997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen R, Chen M, Wang YJ, Kaneki H, Xing L, O’Keefe RJ, et al. Smad6 interacts with Runx2 and mediates Smad ubiquitin regulatory factor 1-induced Runx2 degradation. J Biol Chem. 2006;281:3569–76. doi: 10.1074/jbc.M506761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Xie R, Hou W, Wang B, Shen R, Wang X, et al. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122:1382–9. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 (Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Chen M, Wu Q, Sheu T, Drissi H, Zuscik M, et al. TGF-β activates cyclin D1 expression through a Smad3/β-catenin-dependent mechanism in chondrocytes. J Bone Miner Res. 2006;21:S91. [Google Scholar]
- 32.Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–95. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez Rios M, Sorsa T, Obregon F, Tervahartiala T, Valen-zuela MA, Pozo P, et al. Proteolytic roles of matrix metalloproeinase (MMP)-13 during progression of chronic periodontitis: initial evidence for MMP-13/MMP-9 activation cascade. J Clin Periodontol. 2009;36:1011–7. doi: 10.1111/j.1600-051X.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen CG, Thuillier D, Chin EN, Alliston T. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;64:3278–89. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13–deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–33. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-β (TGFβ) and the TGFβ signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–21. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verrecchia F, Pessah M, Atfi A, Mauviel A. Tumor necrosis factor-α inhibits transforming growth factor-β/Smad signaling in human dermal fibroblasts via AP-1 activation. J Biol Chem. 2000;275:30226–31. doi: 10.1074/jbc.M005310200. [DOI] [PubMed] [Google Scholar]
- 38.Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, et al. A mechanism of suppression of TGF-β/SMAD signaling by NF-κB/RelA. Genes Dev. 2000;14:187–97. [PMC free article] [PubMed] [Google Scholar]
- 39.Seth RB, Chen ZJ. Smads keep TABs on inflammation. Nat Immunol. 2007;8:477–8. doi: 10.1038/ni0507-477. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, et al. Transforming growth factor-β1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132:1603–8. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, James AW, Longaker MT. Transforming growth factor-β1 stimulates chondrogenic differentiation of posterofrontal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:1649–59. doi: 10.1097/PRS.0b013e31818cbf44. [DOI] [PubMed] [Google Scholar]
- 42.Dong Y, Drissi H, Chen M, Chen D, Zuscik MJ, Schwarz EM, et al. Wnt-mediated regulation of chondrocyte maturation: modulation by TGF-β. J Cell Biochem. 2005;95:1057–68. doi: 10.1002/jcb.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Kraan PM, Blaney Davidson EN, Blom A, van den Berg WB. TGF-β signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17:1539–45. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-β and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.