Inhibition of β-catenin signaling with overexpression of ICAT driven by the Col2a1 romoter resulted in dysplastic caudal vertebrae and angulated deformities in mouse tails. The poorly developed sclerotomes failed to resegment and to form complete caudal vertebrae.
Keywords: β-catenin, ICAT, chondrocyte, caudal vertebral dysplasia
Abstract
Study Design.
To inhibit β-catenin specifically signaling in chondrocytes Col2-ICAT transgenic mice were generated. Anomalies in caudal vertebrae were detected during embryonic and postnatal stages of Col2-ICAT transgenic mice.
Objective.
To determine the role of canonical β-catenin signaling in caudal vertebral development.
Summary of Background Data.
β-catenin signaling plays a critical role in skeletal development. Col2-ICAT transgenic mice were generated to selectively block β-catenin signaling by overexpression of the ICAT gene in chondrocytes.
Methods.
Tails of E16.5 transgenic embryos and adult Col2-ICAT transgenic mice and their wild-type littermates were collected and analyzed. Skeletal preparation, 3-dimensional micro-computed tomographic and histological analyses were performed to evaluate changes in the structure of caudal vertebrae. Bromodeoxyuridine labeling was performed to evaluate changes in chondrocyte proliferation in caudal vertebrae.
Results.
Skeletal preparation and 3-dimensional micro-computed tomographic analyses revealed bone deformation and angulated deformities in tail tissue in Col2-ICAT transgenic mice. Histological studies revealed abnormal bone development and dysplastic caudal vertebrae in Col2-ICAT transgenic mice. Inhibition of β-catenin signaling in cartilage resulted in vertebral dysplasia leading to aberrant resegmenting process. Thus, 2 poorly developed sclerotomes failed to fuse to form a complete vertebrae. BrdU labeling revealed a decreased chondrocyte proliferation in both cartilageous templates of transgenic embryos and the growth plate of adult Col2-ICAT transgenic mice.
Conclusion.
Wnt/β-catenin signaling plays an important role in vertebral development. Inhibition of β-catenin signaling in chondrocytes results in caudal vertebra deformity in mice, which may occur as early as in the stage of sclerotome formation.
Level of Evidence: N/A
The metameric vertebral columns develop from the somites, the repeated segmental units in the paraxial mesoderm. Sometogenesis in the mouse embryo initiates with the generation of presumptive somatic mesoderm at the primitive streak and the tail-bud mesenchyme. Somites, which are blocks of mesodermal cells, are formed in a coordinated craniocaudal sequence from the paraxial mesoderm.1–3 Mesenchymal cells in the caudal somites then differentiate into chondrocytes with subsequent formation of a cartilaginous template. Endochondral ossification of such a template eventually leads to the formation of caudal vertebrae.4–6
In the mouse embryo, mesodermal formation begins at embryonic day 6.5 (E6.5).7
The sequential formation of somites along the anterior-posterior axis is under control of multiple signaling gradients involving the Wnt (wingless-related MMTV integration site), fibroblast growth factor, and retinoic acid pathways.8 The Wnt family of signaling molecules have been detected in the primitive streak and tail-bud mesenchyme. Among the Wnt family members detected, Wnt3a is expressed in cells fated to give rise to embryonic mesoderm, from E6.5 on. A null mutation of Wnt3a leads to lack of caudal somites from the level of umbilicus at E12.5, have a disrupted notochord, and fail to form a tail bud.9
The canonical Wnt signaling pathway is mediated by β-catenin, which accumulates in the cytoplasm in the presence of Wnts, and translocates into the nucleus. In the nucleus, β-catenin interacts with a T cell-specific factor or a lymphoid enhancer factor (LEF) to activate the downstream target genes.10 Inhibition of β-catenin and T cell-specific factor (ICAT) is a small peptide that binds the armadillo repeats of β-catenin and interferes with the formation of the β-catenin and T cell-specific factor/LEF complex, thereby disrupting this pathway.11–13 ICAT expression starts at a very low level in the precartilaginous mesenchymal sclerotome, which coincides with the temporal and spatial expression pattern of Col2a1 mRNA during mouse embryonic development (at about E12).14–16
Wnt/β-catenin signaling also plays a role in the differentiation process of mesenchymal progenitors toward chondrocyte lineage. β-catenin is highly expressed in prechondrogenic mesenchymal cells but significantly decreased in differentiated chondrocytes.17 The canonical Wnt signaling represses chondrogenesis, and inactivation of β-catenin in mesenchymal progenitor cells induces chondrocyte differentiation under conditions allowing only osteoblasts to form in vitro.18,19 However, the findings are not always consistent. For example, Yano et al20 have shown that constitutively active LEF-1 in undifferentiated mesenchymal cells promoted chondrogenic differentiation, whereas downregulation of LEF-1 or silencing β-catenin suppressed the chondrocyte gene expression. The expression level of the Wnt mRNA was low in the resting and hypertrophic zones of the growth plate. In contrast, LEF-1 expression is much higher in proliferative and prehypertrophic zones.21 Inhibition of Wnt/β-catenin signaling or inactivation of β-catenin leads to reduced chondrocyte proliferation and increased chondrocyte apoptosis.22–24 Despite these findings, the role of the Wnt/β-catenin signaling pathway in resegmentation process remains largely undefined.
A previous study has demonstrated that cartilage-specific inhibition of β-catenin in the Col2-ICAT transgenic mice results in severe osteoarthritis-like phenotype.25 In this study, we aimed to investigate the role of β-catenin signaling in the development of caudal vertebrae.
MATERIALS AND METHODS
Col2-ICAT Transgenic Mice and Genotyping
The use of animals was approved by the Shanghai Laboratory Animal Use Committee. The Col2-ICAT transgenic mouse (C57BL/6J) was generated and reported before.24,25 The Flag-tagged ICAT (Flag-ICAT) complementary DNA includes the 5′ Nde I site Col2a1 promoter, β-globin intron cassette, SV40 poly (A), and Col2a1 enhancer. The generation of 2 separate lines of Col2-ICAT transgenic mice and their wild-type (WT) littermates were genotyped by polymerase chain reaction.23,24
Skeletal Preparation
Skeletal preparation was performed to compare possible differences between E16.5 Col2-ICAT transgenic and WT embryos (n = 6). The phenotype of 6-month-old Col2-ICAT transgenic mice and their WT littermates (n = 6) were also analyzed. The skin, viscera, and adipose tissue were carefully removed after they were killed. The whole skeletons were fixed in 95% ethanol for 2 to 5 days followed by fixation in acetone for another 1 to 2 days, and stained with 0.015% Alcian Blue and 0.005% Alizarin Red for 1 to 3 days. Images of the mouse skeletons were captured with a camera (Sony H10, Tokyo, Japan).
Three-Dimensional Reconstruction Analyses
The caudal vertebrae from 6-month-old Col2-ICAT transgenic mice (n = 6) and their WT littermates (n = 6) were dissected, and fixed in 4% paraformaldehyde overnight followed by washing for 2 hours. Three-dimensional reconstruction analyses were performed with a Micro-CT 80 scan machine (SCANO Medical AG, Bassersdorf, Switzerland). The deformed regions were first located with scout views of the whole caudal vertebrae. The abnormal bones and part of the neighboring vertebrae underwent fine scanning for 160 slices with 20-μm slice increments. The x-ray source voltage was 70 kVp, the source current was 114 μA, and the integration time was 400 ms. A reconstruction of the bitmap data set was used to build the 3-dimensional images.
Histological Evaluation
Tail samples from 6-month-old mice of both genotypes (WT and Col2-ICAT transgenic) were subjected to histological analysis with different staining methods to reveal the potential pathological changes. The caudal vertebrae of E16.5 and 6-month-old WT and Col2-ICAT transgenic mice were fixed in 4% paraformaldehyde, decalcified, dehydrated, and embedded in paraffin. Serial midsagittal sections (6-μm thick) of the caudal vertebrae were cut and stained with hematoxylin/eosin, the widely used staining method in histological diagnosis, and safranin O/fast green, a common staining method for cartilage and bone, respectively. A morphometric study was performed using a light microscope (Olympus B×50; Tokyo, Japan) with camera (Olympus DP71; Tokyo, Japan) and image analysis system (CMIAS-99B; Beijing, China).
BrdU Labeling and Staining
For adult mice, bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO) was intraperitoneally injected into 6-month-old WT and Col2-ICAT transgenic mice 1 day and 4 hours before they were killed (10 mg/mL, 100 mg/kg body weight). For E16.5 embryos, Col2-ICAT transgenic mice were bred with WT mice, and the pregnant mice were intraperitoneally injected with BrdU 2 hours before they were killed. The caudal vertebrae of adult mice and embryos were collected and fixed in 4% paraformaldehyde, decalcified, dehydrated, and embedded in paraffin. Serial midsagittal sections (6-μm thick) of the caudal vertebrae were cut and stained using a BrdU immunohistochemistry kit (Chemicon, Billerica, MA), for cell proliferation.
Statistical Analysis
Data were expressed as means ± standard error of the mean. An unpaired Student t test was performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered statistically significant.
RESULTS
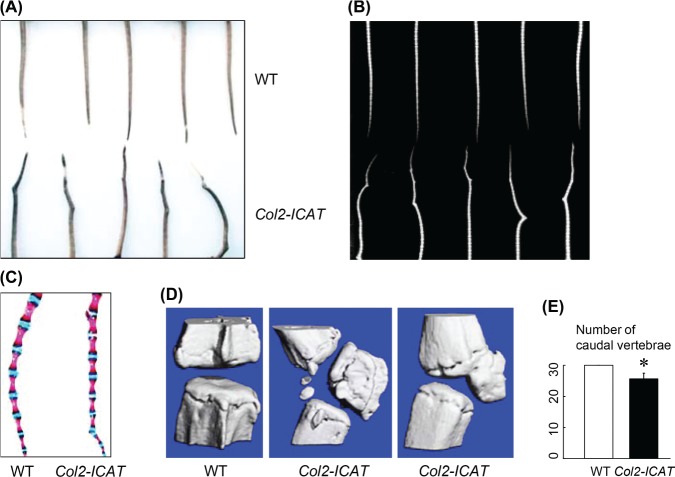
The mice were able to ambulate and move their tails, suggesting that the neural tube development had not been affected. We observed that all Col2-ICAT transgenic mice developed at least one angulated deformity in their distal tails (Figure 1A). Radiographical images showed an aberrant vertebral development, leading to reduced lengths of the caudal vertebrae (Figure 1B). Skeletal preparation and 3-dimensional (3D) reconstruction analyses showed the paraxial locations of the sesamoid-like bones in most transgenic mice. The deformed bones were either solitary or attached to neighboring vertebra, and in some cases, single or multiple bones with different sizes were dislocated on the opposite side (Figure 1C, D). All Col2-ICAT transgenic mice showed decreased numbers of the caudal vertebrae compared with their WT littermates (Figure 1E, unpaired Student t test, *P < 0.05, n = 6).
Figure 1.
Col2-ICAT transgenic mice display an aberrant vertebral development resulting in angulated deformity in tails as evidenced by macroscopic observation (A) and radiographic analysis (B). The results of whole-mount Alizarin red/Alcian blue staining showed abnormal bone formation in the tails of the Col2-ICAT transgenic mice (C). The results were further confirmed by micro-CT analysis (D). The quantitative data showed that numbers of caudal vertebrae of the Col2-ICAT transgenic mice were significantly reduced compared with the WT littermates (E). P < 0.05, unpaired Student t test (n = 6). WT indicates wild-type; CT, computed tomographic.
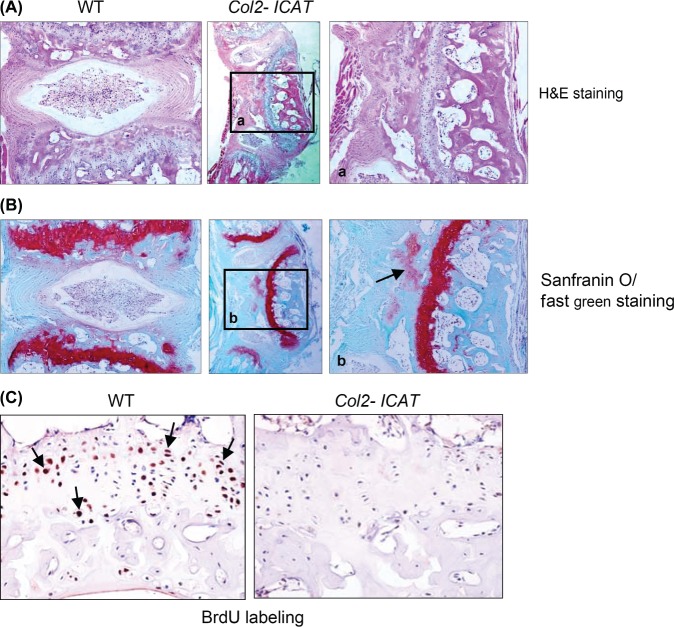
The abnormal bones displayed some histological characteristics of vertebrae, including cartilage endplate, endochondral ossification, and intervertebral disc tissue (nucleus pulposus and annulus fibrosus) between the abnormal bone and the consecutive vertebrae (Figure 2A, B). In some cases, sparse cartilaginous tissues were detected opposite to the abnormal vertebra, and ossification in such tissue was rare. BrdU labeling revealed a significant reduction in the number of positive cells in the growth plate chondrocytes of Col2-ICAT transgenic mice compared with that in the WT littermates (Figure 2C).
Figure 2.
Tail-tissue deformity of adult Col2-ICAT transgenic mice. Histological analysis with H&E (A) and safranin O/fast green (B) staining demonstrated the dysplasia of caudal vertebrae in Col2-ICAT transgenic mice compared with their WT littermates. Disorganized bone due to vertebral dysplasia exhibits a typical cartilage endplate, endochondral ossification, and IVD-like structure between the abnormal bone and the adjacent vertebra. Sparse cartilaginous tissue was also found opposite to the abnormal vertebra with limited bone formation inside (black arrow). Right and left panels, ×100, middle panels, ×40. The results of BrdU labeling demonstrated that chondrocyte proliferation in growth plate of caudal vertebrae from the Col2-ICAT transgenic mice was significantly reduced compared with their WT littermates (×200, C). H&E indicates hematoxylin/eosin; IVD, intervertebral disc; BrdU, bromodeoxyuridine; WT, wild-type.
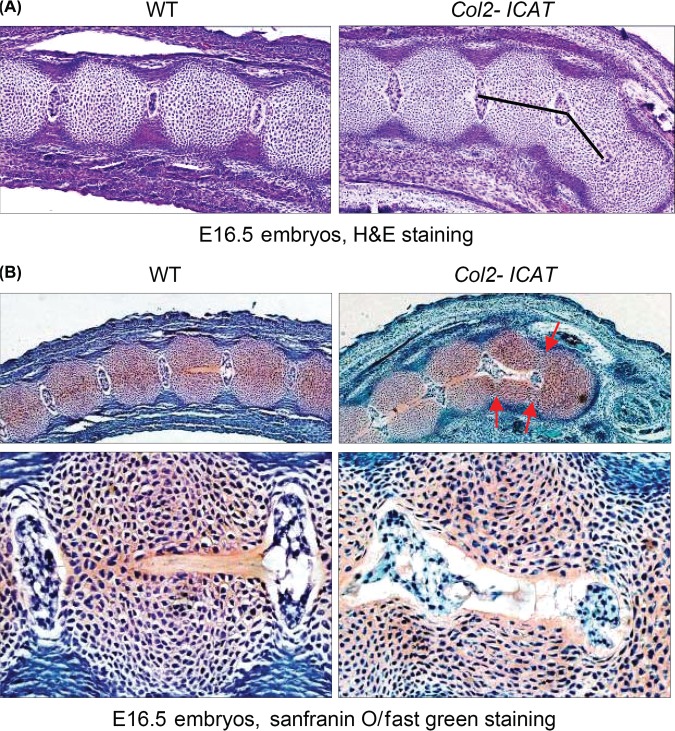
The E16.5 Col2-ICAT transgenic embryos developed macroscopic deformities in their tails (Figure 3A). Alcian blue staining of the caudal vertebrae showed that at the embryonic E16.5 stage, the cartilaginous templates have already formed although endochondral ossification has not yet been initiated (Figure 3B). The proliferation of the chondrocytes was reduced in the caudal vertebrae of Col2-ICAT transgenic embryos compared with that in WT littermates, as evidenced by significant lower numbers of BrdU positive cells (Figure 3C, D, unpaired Student t test, *P < 0.05, n = 6).
Figure 3.
Defects in the development of caudal vertebrae at early embryonic stage. Macroscopic deformities (the white arrows) in tails were observed in E16.5 Col2-ICAT transgenic embryos (A). The results of Alizarin red/Alcian blue staining showed angulated tail and ectopic caudal vertebrae of E16.5 Col2-ICAT transgenic embryos (B) (×100). Cell proliferation was reduced in cartilaginous template of caudal vertebrae of E16.5 Col2-ICAT transgenic embryos compared with WT embryos (×400, C). The BrdU positive cells were significantly decreased in Col2-ICAT transgenic embryos compared with wild-type controls (D). *P < 0.05, unpaired Student t test (n = 6). BrdU indicates bromodeoxyuridine; WT, wild-type.
A normal caudal vertebra is developed from 2 sclerotomes located on the 2 sides of the notochord, and these 2 sclerotomes become gradually fused in the middle. Histological examination demonstrated that the defective resegmentation of the caudal vertebrae in the Col2-ICAT transgenic embryos resulted in an axis excursion to 1 side leading to angle formation. The poorly developed semicolumn failed to fuse with the other half to form a complete vertebra, thereby prevent the proper translocation of the notochord to the nucleus pulposus (Figure 4A, B).
Figure 4.
Ectopic resegmentation and formation of caudal vertebrae were observed in Col2-ICAT transgenic embryos. Histological analyses showed that ectopic resegmentation (arrows), axis excursion (A) as well as poorly formed dysplastic caudal vertebrae were observed in Col2-ICAT transgenic embryos (B). A, ×100; B, upper panel, ×100; lower panel, ×400. H&E indicates hematoxylin/eosin; WT, wild-type.
DISCUSSION
Wnt/β-catenin/Axin2 signaling molecules have been reported to be involved in the oscillatory process for segmentation.26 However, their precise role in segmentation remains largely undefined. Abnormal development of the caudal vertebrae was observed in the mice with aberrant Wnt/β-catenin signaling. Wnt3a mutant mice display a complete absence of tail-bud development.9,27 In contrast, the mice with the gain-of-function mutation in low-density lipoprotein receptor-related protein 6 (Lrp6), a coreceptor of Wnt signaling, have a neural tube defect, leading to the formation of the crooked tails.28,29 Consistently, mice deficient of Dkk1 (Dickkopf 1), a secreted Wnt antagonist, display vertebral phenotypes, including tail kinks and vertebral fusion.30 Furthermore, inactivation of sFRP (Frizzled-related proteins) 1, 2, and 5, which are the secreted Wnt antagonists, induces the formation of fused somites in early mouse embryos.31 These findings demonstrate the importance of the balanced Wnt signaling in the normal development of caudal vertebrae.
In this study, we provide new evidence of the role of β-catenin signaling in the pathogenesis of vertebral dysplasia. Our results reveal a reduced proliferation of the chondrocytes isolated from the caudal vertebrae in Col2-ICAT transgenic mice. Specific inhibition of β-catenin signaling in type II collagen (Col2) expressing chondrocytes affects early vertebral development when sclerotomes form. We have described previously that the mesenchymal sclerotomes express a low level of Col2.23 Further, cartilage-specific inhibition of β-catenin in the Col2-ICAT transgenic mice results in severe osteoarthritis-like phenotype in some, but not all of Col2-ICAT transgenic mice.24 Interestingly, all Col2-ICAT transgenic mice have deformities in the caudal vertebra including angulated and/or shortened tails during embryonic development. Osteoarthritis pathogenesis differs from that of vertebral dysplasia in that it develops in adults, and is influenced by multiple factors such as activity level, body weight, and sex.
Our results suggest that the reduced chondrocyte proliferation in the endplates of the Col2-ICAT transgenic mice is the critical defect responsible for vertebral dysplasia. Because the tail deformities in the Col2-ICAT transgenic mice were noticed immediately after birth, we postulated that such deformities may result from defective vertebral development. Therefore, we examined changes in caudal vertebrae in transgenic embryos.
In addition to chondrocyte proliferation, mesenchymal differentiation toward chondrocyte lineage may also be affected by inhibited β-catenin signaling. Thus, the poorly developed sclerotomes cannot fuse with the opposite parts to form a complete vertebra. Instead, they gradually develop into sparse cartilage tissues with very limited or no endochondral ossification. The opposite parts of the sclerotome may continue to develop after fusion failure, eventually leading to the formation of an abnormal vertebral column in a paraxial location. The results suggest that abnormal bone formation in the Col2-ICAT transgenic mice is deformed caudal vertebrae, but not ectopic bone.
The abnormal changes are observed only in the tail region, and no developmental defects are detected in other regions of the spinal column such as cervical, thoracic, and lumbar columns. ICAT expression starts at about E12 at a very low level in the precartilaginous mesenchymal sclerotome, consistent with the temporal and spatial expression pattern of Col2a1 mRNA during mouse embryonic development. Because ICAT inhibition of β-catenin signaling is leaky and starts at a later stage than Wnt3a, which is detected as early as E6.5, the tail deformity described in this study is milder than that of Wnt3a mutation mice. The neural tube development was not affected in our Col2-ICAT transgenic mice, likely because the ICAT gene express at a later stage (E12), after completion of neural tube closure (E10, about 26–28 somites).32
CONCLUSION
Tissue-specific inhibition of β-catenin signaling in cartilage causes the deformities of caudal vertebrae in mice, suggesting that β-catenin signaling plays a critical role in the caudal vertebral development.
Key Points
Chondrocyte-specific inhibition of β-catenin signaling results in caudal vertebra dysplasia in mice.
Choncrocyte proliferation is decreased in both cartilageous templates of transgenic embryos and the growth plate of adult Col2-ICAT transgenic mice.
The poorly developed sclerotomes in Col2-ICAT transgenic mice failed to resegment and form regular caudal vertebrae.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
National Basic Research Program of China (973 Program, 2010CB530400); Key Program of Natural Science Foundation of China (30930111); National Institute of Health (NIH) (AR055915 and AR054465); Natural Science Foundation of Shanghai (12ZR1450400); Natural Science Foundation of China (81102604); and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, K08 HD049598) funds were received, in part, to support this work.
Relevant financial activities outside the submitted work: board membership, consultancy.
References
- 1.Flint OP, Ede DA, Wilby OK, et al. Control of somite number in normal and amputated mouse embryos: an experimental and a theoretical analysis. J Embryol Exp Morph 1978;45:189–202 [PubMed] [Google Scholar]
- 2.Alexander Aulehla and Olivier Pourquié. Signaling gradients during paraxial mesoderm development. Cold Spring Harb Perspect Biol 2010;2:a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam PPL. The control of somitogenesis in mouse embryos. J Embryol Exp Morph 1981;65:103–28 [PubMed] [Google Scholar]
- 4.Erlebacher A, Filvaroff EH, Gitelman SE, et al. Toward a molecular understanding of skeletal development. Cell 1995;80:371–8 [DOI] [PubMed] [Google Scholar]
- 5.Karsenty G. Genetics of skeletogenesis. Dev Genet 1998;22:301–13 [DOI] [PubMed] [Google Scholar]
- 6.Hall BK, Miyake T. Craniofacial development of avian and rodent embryos. Methods Mol Biol 2000;135:127–37 [DOI] [PubMed] [Google Scholar]
- 7.Snow MHL. Gastrulation in the mouse: regionalization of the epiblast. J Embryol Exp Morph 1977;42:293–303 [Google Scholar]
- 8.Aulehla A, Pourquié O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb Perspect Biol 2001;2:a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greco TL, Takada S, Newhouse MM, et al. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev 1996;10:313–24 [DOI] [PubMed] [Google Scholar]
- 10.Takada R, Hijikata H, Kondoh H, et al. Analysis of combinatorial effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and dishevelled phosphorylation. Genes Cells 2005;10:919–28 [DOI] [PubMed] [Google Scholar]
- 11.Tago K, Nakamura T, Nishita M, et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev 2000;14: 1741–9 [PMC free article] [PubMed] [Google Scholar]
- 12.Graham TA, Clements WK, Kimelman D, et al. The crystal structure of the beta-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Mol Cell 2002;10:563–71 [DOI] [PubMed] [Google Scholar]
- 13.Daniels DL, Weis WI. ICAT inhibits beta-catenin binding to TCF/LEF-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell 2002;10:573–84 [DOI] [PubMed] [Google Scholar]
- 14.Peters H, Wilm B, Sakai N, et al. Pax1 and Pax9 synergistically regulate vertebral column development. Development 1999;126:5399–408 [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Lu N, Eberspaecher H, et al. A new long form of c-Maf cooperates with Sox9 to activate the type II collagen gene. J Biol Chem 2002;277:50668–75 [DOI] [PubMed] [Google Scholar]
- 16.Kravis D, Upholt WB. Quantitation of type II procollagen mRNA levels during chick limb cartilage differentiation. Dev Biol 1985;108:164–72 [DOI] [PubMed] [Google Scholar]
- 17.Ryu JH, Kim SJ, Kim SH, et al. Regulation of the chondrocyte phenotype by beta-catenin. Development 2002;129:5541–50 [DOI] [PubMed] [Google Scholar]
- 18.Reinhold MI, Kapadia RM, Liao Z, et al. The Wnt-inducible transcription factor twist1 inhibits chondrogenesis. J Biol Chem 2006;281:1381–8 [DOI] [PubMed] [Google Scholar]
- 19.Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005;8:739–50 [DOI] [PubMed] [Google Scholar]
- 20.Yano F, Kugimiya F, Ohba S, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun 2005;333:1300–8 [DOI] [PubMed] [Google Scholar]
- 21.Andrade AC, Nilsson O, Barnes KM, et al. Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone 2007;40:1361–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone 2006;39:5–16 [DOI] [PubMed] [Google Scholar]
- 23.Akiyama H, Lyons JP, Mori-Akiyama Y, et al. Interactions between Sox9 and beta- catenin control chondrocyte differentiation. Genes Dev 2004;18:1072–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Zhu M, Awad H, et al. Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. J Cell Sci 2008;121:1455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu M, Chen M, Zuscik M, et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 2008;58:2053–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldbeter A, Pourquié OJ. Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways. Theor Biol 2008;252:574–85 [DOI] [PubMed] [Google Scholar]
- 27.Aulehla A, Wehrle C, Brand-Saberi B, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell 2003;4:395–406 [DOI] [PubMed] [Google Scholar]
- 28.Carter M, Chen X, Slowinska B, et al. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc Natl Acad Sci U S A 2005;102:12843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray JD, Nakouzi G, Slowinska-Castaldo B, et al. Functional interactions between the LRP6 WNT co-receptor and folate supplementation. Hum Mol Genet 2010;19:4560–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development 2004;131:2543–52 [DOI] [PubMed] [Google Scholar]
- 31.Satoh W, Matsuyama M, Takemura H, et al. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis 2008;46:92–103 [DOI] [PubMed] [Google Scholar]
- 32.Gray J, Ross ME. Neural tube closure in mouse whole embryo culture. J Vis Exp 2011;3132. [DOI] [PMC free article] [PubMed] [Google Scholar]