Summary
Interactions between commensals and the host impact the metabolic and immune status of metazoans. Their deregulation is associated with age-related pathologies like chronic inflammation and cancer, especially in barrier epithelia. Maintaining a healthy commensal population by preserving innate immune homeostasis in such epithelia thus promises to promote health and longevity. Here we show that in the aging intestine of Drosophila, chronic activation of the transcription factor Foxo reduces expression of Peptidoglycan Recognition Protein SC2 (PGRP-SC2), a negative regulator of IMD/Relish innate immune signaling, and homologue of the anti-inflammatory molecules PGLYRP1-4. This repression causes deregulation of Rel/NFkB activity, resulting in commensal dysbiosis, stem cell hyperproliferation, and epithelial dysplasia. Restoring PGRP-SC2 expression in enterocytes of the intestinal epithelium, in turn, prevents dysbiosis, promotes tissue homeostasis and extends lifespan. Our results highlight the importance of commensal control for lifespan of metazoans, and identify SC-class PGRPs as longevity-promoting factors.
Introduction
Loss of proliferative homeostasis and regenerative capacity in high-turnover tissues is a hallmark of aging, yet the causes of this decline are only beginning to be understood. Chronic inflammation is associated with this loss of homeostasis and with increased cancer incidence in aging organisms (Grivennikov et al., 2010). This is particularly significant in barrier epithelia like the intestinal epithelium, which mount frequent and required immune responses to pathogenic microorganisms, but also engage in mutually beneficial interactions with commensals that shape the host immune system and provide essential metabolic functions (Clemente et al., 2012; Hooper et al., 2012; Nicholson et al., 2012; Shin et al., 2011; Storelli et al., 2011). Changes in commensal populations (‘dysbiosis’) are associated with disorders like inflammatory bowel disease (IBD), autoimmune and allergic diseases, obesity and diabetes (Clemente et al., 2012), and potentially cause chronic inflammation and cancer (Kaser et al., 2010).
The effects of aging on the microbiota, and the consequences of microbiome changes on tissue homeostasis in the aging intestine remain unclear. Recent studies that have characterized gut microbiota composition in the elderly have found that the microbiota of older people is different and more diverse than that of younger adults (Claesson et al., 2011), and that microbiota composition in the elderly correlates significantly with measures of frailty and co-morbidity (Claesson et al., 2012). It has been proposed that the inflammation associated with dysbiosis promotes the development of various age-related diseases of the host, and that manipulating host/commensal interactions might be a viable avenue to promote healthy aging (Biagi et al., 2013; Ottaviani et al., 2011). Better mechanistic insight into the interactions between the microflora, innate immune responses, as well as regenerative processes in the intestinal epithelium is thus key (Clemente et al., 2012).
The Drosophila intestine is a productive model to characterize age-related changes in host-commensal interactions, innate immune signaling and regenerative capacity (Buchon et al., 2013; Chambers and Schneider, 2012). The intestinal epithelium is constantly being renewed in a process that mechanistically and morphologically resembles cell turnover in the intestinal epithelium of mammals (Buchon et al., 2013; Biteau et al., 2011a; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Intestinal Stem Cells (ISCs) are the only dividing cells in the intestinal epithelium and can give rise to at least two differentiated intestinal cell types: enteroendocrine cells (EEs) and enterocytes (ECs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). This lineage is critical for normal tissue turnover, as well as for epithelial recovery after damage or infection (Buchon et al., 2009a; Buchon et al., 2009b; Jiang et al., 2009), but is deregulated in the aging intestine, where ISC proliferation strongly increases, and polyploid, mis-differentiated cells accumulate in the epithelium (Biteau et al., 2008; Biteau et al., 2010; Buchon et al., 2009a). This dysplastic phenotype disrupts epithelial function, resulting in age-related metabolic decline in the whole fly, as well as loss of the intestinal barrier function (Biteau et al., 2010; Rera et al., 2011; Rera et al., 2012). Intestinal dysplasia influences Drosophila lifespan, as limiting the rate of ISC proliferation in the aging intestine is sufficient to extend lifespan (Biteau et al., 2010; Rera et al., 2011).
Control of ISC proliferation by microbiome and innate immune homeostasis
ISC proliferation rates are regulated by multiple stress and growth factor signaling pathways, including the JNK, Jak/Stat, p38 MAPK, and EGFR pathways, whose activity is influenced by the commensal microflora and by infection with pathogenic bacteria (Amcheslavsky et al., 2009; Apidianakis et al., 2009; Biteau et al., 2008; Biteau and Jasper, 2011; Biteau et al., 2010; Buchon et al., 2009a; Buchon et al., 2010; Buchon et al., 2009b; Chatterjee and Ip, 2009; Jiang et al., 2011; Jiang et al., 2009). In young flies, commensal bacteria maintain basal activation of JNK and Jak/Stat activities in ISCs, ensuring low levels of epithelial renewal (Buchon et al., 2009a). In older flies, however, this stimulation of JNK and JAK/Stat signaling becomes de-regulated, inducing the intestinal dysplasia described above. Accordingly, axenically aged flies have significantly fewer mitotic ISCs than conventionally reared animals, but the physiologic consequences of these changes have not yet been explored (Buchon et al., 2009a). Interestingly, the number of microorganisms found in the lumen of the gut significantly increases with age (Ren et al., 2007), suggesting that an age-related impairment in the ability to manage the intestinal microflora is the underlying cause of intestinal dysplasia, and may limit lifespan. The causes of this impairment remain unclear.
Management of the commensal flora and innate immune responses to pathogens are achieved primarily by two strategies acting in Drosophila ECs: Expression and activation of Dual Oxidase (Duox), which initiates an oxidative burst response, producing high levels of reactive oxygen species (ROS) (Ha et al., 2009a; Ha et al., 2009b; Ha et al., 2005), and activation of the immune deficiency (IMD/Relish) pathway, which activates the NFkB-like transcription factor Relish and promotes the expression of antimicrobial peptides (AMPs) (Buchon et al., 2009a; Leulier and Royet, 2009). Duox is regulated transcriptionally by a p38 MAPK signaling pathway and is activated by Gαq-PLCβ-Ca2+ signaling in response to bacterially derived Uracil (Ha et al., 2009a; Ha et al., 2009b; Lee et al., 2013). Flies lacking Duox activity have short life spans due to uncontrollable propagation of dietary yeast and due to an inability to control mutualistic and infectious bacteria (Ha et al., 2009a; Ha et al., 2009b). The IMD/Relish pathway is triggered by bacteria-derived peptidoglycan through activation of the peptidoglycan recognition protein (PGRP-LC) receptor (Hoffmann, 2003; Kim and Kim, 2005; Sorbara and Philpott, 2011). It is required for gut immunity against ROS-resistant pathogens and has been proposed to complement Duox-mediated immune responses (Ryu et al., 2006; Ryu et al., 2008). However, chronic activation of IMD/Relish signaling in the gut can sensitize flies to bacterial infection and shorten lifespan (Bonnay et al., 2013; Maillet et al., 2008). In aging flies, AMP expression significantly increases both systemically and in the gut, suggesting a link between chronic activation of the IMD/Relish pathway and age-related changes in the gut (Libert et al., 2006; Ren et al., 2007).
Here, we characterize the age-related breakdown of intestinal innate immune homeostasis in aging Drosophila. We identify an interaction between the transcription factor Foxo and the IMD/Rel pathway, driven by Foxo-mediated repression of peptidoglycan recognition proteins (PGRPs) of the SC class, that promotes commensal dysbiosis and epithelial dysplasia in aging guts. Strikingly, over-expressing PGRP-SC2 in ECs prevents age-associated dysbiosis and dysplasia, and extends lifespan in conventionally, but not in axenically reared animals. Our findings suggest that a breakdown of host/commensal interactions is central to age-associated tissue degeneration in barrier epithelia, and identify strategies to promote tissue homeostasis by improving these interactions, extending lifespan.
Results
Deregulation of the commensal microflora in aging uninfected flies
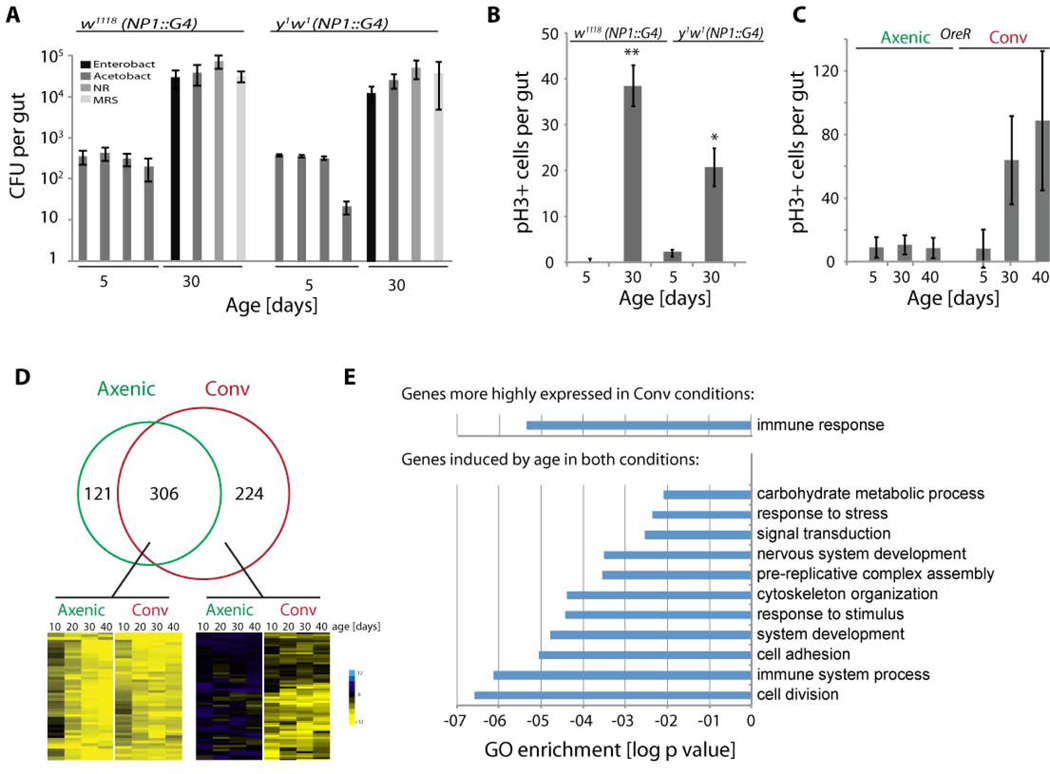
Since Drosophila lifespan is impacted by intestinal dysplasia (Biteau et al., 2010), and this dysplasia can be prevented by maintaining flies axenically (Buchon et al., 2009a), we sought to identify the causes for the age-related deregulation of commensal control. We first confirmed that age-related dysplasia was associated with increased microbial loads in various genetic backgrounds. We quantified overall microbial load by measuring colony-forming units (CFUs) in dissected guts of young and old age, using selective plates to identify Enterobacteriaceae, Lactobacillae, Acetobacteriaceae, and other bacteria grown on nutrient rich medium (using these selective plates, the majority of the commensal microbiome of flies can be monitored)(Ryu et al., 2008)(Fig. 1A). Confirming previous findings (Buchon et al., 2009a; Ren et al., 2007), the number of CFUs in the gut of conventionally reared flies increased exponentially for all analyzed bacterial phylotypes in the first 30 days of age (Fig. 1A and Fig. 2C). Epithelial dysplasia correlated with this dysbiosis in all tested genetic backgrounds, and, consistent with (Buchon et al., 2009a), was not observed in axenically reared animals (Fig. 1B, C, 2C–E, and S1). We used two different techniques for axenic rearing: maintaining flies in sterile conditions, or in media containing a cocktail of antibiotics (Ryu et al., 2008). In both conditions, low commensal numbers correlated with ISC quiescence (Fig. 1C, S1C).
Figure 1. Dysbiosis, ISC hyperproliferation, and changes in gene expression in axenically and conventionally aging intestines.
A) Colony-forming units (CFUs) in intestinal extracts of NP1∷Gal4 transgenic lines in y1w1 and w1118 background. See Fig. S1A for OreR flies. Midgut homogenates from flies at 5 days or 30 days of age were plated on nutrient rich medium (NR), or on selective plates allowing growth of Lactobacilli (MRS Agar), Acetobacteria or Enterobacteria.
B) Quantification of mitotic figures (cells positive for phosphorylated Histone H3; pH3+) in guts of the same genotypes as in A.
C) Quantification of mitotic figures in OreR flies of indicated ages, aged axenically (with antibiotics) or conventionally. See Fig. S1B for flies maintained on sterile food without antibiotics.
D) Venn diagram comparing the number of genes significantly induced in conventionally or axenically aging flies as identified by RNASeq. Genes were classified as induced when expression levels were are least 3 times higher than at 2 days of age in at least three older timepoints. Only genes with combined expression values above 10 RPKM were considered. Outtakes of hierarchical clustering highlighting examples of genes induced in both axenic and conventional conditions (left), or induced selectively in conventional conditions (right). Hierarchical clustering was performed on log2 values of gene expression ratios between indicated ages and 2d old samples.
E) GO enrichment analysis using Flymine.org of 67 genes expressed at least 3 times higher in guts of conventionally reared animals at at least 3 timepoints (upper chart). GO enrichment analysis of 112 genes induced more than 3 fold in both conditions compared to 2 day old samples at all four timepoints (lower chart).
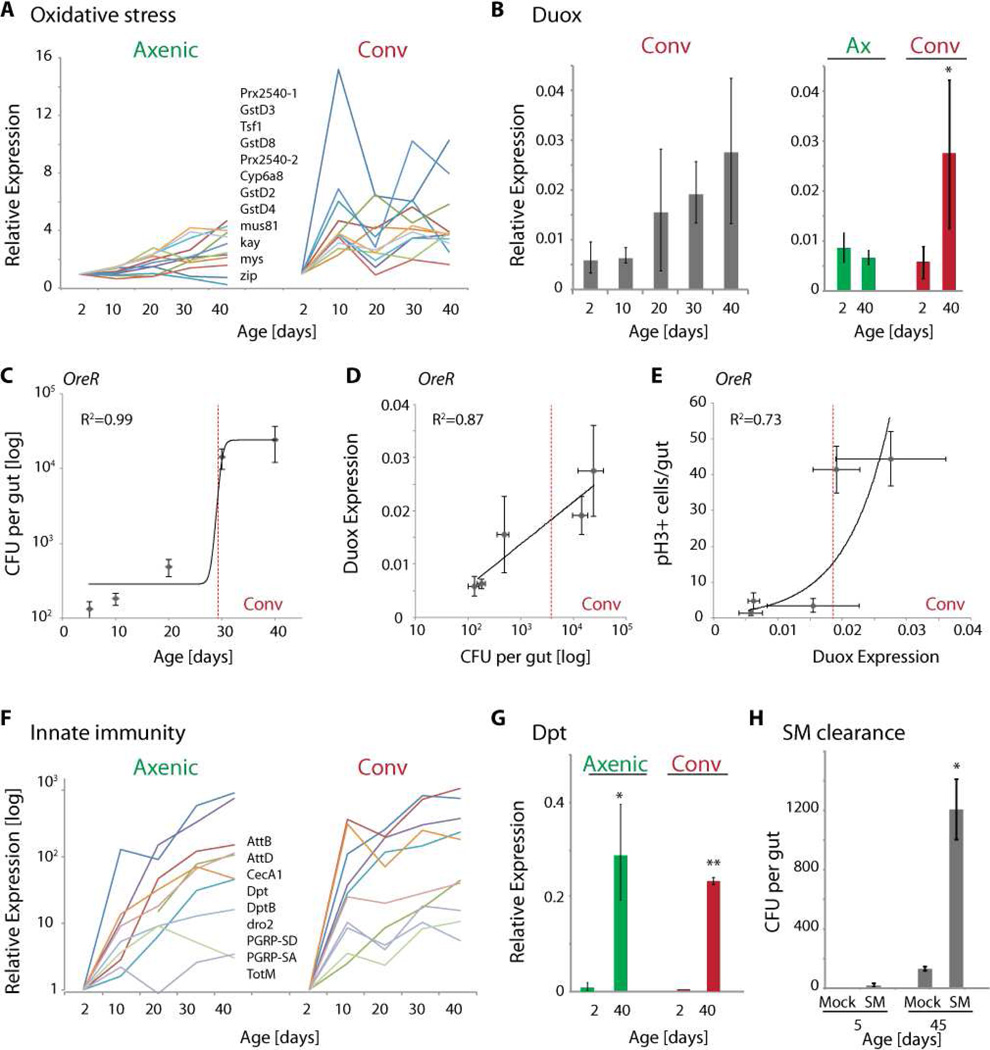
Figure 2. Oxidative stress response, IMD/Rel activity and innate immune dysfunction in aging intestines.
A) Oxidative stress response genes, as well as JNK target genes (mys, zip, kay) are induced in conventionally, but not in axenically aging intestines. Gene expression trajectories from RNAseq using RPKM values normalized to 2 day old samples.
B) qRT-PCR detecting duox expression relative to actin5C. Averages and standard deviations (N=3; * p<0.05, Student’s Ttest).
C–E) Scatter plots relating CFUs with age, Duox expression with CFUs, and mitotic figures with Duox expression in intestines of wild-type (OreR) flies. Fitted curves with R2 values are indicated. The dotted red line indicates a threshold value for Duox expression at which mitotic figures increase strongly.
F) Expression trajectories of innate immune response genes in axenically and conventionally reared flies. Log scale.
G) qRT-PCR detecting Dpt expression relative to actin5C. Averages and standard deviations (N=3; ** p<0.01, * p<0.05; Student’s Ttest).
H) S. Marcescens clearance: Bacterial load (CFU/gut) determined after feeding axenically reared young (5 day old) or old (45 day old) animals S.Marcescens (flies were fed 700µl of 5% sucrose containing a 100 fold concentrated suspension of bacteria grown to OD600=1) for 1 day, then allowing a 3 day recovery period. Mock treated animals were fed 5% sucrose solution.
See also Figure S2.
Transcriptome profiles comparing conventionally and axenically reared intestines
Since dysbiosis thus causes epithelial dysplasia, the origin of dysbiosis has to be determined to understand the etiology of age-related degeneration in the fly intestine. Towards this goal, we compared expression profiles of intestines of axenically and conventionally aging flies. We hypothesized that age-related changes in gene expression observed exclusively in conventionally reared animals are likely to represent epithelial responses to the expanding commensal population (i.e. events that occur as a consequence of, rather than cause commensal dysbiosis). Changes in epithelial gene expression observed in both conventionally reared and axenically reared animals, on the other hand, are expected to inform about the underlying epithelial changes causing dysbiosis.
Using RNAseq, we obtained expression data for about 8000 genes in each sample of conventionally or axenically reared flies at 2, 10, 20, 30, and 40 days of age (Fig. 1D). While a large number of ‘housekeeping’ genes did not significantly change with age (see examples in Fig. S1D), we identified and characterized groups of genes with similar age-associated increases in expression using hierarchical clustering. Functional classification of these genes and gene ontology enrichment analysis is expected to highlight specific functional changes in aging intestines. The expression profiles of selected representative genes in these groups were validated independently using qRT-PCR (Figs. 2B, D, S1E, S2, 3A, and not shown). Interestingly, the number of genes induced with age in both conditions, or induced exclusively in conventionally reared animals, was much higher than the number of genes found to be significantly induced only in the axenically reared animals (Fig. 1D). This observation supports the idea that aging-induced genes common to both conditions would reveal a transcriptional program underlying age-related epithelial changes, while aging-induced genes specific to conventionally reared animals would reveal the epithelial response to commensals.
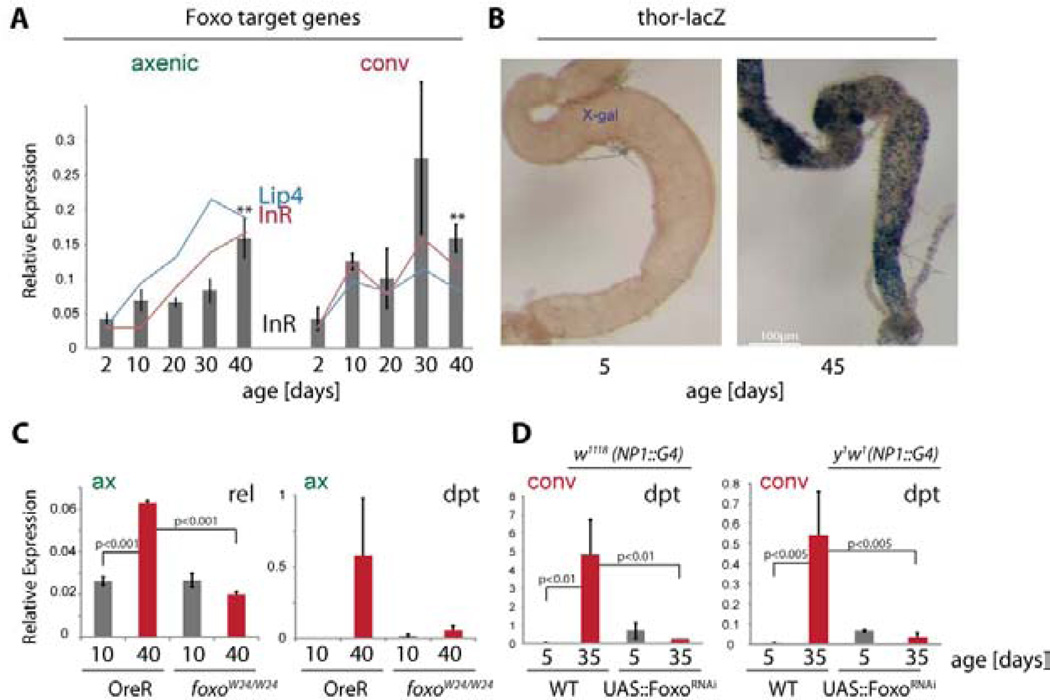
Figure 3. Foxo activation in aging intestines activates IMD/Rel signaling.
A) Expression of Foxo target genes Lip4 and InR in axenically and conventionally reared animals as determined by RNASeq (lines) and qRT-PCR (for InR, relative to actin5C, bars). Averages and standard deviations (N=3; ** p<0.01, Student’s Ttest).
B) X-Gal staining showing activation of thor-lacZ in aging intestines.
C) qRT-PCR detecting Rel and Dpt expression relative to actin5C in wild-type (OreR) or foxoW24 aged axenically. Averages and standard deviations (N>=3; p values from Student’s Ttest).
D) qRT-PCR detecting Dpt expression relative to actin5C in animals expressing FoxoRNAi using NP1∷Gal4 in w1118 (left) or y1w1 (right) backgrounds and aged conventionally. Averages and standard deviations (N>=3; p values from Student’s Ttest).
See also Figure S3.
When absolute expression levels were compared, the group of genes more highly expressed in conventionally compared to axenically reared animals across all ages was enriched in genes classified as ‘immune response’ genes (Fig. 1E). The group of genes induced in both axenically and conventionally aging guts, in turn, was enriched in genes belonging to GO categories commonly associated with aseptic wounding responses (GO categories ‘response to stress’, ‘cytoskeletal organization’ and ‘cell adhesion’, compare with (Boutros et al., 2002)), indicating that tissue damage in the gut increases with age independently of bacteria (Fig. 1E). This was consistent with the fact that cytokines commonly associated with wounding responses (Upd 1 and 2, Pvf 1and 2), and markers of JAK/Stat activation (Socs36E) were also significantly induced under both aging conditions (Fig. S1E). Upds, which activate JAK/Stat signaling, are secreted by damaged ECs, triggering compensatory proliferation of ISCs (Jiang et al., 2009).
A significant difference between axenically and conventionally reared guts was observed in a group of genes involved in oxidative stress responses and in responses to JNK activation (Jasper et al., 2001; Wang et al., 2003). These genes were induced with age in conventionally but not axenically reared animals (Fig. 2A), a finding consistent with the bacteria-dependent induction of Dual Oxidase (Duox) (Fig. 2B, S2A) and the increase in ROS observed in aging intestines (Hochmuth et al., 2011). These results are further consistent with the lack of age-related dysplasia in both axenic conditions and when ROS concentration is reduced in the gut (Fig. 1C) (Buchon et al., 2009a; Hochmuth et al., 2011), and support a model in which age-related dysbiosis induces expression of Duox, resulting in ISC hyper-proliferation due to increased ROS production. Accordingly, strong correlations between CFUs and age (R2=0.99), duox expression and CFUs (R2=0.87), and between duox expression and mitotic figures (R2=0.73) were observed (Fig. 2C–E). These correlations suggest a refractory period for the increased proliferative activity of ISCs, which is induced only after a threshold for duox expression is reached. Interestingly, this threshold was reached at an age when mortality within a wild-type population starts to increase, supporting a causal relationship between commensal dysbiosis, loss of redox homeostasis, dysplasia, and increased mortality (see Fig.6). The underlying cause for the age-related commensal dysbiosis that induces this degenerative condition, however, remained unclear.
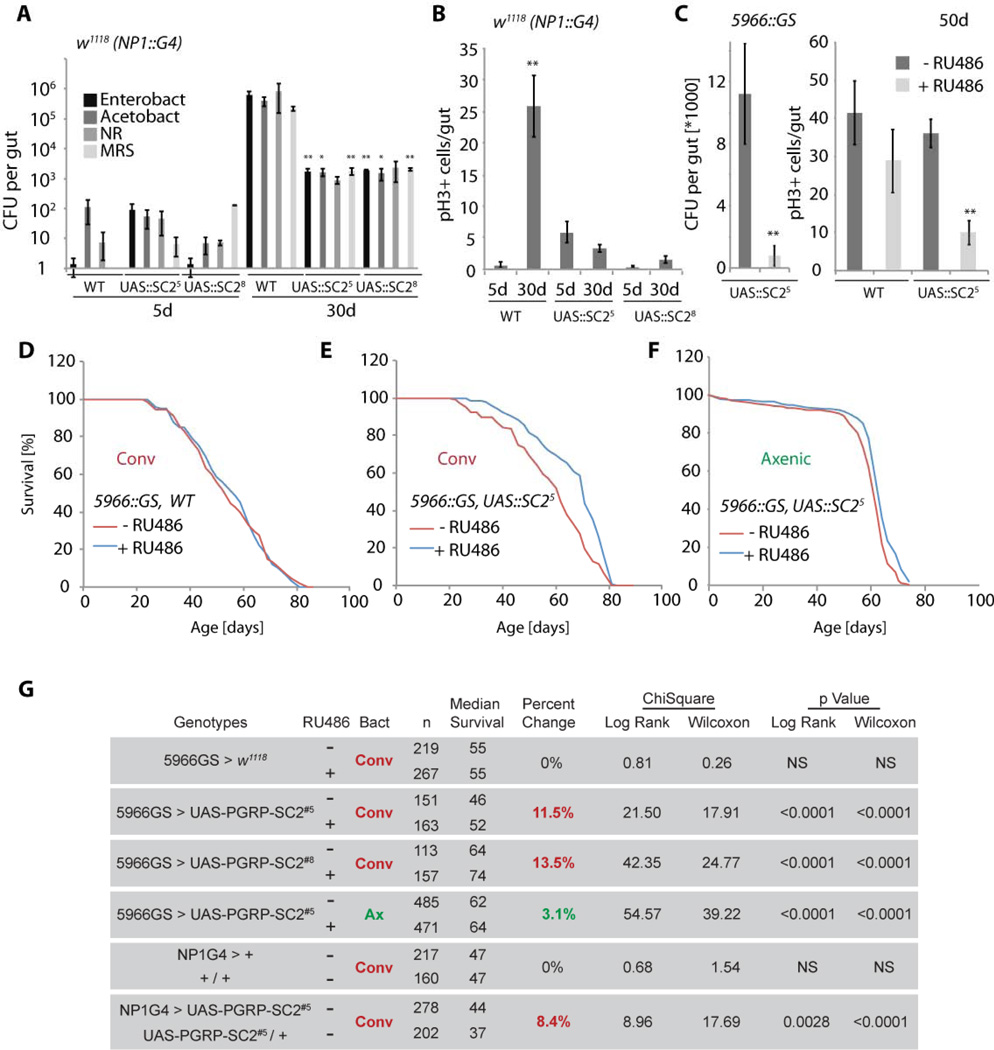
Figure 6. Over-expression of PGRP-SC2 in ECs reduces dysbiosis, ISC overproliferation, and extends lifespan.
A, B) Age-related changes in gut commensal numbers (A) and ISC proliferation (B) in PGRP-SC2 gain of function conditions. Intestinal CFUs (averages and SEM) quantified in wild-type, or PGRP-SC2 over-expressing animals (using NP1∷Gal4 in the w1118 background). Two independent UAS∷PGRP-SC2 transgenic lines were used. Averages and SEM are shown (*p<0.05; **p<0.01).
C) Quantification of intestinal CFUs (left; averages and SEM, NR plates) and ISC proliferation (right, averages and SEM) in 50 day old intestines of animals over-expressing PGRP-SC2 in ECs using the RU486-inducible 5966GS driver.
D–F) Mortality trajectories of females over-expressing PGRP-SC2 in ECs using the RU486-inducible 5966GS driver in conventional (E) or axenic (F) conditions. Axenic flies were kept on sterile food in a laminar flow hood without addition of antibiotics. Note that RU486 treatment does not affect lifespan in wild-type conditions (F).
G) Table summarizing parameters and statistics of the demographies shown in D–F, as well as of flies over-expressing PGRP-SC2 using NP1∷Gal4. PGRP-SC2 lines were backcrossed 7 generations into the w1118 background and crossed to similarly backcrossed heterozygous NP1∷Gal4 (see graphs in Fig. S6). Mortality of sibling populations with or without the NP1∷Gal4 transgene was compared.
See also Figure S6.
Age-related immunosenescence
Surprisingly, the group of genes induced with age in both conditions was also enriched in genes classified as ‘immune response’ genes (Fig. 1E). This includes AMP-encoding genes, although their induction was generally delayed and their expression levels were reduced in axenically, compared to conventionally aged animals (Fig. 2F,G, S2). Since AMP genes (like Diptericin, Dpt) are regulated by the IMD/Rel pathway, these results indicated that Rel activity is increased in the intestinal epithelium of both conventionally and axenically reared animals. This is consistent with the fact that rel itself is transcriptionally induced in axenically aging guts (see Fig. 3C, Fig. S2B). Interestingly, this age-related activation of IMD/Rel signaling in axenically reared flies is associated with an acquired deficiency in intestinal immune function, as illustrated by the fact that the ability to clear the moderately pathogenic bacterium S. Marcescens from the gut is impaired in old flies (Fig. 2H), while resistance to S.Marcescens or Erwinia carotovora carotovora 15 – induced mortality decreases (Fig. S2D). These defects are consistent with previously reported activation of Rel in aging tissues and concurrent increases in bacterial load (Brummel et al., 2004; Buchon et al., 2009a; Libert et al., 2006; Ren et al., 2007; Ryu et al., 2008), and with the observation that chronic activation of IMD/Rel signaling sensitizes flies to infection (Bonnay et al., 2013; Maillet et al., 2008). Activation of IMD/Rel signaling may thus constitute a cause for the observed dysbiosis, a hypothesis that we decided to test by exploring the regulation of Rel activity in the aging intestinal epithelium.
EC-specific Foxo activity induces immunosenescence
Rel activation and AMP expression are not only triggered by bacterial antigens, but are also modulated by the insulin/IGF signaling (IIS) pathway (Becker et al. 2010; Diangelo et al., 2009; Karpac et al., 2011; Libert et al., 2006). IIS-regulated Foxo can transcriptionally activate AMP genes through both Rel/NFkB-dependent and independent mechanisms (Becker et al. 2010), and in the larval fatbody, Foxo induces Rel expression under starvation conditions (Karpac et al., 2011). Among the genes found to be induced in both axenic and conventionally reared flies were Foxo target genes (Fig. 3A), suggesting that Foxo activity increases in the aging intestine. Using qRT-PCR and a thor-lacZ Foxo reporter (Karpac et al., 2011), we confirmed that Foxo activity increases in aging intestines under both axenic conditions and in conventionally reared flies (Fig.3A, B, S3A, B). In related work, we have found that this activation occurs exclusively in ECs, as induction of thor-lacZ can be detected in ECs, but not ISCs or EBs (Karpac et al., 2013). This age-associated chronic activation of Foxo in ECs disrupts their metabolic activity (Karpac et al., 2013), and our results reported here suggest that this chronic activation also deregulates their innate immune function. Supporting this idea, we found that loss of Foxo, either systemically (in foxoW24 loss of function mutants, (Becker et al., 2010; Weber et al., 2005)), or specifically in ECs (in flies expressing FoxoRNAi under the control of the pan-EC driver NP1∷Gal4, see also (Karpac et al., 2013)) reduced the age-related increase in Rel and Dpt expression, in both axenic and conventional conditions (Fig. 3C, D, S3C).
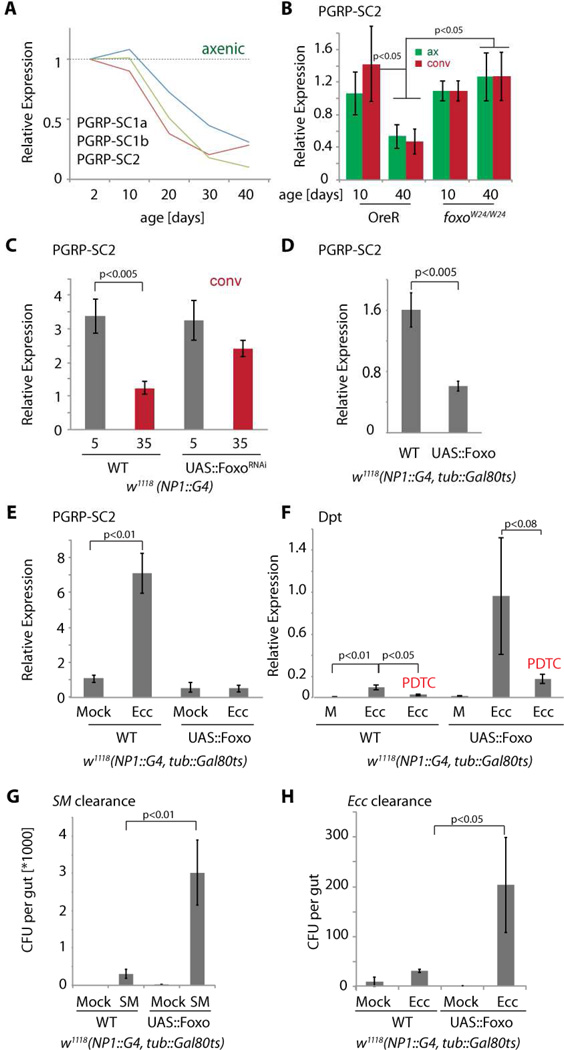
Foxo activity in ECs is thus required for the age-related increase in Rel expression and activity. It remained unclear, however, if Foxo affects the innate immune response in the gut by regulating rel or AMP expression directly, or by influencing the IMD/Rel pathway more generally. Interestingly, our RNASeq experiment indicated that the SC class of peptidoglycan recognition proteins (PGRP-SC 1a, 1b, and 2) was repressed in axenically aging guts (Fig. 4A), and subsequent validation by RT-PCR in various genetic backgrounds found that this repression could be observed reproducibly in both axenic and conventionally aging animals (Fig. 4B, C, S4A). Foxo activity in ECs is both required (Fig. 4B, C, S4A) and sufficient (Fig. 4D, S4B) to inhibit PGRP-SC2 and PGRP-SC1a expression in the gut (the effects of Foxo over-expression are not caused by apoptosis, as the pro-apoptotic Foxo target gene hid was not induced, and gut morphology was preserved; Fig. S4C and not shown).
Figure 4. Foxo mediates age-related repression of PGRP-SC2 expression and impairs innate immune responses.
A) Age-related repression of the three PGRP-SC genes in the gut of axenic flies (from RNASeq; normalized to 2 day olds; PGRP-SC2 is the most highly expressed PGRP-SC).
B) qRT-PCR detecting PGRP-SC2 relative to actin5C in OreR or foxoW24 aged axenically (green) or conventionally (red). Averages and SEM (N>=8; p values from Student’s Ttest).
C) qRT-PCR analysis as described above.
D) PGRP-SC2 expression in animals over-expressing Foxo in ECs. Animals were reared at 18°C and Foxo expression was induced in adults by shifting animals to 29°C for 5 days. qRT-PCR analysis as described above.
E) Induction of PGRP-SC2 by Erwinia Carotovora Carotovora (ATCC #15359) infection in animals over-expressing Foxo in ECs. Animals were reared at 18°C and Foxo expression was induced in adults by shifting animals to 29°C for 5 days. qRT-PCR analysis as described above.
F) Induction of Dpt in response to Ecc infection in animals over-expressing Foxo in ECs with or without the Relish inhibitor Pyrrolidine dithiocarbamate (PDTC). Culture conditions and qRT-PCR analysis as describe above.
G, H) Clearance of Serratia marcescens (SM, G) or Ecc (H) from guts of animals over-expressing Foxo in ECs.
See also Figure S4.
PGRP-SCs are catalytic PGRPs that can scavenge peptidoglycan and act as feedback inhibitors of the IMD/Rel innate immune signaling pathway, ensuring immune homeostasis (Bischoff et al., 2006; Paredes et al., 2011). The vertebrate homologues of PGRP-SCs, PGLYRP1-4, limit intestinal inflammation in rodents (Saha et al., 2010). In response to bacterial infection, these genes are induced, limiting the innate immune response and preventing excessive inflammation (Bischoff et al., 2006; Paredes et al., 2011). Over-expression of Foxo in ECs, however, strongly suppressed infection-induced expression of PGRP-SC2 in the gut (using Ecc infection, Fig. 4E), resulting in elevated dpt expression that could be dampened by treating animals with the Rel inhibitor PDTC (Moskalev and Shaposhnikov, 2011; Tanenhaus et al., 2012)(Fig. 4F). Increased AMP expression in Foxo gain-of-function conditions was thus Rel-dependent, and, as in old flies, was associated with an inability to efficiently clear bacteria (Fig. 4G, H). Taken together, these findings indicate that limiting Foxo activity in ECs is critical to ensure moderate and effective innate immune responses in the intestinal epithelium.
Age-related deregulation of commensal microbiota by Foxo limits lifespan
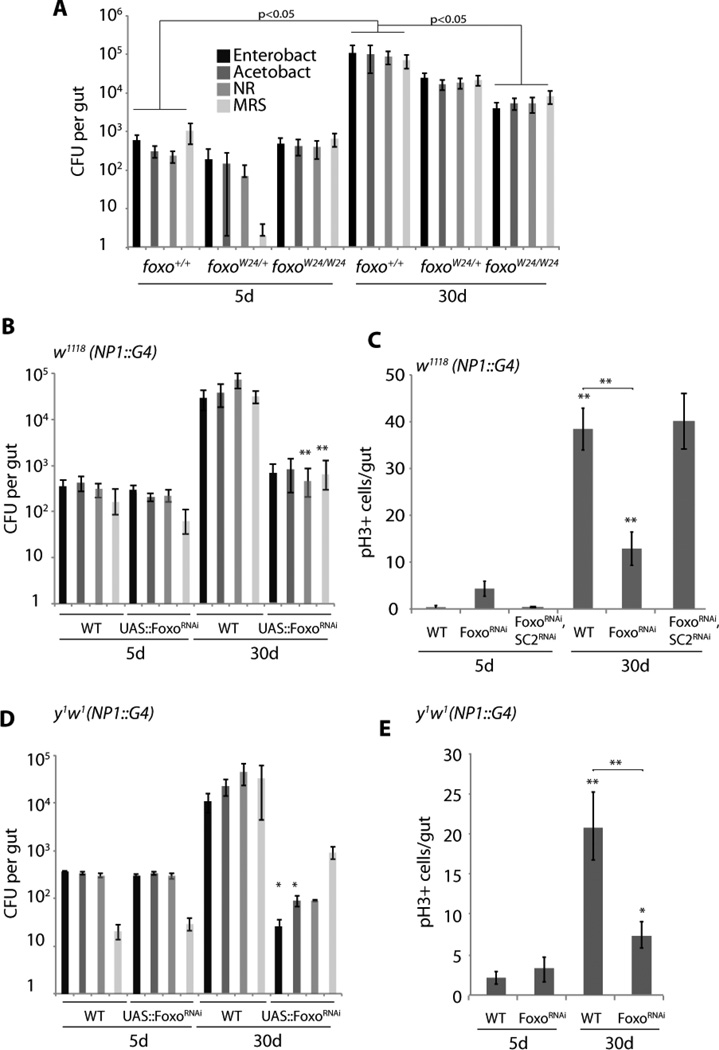
To test whether the repression of PGRP-SCs by Foxo contributes to age-related dysbiosis and dysplasia, we analyzed the commensal population in Foxo deficient flies. In all tested genetic backgrounds, loss of Foxo activity in ECs resulted in a strong reduction in CFUs in old intestines, confirming that chronic Foxo activity contributes significantly to dysbiosis (Fig. 5A, B, D). Since the FoxoRNAi construct also affects expression of one off-target gene, nebbish (Karpac et al., 2013), we confirmed that nebbish repression does not impact the commensal microbiota by specifically targeting nebbish in ECs using a separate nebbish RNAi construct (Fig. S5C).
Figure 5. Knockdown Foxo in Enterocytes reduces dysbiosis and ISC overproliferation.
A) Age-related changes in gut commensal numbers in foxo loss of function conditions. Intestinal CFUs (averages and SEM) quantified in wild-type, foxoW24 hererozygous, or foxoW24 homozygous sibling animals of the indicated ages. foxoW24 was backcrossed for 5 generations into the y1w1 background (**p<0.01 relative to wild-type).
B–C) Intestinal CFUs (B) and mitotic ISCs (C) quantified from animals of the indicated ages in which Foxo (or Foxo and PGRP-SC2) was knocked down specifically in ECs (using NP1∷Gal4 backcrossed into w1118). Averages and SEM are shown (N>17 for each data point).
D–E) As above, using NP1∷Gal4 backcrossed into the y1w1 background (*p<0.05; **p<0.01).
See also Figure S5.
Concurrent with the reduced number of commensal bacteria, animals in which Foxo was suppressed in ECs exhibited significantly reduced ISC proliferation, supporting the idea that preventing the accumulation of commensals in the aging gut is sufficient to limit dysplasia (Fig. 5C, E; mitotic figures were not affected by expression of nebbish RNAi, Fig. S5D). When PGRP-SC2 was knocked down in ECs, in turn, dysbiosis and dysplasia were increased compared to age-matched wild-type controls (Fig S5A, B). Importantly, knockdown of Foxo did not rescue age-associated dysplasia in PGRP-SC2 loss of function conditions (Fig. 5C), confirming epistasis of Foxo and PGRP-SC2 in the control of intestinal homeostasis.
We further assessed if specifically restoring the expression of PGRP-SC2 in the aging intestine is sufficient to promote commensal homeostasis and prevent dysplasia. Since PGRP-SC2 is the most highly expressed PGRP-SC gene in the young gut (based on RNASeq data, PGRP-SC2 is expressed about 100 times higher in the gut than PGRP-SC1a, and 500 times higher than PGRP-SC1b), we focused on restoring expression of this gene. Using two independent PGRP-SC2 transgenes, we found that over-expression of PGRP-SC2 in ECs (using NP1∷Gal4) prevented dysbiosis, limited dpt expression, and reduced dysplasia (Fig. 6A, B, S6A). Similar reduction in dysbiosis and dysplasia was observed when PGRP-SC2 was expressed using an EB/EC-specific RU486-inducible driver, 5966GS (Fig. 6C, S6B)(Mathur et al., 2010).
Since intestinal proliferative homeostasis correlates with longevity (Biteau et al., 2010), we assessed if promoting innate immune function by expressing PGRP-SC2 in ECs would also be sufficient to extend lifespan. Indeed, both when expressed constitutively in ECs using isogenized lines of NP1∷Gal4, or when expressed only in adults in an RU486-inducible manner using 5966GS, both PGRP-SC2 transgenes were sufficient to significantly extend Drosophila lifespan compared to isogenic sibling controls (Fig. 6 D–G, S6C). This lifespan extension by PGRP-SC2 was strongly reduced when animals were reared axenically, highlighting the critical role of PGRP-SC2-induced changes in the commensal bacterial population for its effects on mortality (Fig. 6F–G).
Discussion
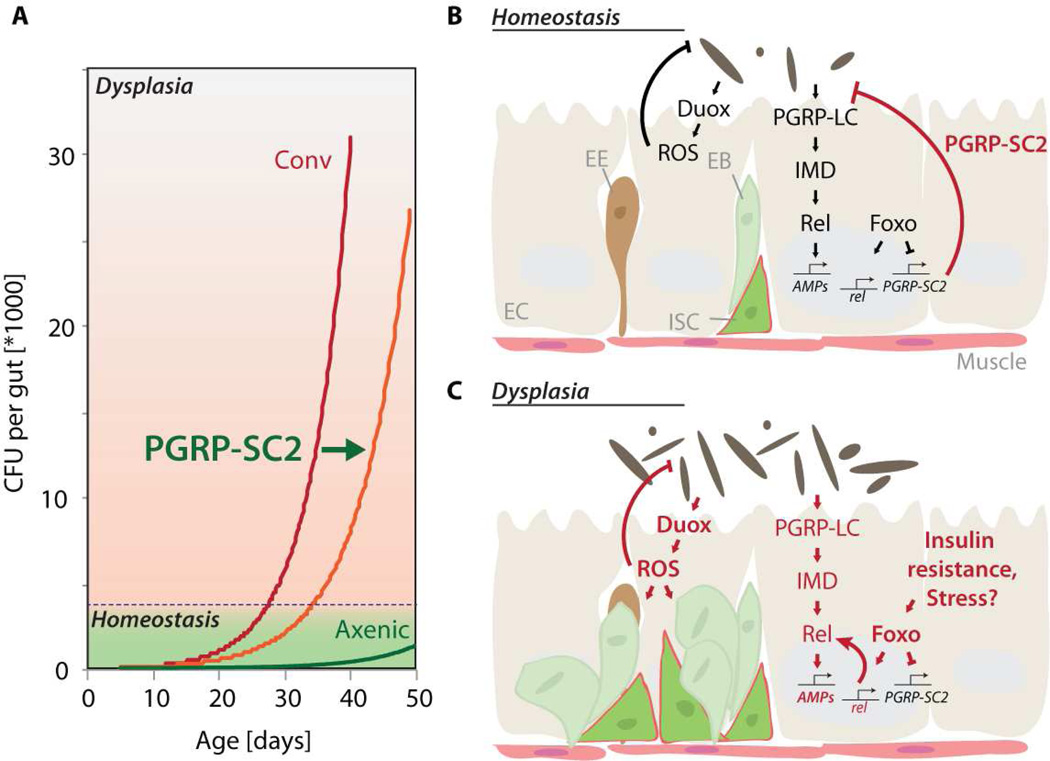
Our results provide a model for the development of age-related dysplasia in the aging intestine, and demonstrate that improving host-commensal interactions in aging barrier epithelia can promote health and lifespan. We propose that chronic activation of Foxo in ECs of aging animals initiates a breakdown of IMD/Rel homeostasis by suppressing expression of SC-class PGRPs, thus compromising the efficacy of the innate immune response. The resulting dysbiosis, in turn, stimulates an inflammatory response by activating Duox and promoting ROS production, thus triggering ISC over-proliferation and epithelial dysplasia (Fig. 7).
Figure 7. Model for the age-related deregulation of innate immune and epithelial homeostasis in the Drosophila intestine.
A) Model depicting the relationship between age, commensal dysbiosis, and intestinal dysplasia. Our results indicate that when intestinal CFUs and associated Duox expression reach a threshold, ISCs overproliferate, causing dysplasia. Over-expression of PGRP-SC2 delays the accumulation of commensals, resulting in delayed acquisition of dysplasia, extending lifespan.
B, C) Proposed mechanism causing immune dysfunction and commensal dysbiosis in the fly intestine. In young animals, PGRP-SC2 maintains immune homeostasis by limiting IMD/Rel activity. Foxo-mediated suppression of PGRP-SC2 allows adjusting responsiveness and basal activity of IMD/Rel. Age-associated chronic activation of Foxo in ECs, in turn, represses PGRP-SC2 expression chronically, de-regulating IMD/Rel signaling. The associated immunosenescence causes commensal dysbiosis, which chronically activates Duox – mediated ROS production, triggering ISC over-proliferation and dysplasia.
While the role of ROS in stimulating ISC proliferation is well established, an alternative explanation for the effects of reducing bacterial numbers on ISC activity are changes in the concentration of essential metabolites provided by commensal bacteria. Metabolic consequences of commensal bacteria are being explored in various contexts (Clemente et al., 2012; Hooper et al., 2012; Nicholson et al., 2012; Shin et al., 2011; Storelli et al., 2011), and the effects of bacterial metabolites on ISC activity are a fascinating area to explore in future studies.
The relationship between Foxo activation, the loss of immune function, dysbiosis, and intestinal dysplasia highlights the delicate balance that has to be maintained to ensure long-term homeostasis of barrier epithelia. Our observations point to a critical role of SC class PGRPs in maintaining intestinal homeostasis, a role that is consistent with studies showing that flies lacking these PGRPs experience deleterious immune responses to innocuous infections (Paredes et al., 2011). Interestingly, our data suggest that PGRP-SC2 expression in ECs primarily affects mortality in mid-life, and does not significantly increase maximal lifespan, indicating that PGRP-SC2-mediated control of intestinal immune homeostasis primarily affects healthspan of the animal and not the overall rate of aging. This critical role of amidase PGRPs likely extends to the vertebrate PGRP-SC homologues PGLYRP1-4, as these promote a normal gut flora and limit inflammation in a rodent model (Saha et al., 2010). Furthermore, mutations in the peptidoglycan recognition protein NOD2 are some of the most commonly found gene variants associated with Crohn’s disease, while interactions between NOD2 and PGLYRPs shape the inflammatory response to microorganisms (Saha et al., 2009; Sorbara and Philpott, 2011).
Foxo-mediated regulation of innate immune homeostasis and longevity
The age-related repression of PGRP-SCs and the dysbiosis and dysplasia caused by chronic Foxo activation in fly ECs are reminiscent of inflammatory conditions induced by excessive or chronic Foxo activation in mammals (Jonsson et al., 2005; Peng, 2010). Foxo activity is commonly associated with increased lifespan in invertebrates, but can have context-dependent and pleiotropic consequences for cell and tissue function, mediated by a wide array of Foxo target genes (Becker et al. 2010; Biteau et al., 2011b; Zhang et al., 2013). Accordingly, Foxo activity has complex consequences for metabolic, immune, and proliferative homeostasis of the intestinal epithelium: Foxo activation in ISCs or EBs prevents ISC proliferation, and constitutive activation of Foxo in these cells inhibits epithelial regeneration and reduces lifespan (Amcheslavsky et al., 2009; Biteau et al., 2010; Kapuria et al., 2012; McLeod et al., 2010; O'Brien et al., 2011). Over-expression of selected Foxo target genes in ISCs, in turn, can prevent age-related hyperproliferation of ISCs and intestinal dysplasia, resulting in lifespan extension (Biteau et al., 2010). In ECs, activation of Foxo is part of a metabolic response to changing nutrient conditions, but chronic activation of Foxo in these cells leads to a deregulation of gastric lipases, disrupting lipid homeostasis in the animal (Karpac et al., 2013). The inhibition of PGRP-SC2 expression reported here may also constitute an adaptive response to starvation, where Foxo activation boosts the activity of the IMD/Rel pathway to ensure survival under adverse conditions. The combined chronic suppression of PGRP-SC2 and gastric lipase expression by Foxo in aging ECs, however, results in the metabolic and immunological decline of the intestine. Managing Foxo activity in a cell-type specific manner in barrier epithelia is thus critical to ensure tissue health and organismal homeostasis. Interestingly, reducing Foxo activity specifically in ECs, while limiting dysbiosis and dysplasia, does not extend lifespan, but leads to early mortality (not shown). It is likely that this reflects the need for Foxo-mediated metabolic and stress adaptation in ECs of young animals (see also (Karpac et al., 2013)).
The reasons for the increase in Foxo activity in aging ECs are currently under investigation, and we find that JNK signaling appears to contribute to this activation (Karpac et al., 2013). However, a previous study has shown that age-related activation of JNK in the intestinal epithelium is dependent on the presence of commensal bacteria, as maintaining animals axenically reduces activation of JNK in the first 30 days of life (Buchon et al., 2009a). Since we find that Foxo activity increases with age in both conventionally and axenically reared animals, it is unlikely that JNK plays an exclusive role in the age-related activation of Foxo in ECs. Our RNAseq results, which indicate a significant age-related up-regulation of tissue damage and wounding response genes in both conventionally and axenically reared animals, suggest that a general accumulation of molecular and tissue damage might contribute to the activation of Foxo in ECs. Alternatively, local or systemic metabolic imbalances (such as insulin resistance), may contribute to chronic Foxo activation. Further studies using axenically reared flies will be necessary to determine the role of JNK, and of other stress signaling pathways, in elevating Foxo activity in ECs during aging.
Immunosenescence and inflammation in the aging intestine
It is likely that the benefits of limiting IMD/Rel signaling for homeostasis of barrier epithelia are conserved. Chronic inflammation and imbalanced Rel/NFkB signaling are major risk factors underlying aging and age-related diseases (Chung et al., 2009). Accordingly, over-activation of Rel/NFkB signaling, which in aging flies occurs both systemically and in the gut (Libert et al., 2006; Ren et al., 2007), has deleterious consequences, and has been associated with the pathogenesis of IBDs in vertebrates (Bonnay et al., 2013; Fukuyama et al., 2005; Kaser et al., 2010; Maillet et al., 2008; Nenci et al., 2007; Tominaga et al., 2007). Similarly, over-activation of Nox (NADPH oxidase) / Duox has been implicated in gastric adenocarcinomas in humans (Fukuyama et al., 2005; Tominaga et al., 2007). Our results indicated that IMD/Rel over-activation in ECs is an age-related condition that occurs both in conventionally and (albeit in a delayed fashion, see also (Buchon et al., 2009a)) in axenically reared animals, and thus drives age-related immune senescence.
It is puzzling that Rel-induced AMPs are vigorously expressed in the gut of old, conventionally reared animals, while commensal bacteria accumulate. This disconnect between high levels of AMP expression and bacterial accumulation has been reported before (Brummel et al., 2004; Libert et al., 2006; Ren et al., 2007), and might reflect the proliferation of AMP insensitive bacteria, or other, posttranscriptional, perturbations of AMP function that occur in aging flies. Such perturbations may include a deficiency in translation, as reported in the intestinal epithelium of animals infected with P. entomophila (Chakrabarti et al., 2012), or defects in secretion of AMPs. Alternatively, high levels of AMPs might influence commensal composition, but not overall load, resulting in the selection of deleterious strains, as has been described for animals deficient for the homeobox transcription factor Caudal (Ryu et al., 2008). Further studies are needed to resolve this question.
Extending lifespan by preventing commensal dysbiosis
Our study demonstrates that regulating host/commensal interactions in barrier epithelia can significantly delay age-related pathologies and extend lifespan. Previous work has demonstrated the complex relationship between bacterial load and longevity, establishing that bacteria can have both beneficial and deleterious consequences for flies, and that completely eliminating pathogenic and commensal bacteria does not lead to lifespan extension (Ryu et al., 2008; Shin et al., 2011; Storelli et al., 2011; Brummel et al., 2004; Ren et al., 2007). We show here, however, that lifespan extension can be achieved by improving innate immune homeostasis to stabilize the gut microbiota rather than by eliminating all bacteria from the gut. The fact that PGRP-SC2 over-expression fails to extend lifespan in axenic conditions supports this interpretation. Recently identified age-related changes in the human commensal population (Claesson et al., 2011; Claesson et al., 2012) indicate that such interventions may be able to also allay age-related pathologies and increase health- and lifespan in humans (Biagi et al., 2013; Ottaviani et al., 2011).
Materials and Methods
Drosophila stocks and culture
The following strains were obtained from the Bloomington Drosophila Stock Center: w1118, y1w1, OreR, NP1∷Gal4, tub-Gal80ts. UAS-FoxoRNAi (transformant ID 106097-CG3143), UAS-NebbishRNAi (108138-CG10718), and UAS-PGRP-SC2RNAi (6444) were obtained from the Vienna Drosophila RNAi Center. foxoW24 (gift from M. Tatar) was backcrossed into y1w1 background for 10 generations to obtain foxo+/+, foxow24/+ and foxow24/w24 flies. UAS-Foxo was a gift from M. Tatar. UAS-PGRP-SC2 transgenic lines were generated as described in Supplemental Information. Flies were raised on standard yeast/molasses based food (see Supplemental data for recipe).
Axenic fly cultures were generated with or without antibiotics as described in (Bakula, 1969; Brummel et al., 2004; Ryu et al., 2008). See Supplemental Methods for detailed description.
Immunostaining and microscopy
Intact guts were dissected, fixed, and stained by immunohistochemistry following standard protocols. See Supplemental Information for details.
RNAseq analysis
Intact fly intestines were dissected in PBS. Total RNA was extracted using Trizol reagent and used as template to generate RNASeq libraries for Illumina sequencing. Illumina HiSeq sequencing was performed at the University of Rochester Genomics Core Facility. Between 6 and 10 Million 72bp reads were generated and mapped to the Drosophila genome Release 5.46. Expression was recorded as RPKM: reads per kbp per million reads.
qRT-PCR analysis
Total RNA was extracted from dissected guts (7 per sample) using Trizol, and cDNA was synthesized using Superscript II (Invitrogen). Real time PCR was performed on a Biorad IQ5 system. See Supplemental Methods for details.
Bacteria culture and commensal quantification
Serratia Marcescens (NO. 21074) and Erwinia Carotovora Carotovora (NO. 15359) strains were obtained from ATCC.
To culture commensal bacteria, dissected midguts were homogenized in 100µl PBS and plated onto selective plates (see recipes in Supplemental Methods). Plates were incubated at 30°C for 36 – 48 hours.
Oral infection was performed as described (Buchon et al., 2010). Flies were fed for 24 hours either 700µl of concentrated bacteria cultures (OD100) or an equal volume of 5% sucrose as control. At least 3 vials (cohorts of 15 flies per vial) were used for each genotype. See Supplemental Methods for clearance experiments.
Lifespan analysis
60 virgins (5966∷GS homozygotes or w1118;NP1∷Gal4/+ heterozygotes) were crossed to 10–15 w1118;UAS∷PGRP-SC2 homozygous males (backcrossed 7 generations into w1118) or w1118 males. Progeny of these crosses was collected for 4 to 5 days after the first fly hatched. Flies were allowed to mate for 2 days at room temperature. Siblings were then separated according to sex and genotype into cages (20–50 flies/cage) and aged at 25°C. Dem ographic data was analyzed using Prism statistical software. See supplemental methods for additional fly handling information.
Supplementary Material
Highlights.
-
-
Age-associated commensal dysbiosis causes intestinal epithelial dysplasia in flies
-
-
Dysbiosis is caused by chronic activation of Foxo in aging enterocytes
-
-
Foxo perturbs intestinal immune homeostasis by repressing PGRP-SC2
-
-
Over-expression of PGRP-SC2 in enterocytes prevents dysbiosis and extends lifespan
Acknowledgements
This work was supported by the National Institute on Aging (NIH RO1 AG02812), the Ellison Medical Foundation (AG-SS-2224-08), and by an AFAR/Ellison postdoctoral fellowship to J.K. We would like to thank Olga Dunaevsky for technical assistance, and Drs. B. Ohlstein, N. Perrimon, M. Tatar, D.L. Jones, H. Hayashi, the Vienna Drosophila RNAi Center and the Bloomington Stock Center for flies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A. 2009;106:20883–20888. doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol. 1969;14:365–374. doi: 10.1016/0022-2011(69)90163-3. [DOI] [PubMed] [Google Scholar]
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Schultze JL, Hoch M. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res. 2013;69:11–20. doi: 10.1016/j.phrs.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011a;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Hwangbo D, Jasper H. Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol. 2011b;46:349–354. doi: 10.1016/j.exger.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnay F, Cohen-Berros E, Hoffmann M, Kim SY, Boulianne GL, Hoffmann JA, Matt N, Reichhart JM. big bang gene modulates gut immune tolerance in Drosophila. Proc Natl Acad Sci U S A. 2013;110:2957–2962. doi: 10.1073/pnas.1221910110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- Brummel T, Ching A, Seroude L, Simon AF, Benzer S. Drosophila lifespan enhancement by exogenous bacteria. Proc Natl Acad Sci U S A. 2004;101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009a;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature reviews Microbiology. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009b;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Liehl P, Buchon N, Lemaitre B. Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe. 2012;12:60–70. doi: 10.1016/j.chom.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Chambers MC, Schneider DS. Pioneering immunology: insect style. Curr Opin Immunol. 2012;24:10–14. doi: 10.1016/j.coi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diangelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama M, Rokutan K, Sano T, Miyake H, Shimada M, Tashiro S. Overexpression of a novel superoxide-producing enzyme, NADPH oxidase 1, in adenoma and well differentiated adenocarcinoma of the human colon. Cancer Lett. 2005;221:97–104. doi: 10.1016/j.canlet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009a;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol. 2009b;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:1–12. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Benes V, Schwager C, Sauer S, Clauder-Munster S, Ansorge W, Bohmann D. The genomic response of the Drosophila embryo to JNK signaling. Dev Cell. 2001;1:579–586. doi: 10.1016/s1534-5807(01)00045-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- Kapuria S, Karpac J, Biteau B, Hwangbo D, Jasper H. Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 2012 doi: 10.1371/journal.pgen.1003045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Biteau B, Jasper H. Misregulation of an adaptive metabolic response contributes to the age-related disruption of lipid homeostasis in Drosophila. Cell reports. 2013;4:1250–1261. doi: 10.1016/j.celrep.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Younger A, Jasper H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Dev Cell. 2011;20:841–854. doi: 10.1016/j.devcel.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Kim YJ. Overview of innate immunity in Drosophila. J Biochem Mol Biol. 2005;38:121–127. doi: 10.5483/bmbrep.2005.38.2.121. [DOI] [PubMed] [Google Scholar]
- Lee KA, Kim SH, Kim EK, Ha EM, You H, Kim B, Kim MJ, Kwon YG, Ryu JH, Lee WJ. Bacterial-derived Uracil as a Modulator of Mucosal Immunity and Gut-Microbe Homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Leulier F, Royet J. Maintaining immune homeostasis in fly gut. Nat Immunol. 2009;10:936–938. doi: 10.1038/ni0909-936. [DOI] [PubMed] [Google Scholar]
- Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J. The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe. 2008;3:293–303. doi: 10.1016/j.chom.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Moskalev A, Shaposhnikov M. Pharmacological inhibition of NF-kappaB prolongs lifespan of Drosophila melanogaster. Aging (Albany NY) 2011;3:391–394. doi: 10.18632/aging.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ottaviani E, Ventura N, Mandrioli M, Candela M, Franchini A, Franceschi C. Gut microbiota as a candidate for lifespan extension: an ecological/evolutionary perspective targeted on living organisms as metaorganisms. Biogerontology. 2011;12:599–609. doi: 10.1007/s10522-011-9352-5. [DOI] [PubMed] [Google Scholar]
- Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity. 2011;35:770–779. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Peng SL. Forkhead transcription factors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:482–485. doi: 10.1016/j.biocel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–152. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr, Jones DL, Walker DW. Modulation of Longevity and Tissue Homeostasis by the Drosophila PGC-1 Homolog. Cell Metab. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Ha EM, Oh CT, Seol JH, Brey PT, Jin I, Lee DG, Kim J, Lee D, Lee WJ. An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. Embo J. 2006;25:3693–3701. doi: 10.1038/sj.emboj.7601233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Saha S, Jing X, Park SY, Wang S, Li X, Gupta D, Dziarski R. Peptidoglycan recognition proteins protect mice from experimental colitis by promoting normal gut flora and preventing induction of interferon-gamma. Cell Host Microbe. 2010;8:147–162. doi: 10.1016/j.chom.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Qi J, Wang S, Wang M, Li X, Kim YG, Nunez G, Gupta D, Dziarski R. PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe. 2009;5:137–150. doi: 10.1016/j.chom.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- Sorbara MT, Philpott DJ. Peptidoglycan: a critical activator of the mammalian immune system during infection and homeostasis. Immunol Rev. 2011;243:40–60. doi: 10.1111/j.1600-065X.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Tanenhaus AK, Zhang J, Yin JC. In vivo circadian oscillation of dCREB2 and NF-kappaB activity in the Drosophila nervous system. PLoS ONE. 2012;7:e45130. doi: 10.1371/journal.pone.0045130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Weber K, Johnson N, Champlin D, Patty A. Many P-element insertions affect wing shape in Drosophila melanogaster. Genetics. 2005;169:1461–1475. doi: 10.1534/genetics.104.027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Judy M, Lee SJ, Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.