Abstract
Acetylcholinesterase inhibitors are first-line therapies for Alzheimer's disease. These drugs increase cholinergic tone in the target areas of the cholinergic neurons of the basal forebrain. Basal forebrain cholinergic neurons are dependent upon trophic support by nerve growth factor (NGF) through its neurotrophin receptor, TrkA. In the present study, we investigated whether the acetylcholinesterase inhibitors donepezil and galantamine could influence neurotrophin receptor signaling in the brain. Acute administration of donepezil (3 mg/kg, i.p.) led to the rapid autophosphorylation of TrkA and TrkB neurotrophin receptors in the adult mouse hippocampus. Similarly, galantamine dose-dependently (3, 9 mg/kg, i.p.) increased TrkA and TrkB phosphorylation in the mouse hippocampus. Both treatments also increased the phosphorylation of transcription factor CREB and tended to increase the phosphorylation of AKT kinase but did not alter the activity of MAPK42/44. Chronic treatment with galantamine (3 mg/kg, i.p., 14 days), did not induce changes in hippocampal NGF and BDNF synthesis or protein levels. Our findings show that acetylcholinesterase inhibitors are capable of rapidly activating hippocampal neurotrophin signaling and thus suggest that therapies targeting Trk signaling may already be in clinical use in the treatment of AD.
Keywords: Trk receptor phosphorylation, donepezil, galantamine, nerve growth factor, acetylcholinesterase inhibitor
1. Introduction
One of the characteristic features of Alzheimer's disease (AD) is the degeneration of cholinergic neurons of basal forebrain (BFCN) (Whitehouse et al., 1982). The BFCN is a neuron population that almost exclusively depends upon the trophic support of nerve growth factor (NGF). These neurons receive NGF from the target areas of cholinergic innervation, such as the hippocampus, and specifically take it up and transport it to their somas in the basal forebrain (Seiler et al., 1984). NGF increases the expression of cholinergic markers (e.g. choline acetyltransferase, ChAT) both in vivo and in vitro (Gnahn et al., 1983; Hefti et al., 1985) and increases the survival of BFCN after fimbrial lesion in vivo (Hefti, 1986). Another member of the neurotrophin family, brain derived neurotrophic factor (BDNF), has similar effects on ChAT activity and BFCN survival (Alderson et al., 1990; Morse et al., 1993; Widmer et al., 1993). Moreover, reducing the activity of NGF or its receptor TrkA in the mouse brain results in cholinergic degeneration reminiscent of AD (Capsoni et al., 2000; Capsoni et al., 2010).
First-line therapies of AD are acetylcholinesterase inhibitors (AChEis). These drugs increase cholinergic tone in the brain and improve cognitive function of patients with mild to moderate AD (Birks, 2006). Although the role of neurotrophins in the pathophysiology of AD remains unclear, neurotrophin signaling is often postulated as a potential target for the development of new drugs to treat this disease (Webster et al., 2008). Before undertaking such an approach, it would be useful to determine whether current pharmacotherapies of AD influence neurotrophin signaling in the brain. Our current understanding of the effects of AChEis on neurotrophins is limited. Although some studies have reported that AChEis have little effect on neurotrophin synthesis and protein levels in aged rats (Hernandez et al., 2006), others have reported restoration of decreased ngf mRNA levels in a rat model of experimental allergic encephalomyelitis (D'Intino et al., 2005). In humans, AChEis have been reported to restore serum BDNF levels in patients with mild AD (Leyhe et al., 2008). Therefore the aim of our study was determine whether two clinically used AChEis, donepezil and galantamine, are capable of activating TrkA and TrkB signaling in the mouse hippocampus.
2. Materials and methods
2.1. Animals
Adult C57BL/6N male mice were obtained from Harlan, The Netherlands or from Biocenter 3, University of Helsinki, Finland. TrkB.TK+ mice overexpressing N-terminally FLAG-sequence tagged full-length TrkB receptor (Koponen et al., 2004) were bred at the University of Helsinki. All experiments were conducted according to the guidelines of the European Communities Council Directive (86/609/EEC) and were approved by the County Administrative board of Southern Finland.
2.2. Drug treatments and tissue sampling
Animals received an acute i.p. injection of galantamine-HBr (3 or 9 mg/kg, Toronto Research Chemicals) or donepezil-HCl (3 mg/kg, Toronto Research Chemicals) 1 hour before sacrifice. Saline was used as a vehicle and as a control. Doses were calculated as the free base. The doses were chosen based on the available pharmacokinetic and pharmacodynamic data to produce robust increase in acetylcholine levels in the mouse brain (Yano et al., 2009). According to previous studies, approximately threefold concentrations of galantamine compared to donepezil are needed to produce a similar level of AChE inhibition (Geerts et al., 2005) whereas the lower galantamine dose is optimal to produce the allosteric modulation of nicotinic receptors (Geerts et al., 2005). In another experiment, animals were chronically treated with galantamine-HBr (3,0 mg/kg once a day, i.p) for 14 days. Similar setup has been previously used to study the cognitive effects of galantamine in a mouse model of AD (Van Dam et al., 2005). Mice were stunned with carbon dioxide 1 hour following the last injection, brains removed and both hippocampi dissected and homogenized in standard NP lysis buffer (137 mM NaCl, 20 mM Tris, pH 8.0, 1% NP-40,10% glycerol, 50 mM sodium fluoride, 2× Complete Mini Protease inhibitor (Roche Diagnostics,Hertforshire, UK), and 2 mM sodium vanadate) or were snap-frozen for later analysis. In the chronic treatment paradigm, neurotrophin protein and mRNA levels were analyzed from the right and left hippocampus, respectively.
2.3. Immunoprecipitation and western blotting
For FLAG immunoprecipitation, 1 mg of protein was incubated overnight with 5 μl of anti-FLAG antibody (M2, Sigma Aldrich). The immunocomplexes were precipitated with 15 μl of Protein-G Sepharose (Invitrogen) followed by thorough washing, and boiling in 2× Laemmli sample buffer. For direct SDS-PAGE, 40 or 50 μg of protein was boiled in an equal volume of 2× Laemmli sample buffer. Proteins were separated by SDS-PAGE and transferred to a PVDF membrane. The membranes were blocked with 3 % bovine serum albumin or milk (1 h, room temperature) and incubated in primary antibody (+4 °C, overnight): anti-pY794 and anti-pY816 against the phosphorylated Trk PLC-γ binding site (1:5000, Rajagopal et al.,2004 Bath et al., 2008), anti-phospho-CREB (1:1000, 8763, Cell Signaling), anti-CREB (1:1000, SC-58, Santa Cruz), anti-phospho-MAPK42/44 (1:1000, Cell Signaling), anti-MAPK42/44 (1:1000, Cell Signaling) anti-phoshpo-AKT (1:1000, #9275S, Cell Signaling), anti-AKT (1:1000, C67E7, Cell Signaling), anti-Trk (1:2000, SC-11, Santa Cruz), anti-BDNF (1:500, SC-546, Santa Cruz), anti-NGF (1:500, SC-549, Santa Cruz) and anti-GAPDH (1:10000, Abnova). After washing, membranes were incubated in HRP-conjugated secondary antibody (1:10000, Biorad; 1 h, room temperature) followed by visualization with an electrochemiluminescence kit (ECL+, Amersham Biosciences) and the detection of luminescence with Fuji LAS-3000 camera. The obtained bands were quantified with ImageJ (NIH) and the intensity of phospho-immunoreactive protein bands was normalized to total Trk, MAPK, AKT and CREB levels, respectively. BDNF and NGF immunoreactive bands were normalized to GAPDH.
2.4. ELISA analyses
For NGF protein analyses, hippocampal samples were acid-treated with 1 M HCl for 15 minutes at room temperature and then neutralized again with 1 M NaOH. NGF was measured with Chemikine™ NGF Sandwich ELISA kit according to the manufacturer's instructions. All measurements were run in triplicate and NGF levels were normalized to the total protein concentration of the samples. BDNF protein concentration was analyzed using two-site enzyme-linked immunosorbent assay as previously described (Karpova et al., 2010).
2.5. Real-time qPCR
RNA was extracted from deep-frozen left hippocampus with Trizol (Invitrogen) according to the manufacturer's instructions. The isolated RNA was treated with DNAse (Roche). Complementary DNA synthesis and PCR were performed with Phusion™ RT-PCR kit (Finnzymes) and with 1 μg of RNA. The following primers were used for cDNA synthesis: NGF: 5′- AGCTTTCTATACTGGCCGCA-3′ and 5′-TCTGTGTACGGTTCTGCCTG-3′. BDNF: 5′-GAAGGCTGCAGGGGCATAGACAAA and 5′-TACACAGGAAGTGTCTATCCTTATG-3′. The primers were designed against neurotrophin coding exons common to known isoforms. The relative levels of neurotrophin messenger RNA in control and treated samples were measured by SYBR green incorporation during the linear phase of the PCR reaction using 2-ΔΔCT method (Livak et al., 2001). Neurotrophin mRNA levels were normalized to those of the housekeeping gene, GAPDH.
2.6. Statistical analyses
Drug treated-groups were compared to saline-treated control groups using unpaired t-test or a 1-way ANOVA followed by a Newman-Keuls post hoc test when appropriate.
3. Results
3.1. Donepezil and galantamine rapidly activate Trk receptors in the mouse hippocampus
We studied the effect of two AChEis on Trk receptor activity in the mouse hippocampus. Adult male mice were acutely treated with donepezil (3 mg/kg) or galantamine (3 or 9 mg/kg). The activation of Trk receptors involves sequential phosphorylation of tyrosine residues in autocatalytic domain of the receptor and of other tyrosines that serve as docking sites for intracellular messengers (Chao, 2003). The phosphorylation status of these tyrosine residues can therefore be used as a marker of receptor activity (Segal et al., 1996). Proteins were separated in SDS-PAGE and the Trk tyrosine phosphorylation was analysed by immunoblotting with antibodies raised against the phosphorylated PLC-γ1 binding site of either TrkA or TrkB-receptor (Bath et al., 2008; Rajagopal et al., 2004). Compared to the vehicle-treated controls, both donepezil and galantamine significantly increased phosphorylation levels of TrkA (Y794) and TrkB (Y816) in the mouse hippocampus (Fig 1A and 1B). Both antibodies also revealed the increased phosphorylation of unknown proteins some of which may represent immature Trk receptor isoforms (Lee et al., 2001).
Figure 1.
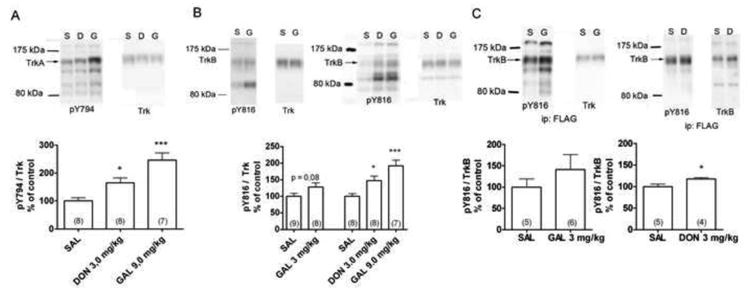
The tyrosine phosphorylation of the PLCγ1 binding site of TrkA (A) and TrkB (B) receptor after acute (1 h) intraperitoneal administration of donepezil (D, DON, 3 mg/kg) or galantamine (G, GAL, 3 or 9 mg/kg) in the adult C57BL6 mouse hippocampus. (C): TrkB receptor pulled down with the anti-FLAG antibody form brain lysates of the TrkB.TK+ mice overexpressing N-terminally FLAG-tagged TrkB receptors in neurons. The phosphorylated Trk receptor levels were normalized to total Trk receptor levels and compared with the phospho-Trk levels in saline treated controls (SAL). The number of animals in each group is indicated inside the bars. * = p< 0,05, ** = p < 0,01, *** = p < 0,001, ANOVA, Newman-Keuls post-hoc test (Figs A and B) or t-test (Fig C).
To further dissect between the contributions of different Trk subtypes, we used an immunoprecipitation approach and transgenic mice overexpressing N-terminally FLAG-tagged TrkB-receptor under Thy1 neuronal promoter (Koponen et al., 2004). These mice were treated with donepezil (3 mg/kg) or galantamine (3 mg/kg) and the TrkB receptors were pulled down from hippocampal homogenate with an antibody against the FLAG epitope tag. Tyrosine phosphorylation of TrkB was then analyzed as above. Donepezil produced a small but significant increase in the phosphorylation of the PLC-γ1 binding site of TrkB (Fig 1C). Galantamine had a similar effect but the difference did not reach statistical significance (Fig 1C). Taken together, our results indicate that AChEis activate both TrkA and TrkB signaling in the mouse hippocampus.
3.2. Activation of Trk downstream signaling pathways by acetylcholinesterase inhibitors
Trk receptors have multiple downstream signaling cascades including the MAPK/ERK, AKT and PLC-γ1 pathways (Chao, 2003). Therefore, we analyzed the activity of several downstream kinases and transcription factor CREB in the mouse hippocampus after acute donepezil and galantamine administration. Both donepezil and galantamine tended to increase the phosphorylation of AKT (p = 0,067, ANOVA) and significantly increased the phosphorylation of CREB when compared to saline-treated controls but there were no changes in MAPK42/44 phosphorylation (Fig 2.).
Figure 2.
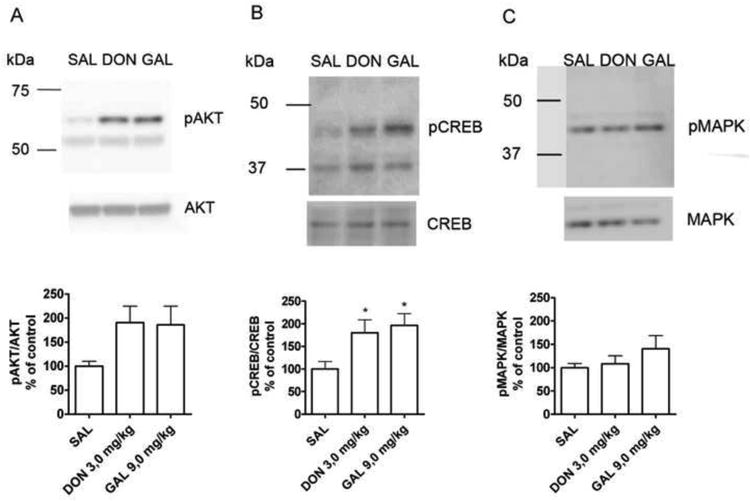
The effect of acute (1 h) donepezil (DON, 3 mg/kg) and galantamine (GAL, 9 mg/kg) treatment on hippocampal AKT, CREB and MAPK signaling in adult mice hippocampus. Donepezil and galantamine tended to increase the phosphorylation of AKT (A, p = 0,067, ANOVA) and induced significant increase in the phosphorylation of CREB (B) when compared to saline (SAL) treated controls. MAPK42/44 activity was not regulated by either treatment (C). Phosphorylated AKT, MAPK and CREB levels were normalized to total AKT, MAPK and CREB levels in each sample. * = p < 0,05 (ANOVA, Newman-Keuls post hoc test). The number of animals in groups were SAL = 8, DON = 8, GAL = 7.
3.3. Galantamine does not have long-term effects on hippocampal neurotrophin synthesis or processing
To investigate whether AChEis could have long-term effects on the levels of brain neurotrophins, we treated mice with galantamine (3 mg/kg, once a day, i.p.) for 14 days. Chronic galantamine treatment did not significantly change BDNF or NGF mRNA or protein levels in the mouse hippocampus (Fig 3). ELISA and PCR methods may not, however, differentiate between the pro and mature forms of neurotrophins. To confirm our findings we performed SDS-PAGE and Western blotting with antibodies against NGF and BDNF. An NGF immunoreactive band corresponding to the molecular weight of proNGF (27 kDa) was detected but it was not regulated by chronic galantamine treatment. Mature NGF was detected only after very long exposure times in low levels that were beyond quantification. On the contrary, mature BDNF (14 kDa) was clearly present in the samples but was not regulated by the galantamine treatment whereas proBDNF was not detected in the samples. In conclusion, chronic galantamine treatment did not have effects on hippocampal neurotrophin synthesis or processing.
Figure 3.
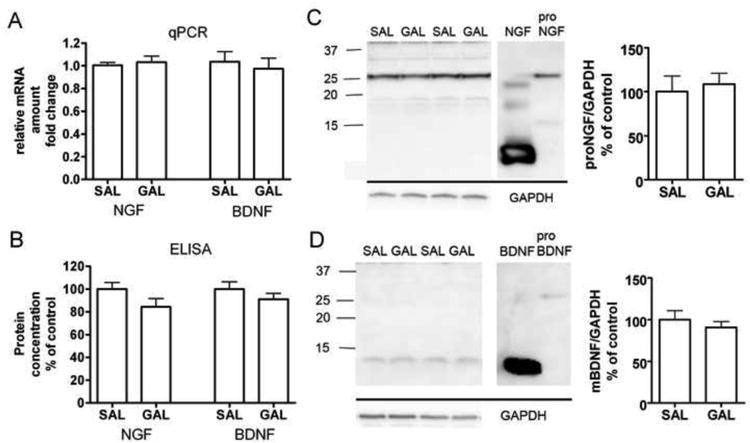
NGF and BDNF messenger RNA and protein levels in the mouse hippocampus after 14 days of galantamine treatment (GAL, 3 mg/kg/day, i.p.). Galantamine did not regulate neurotrophin mRNA (A) or total neurotrophin protein levels (B) when compared to saline (SAL) treated controls. Chronic galantamine treatment did not alter the processing of proneurotrophins to mature neurotrophins in the mouse hippocampus (C, D). Immunoblotting against NGF (C) reveals that the majority of NGF in the hippocampus is in pro form whereas BDNF (D) is present predominantly in mature form. Neurotrophin mRNA levels were quantified with RT-PCR relative to the housekeeping gene GAPDH. In ELISAs, neurotrophin levels were normalized to protein concentrations in the samples. NGF and BDNF immunoreactive bands were normalized to GAPDH band. The number of animals in groups were SAL = 8, GAL = 12.
4. Discussion
The cholinergic neurons of the basal forebrain (BFCN) are among the first neuronal populations that characteristically degenerate in early AD (Whitehouse et al., 1982). BFCNs almost exclusively depend on the trophic effects of NGF for their survival (Capsoni et al., 2010; Hefti, 1986) thus suggesting that the NGF receptor, TrkA, could be a potential target for the development of novel drugs aimed at protecting the BFCN from degeneration. Current first-line medications of AD are AChEis which can improve the cognitive capabilities of patients with mild to moderate AD (Birks, 2006). AChEis have been shown to rescue cholinergic neurons in mice that have reduced NGF activity and that display a phenotype similar to the pathological features and progression of human AD (Capsoni et al., 2002; Capsoni et al., 2004). The aim of the present study was to examine whether AChEis can regulate hippocampal Trk receptor signaling. Indeed, acute treatment with donepezil and galantamine significantly increased the phosphorylation levels of TrkA and TrkB receptors in the mouse hippocampus.
Since TrkA receptors in the adult hippocampus are localized in cholinergic nerve endings (Koh et al., 1989), it is possible to speculate that increased cholinergic tone by AChEi could drive the release of target-derived NGF which could then bind to and activate presynaptic TrkA receptors in the BFCN. The localization of activated TrkB receptors, on the other hand, is unclear since the receptor is widely expressed in hippocampal neurons in addition to BFCN. In addition to neurotrophin binding, there are other possible mechanisms of Trk receptor activation. The G-protein mediated transactivation of Trk receptors in response to adenosine or pituitary adenylate cyclase-activating polypeptide have been previously described (Lee et al., 2001; Rajagopal et al., 2004). It has also been suggested that some neuropsychiatric drugs could directly bind to Trk receptors (Jang et al., 2009). The role of these mechanisms in the activation of Trks by AChEis remains to be investigated. However, galantamine did not induce phosphorylation of Trk receptors in vitro in cultured cortical, hippocampal or basal forebrain neurons (data not shown) thus suggesting that it may not have any direct effects on Trk receptors in vitro and that the effects observed in vivo require the context of intact brain connections. These data are consistent with our observations that while antidepressant drugs activate the autophosphorylation of TrkB when administered to mice in vivo (Saarelainen et al., 2003; Rantamäki et al., 2007) these drugs do not activate TrkB receptors in cultured hippocampal or cortical neurons (Rantamäki et al., 2011).
The AChEi-induced changes in Trk receptor phosphorylation were accompanied by an increase in the phosphorylation of AKT, which is a downstream target of Trk signaling implicated in neuronal survival (Chao, 2003; Takada-Takatori et al., 2006). Although the concomitant and essentially similar changes in the phosphorylation of Trks and AKT suggest a link between the two signaling cascades, Trks certainly are not the only factors that can activate AKT signaling. It remains to be determined whether and to which extent the activation of AKT by AChEis is mediated by Trk receptors. Taken together, the activation of this important pathway which is implicated in neuronal survival and in the neuroprotective effects of AChEis in vitro (Takada-Takatori et al., 2006) could explain the protective effects these drugs show in vivo when NGF activity is reduced (Capsoni et al., 2002).
In addition, both donepezil and galantamine increased the phosphorylation of transcription factor CREB which is crucial for TrkB –dependent hippocampal learning (Bourtchuladze et al., 1994; Minichiello, 2009). This rapid increase in hippocampal pCREB is also sustained in long-term donepezil treatment (Kotani et al., 2006). Our findings indicate that the activation of Trk receptors by AChEis is associated with the concomitant activation of a several important intracellular signaling cascades.
Chronic administration of galantamine did not change hippocampal BDNF or NGF mRNA or protein levels. If anything, NGF protein level appeared to be decreased following galantamine treatment. These findings are in line with previous studies conducted with healthy animals (Hernandez et al., 2006; Kotani et al., 2006). On the other hand, chronic treatment with the AChEis rivastigmine and donepezil have been shown to partially recover ngf mRNA levels and cognitive capabilities in a rat model of experimental allergic encephalomyelitis (D'Intino et al., 2005). Furthermore, Leyhe et al (2008) reported that chronic treatment with donepezil restores BDNF serum concentrations in patients with mild to moderate AD to the levels observed in age-matched controls. Therefore, AChEis may have little long-term effects on brain neurotrophin levels in healthy naïve animals but may augment neurotrophin synthesis in situations where neurotrophin synthesis is compromised. It is becoming increasingly recognized that the processing of proneurotrophins to mature neurotrophins and the balance between Trk and p75 signaling may be a determinant of the survival of BFCN (Capsoni et al., 2010). More detailed analysis by Western blotting revealed that galantamine treatment did not induce any changes in the processing of proneurotrophins to their mature forms. In line with Fahnestock et al (2001), the majority of adult mouse NGF was found to be in pro form. Taken together, our results suggest that AChEis, the drugs of choice for the treatment of AD activate the signaling of brain neurotrophin receptors. These findings call for behavioral studies to investigate whether the activation of Trk signaling correlates with the beneficial effects AChEis show in animal models of memory impairment. These studies need to be extended to aged animals and to animal models of AD to examine whether AChEis are capable of bringing about their effects on neurotrophin signaling also in conditions that model their clinical use in the diseased. Finally, it will be important to investigate whether AChEis influence Trk signaling in human brain and whether this information could be utilized to design better treatment strategies for AD patients.
Highlights.
Galantamine and donepezil activated TrkA and TrkB receptors in the mouse hippocampus
Galantamine and donepezil increased the phosphorylation of AKT and CREB in the mouse hippocampus
Chronic galantamine did not regulate BDNF or NGF synthesis in the mouse hippocampus
Acknowledgments
The authors would like to thank Outi Nikkilä for her excellent technical support and Sissi Pastell and Virpi Nousiainen for taking care of the animals and Dr. Olivia F. O'Leary for language revision. This study was financially supported by the Memories project of the EU FP6 (LHSM-CT-2006-037831), Academy of Finland Center of Excellence Programme, Finnish Graduate School of Neurosciences and the Sigrid Juselius Foundation.
Role of funding source: Funding source had no role in designing the study; in the collection, analysis, and interpretation of data; in writing the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest: H.A., K.M., T.R., L.L., M.H., M.C., U.A. and E.C. declare that they don't have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. Journal of Neuroscience. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for alzheimer's disease. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, Cattaneo A. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6826–6831. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Giannotta S, Cattaneo A. Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12432–12437. doi: 10.1073/pnas.192442999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Giannotta S, Stebel M, Garcia AA, de Rosa R, Villetti G, Imbimbo BP, Pietra C, Cattaneo A. Ganstigmine and donepezil improve neurodegeneration in AD11 antinerve growth factor transgenic mice. American Journal of Alzheimer's Disease and Other Dementias. 2004;19:153–160. doi: 10.1177/153331750401900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Tiveron C, Vignone D, Amato G, Cattaneo A. Dissecting the involvement of tropomyosin-related kinase A and p75 neurotrophin receptor signaling in NGF deficit-induced neurodegeneration. Proceedings of the National Academy of Sciences. 2010;107:12299–12304. doi: 10.1073/pnas.1007181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. 2003;4:309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- D'Intino G, Paradisi M, Fernandez M, Giuliani A, Aloe L, Giardino L, Calzà L. Cognitive deficit associated with cholinergic and nerve growth factor down-regulation in experimental allergic encephalomyelitis in rats. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3070–3075. doi: 10.1073/pnas.0500073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Molecular and Cellular Neuroscience. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat P, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Research. 2005;1033:186–193. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Gnahn H, Hefti F, Heumann R, Schwab ME, Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: Evidence for a physiological role of NGF in the brain? Brain Research. 1983;285:45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Hamner S, Arumae U, Li-Ying Y, Sun YF, Saarma M, Lindholm D. Functional characterization of two splice variants of rat bad and their interaction with bcl-w in sympathetic neurons. Molecular and Cellular Neuroscience. 2001;17:97–106. doi: 10.1006/mcne.2000.0905. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. Journal of Neuroscience. 1986;6:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Hartikka J, Eckenstein F, Gnahn H, Heumann R, Schwab M. Nerve growth factor increases choline acetyltransferase but not survival or fiber outgrowth of cultured fetal septal cholinergic neurons. Neuroscience. 1985;14:55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Gearhart DA, Parikh V, Hohnadel EJ, Davis LW, Middlemore ML, Warsi SP, Waller JL, Terry AV., Jr Comparison of galantamine and donepezil for effects on nerve growth factor, cholinergic markers, and memory performance in aged rats. Journal of Pharmacology and Experimental Therapeutics. 2006;316:679–694. doi: 10.1124/jpet.105.093047. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Chan CB, Weinshenker D, Hall RA, Xiao G, Ye K. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chemistry & Biology. 2009;16:644–656. doi: 10.1016/j.chembiol.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Rantamaki T, Di Lieto A, Lindemann L, Hoener MC, Castren E. Darkness reduces BDNF expression in the visual cortex and induces repressive chromatin remodeling at the BDNF gene in both hippocampus and visual cortex. Cellular and Molecular Neurobiology. 2010;30:1117–1123. doi: 10.1007/s10571-010-9544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Oyler GA, Higgins GA. Localization of nerve growth-factor receptor messenger-rna and protein in the adult-rat brain. Experimental Neurology. 1989;106:209–221. doi: 10.1016/0014-4886(89)90154-4. [DOI] [PubMed] [Google Scholar]
- Koponen E, Võikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castrén E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB–PLCγ pathway, reduced anxiety, and facilitated learning. Molecular and Cellular Neuroscience. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–514. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of trk neurotrophin receptors in the absence of neurotrophins. Proceedings of the National Academy of Sciences. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early alzheimer's disease. European Archives of Psychiatry & Clinical Neuroscience. 2008;258:124–128. doi: 10.1007/s00406-007-0764-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-delta delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nature Reviews Neuroscience. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Morse J, Wiegand S, Anderson K, You Y, Cai N, Carnahan J, Miller J, DiStefano P, Altar C, Lindsay R. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. Journal of Neuroscience. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Chen Z, Lee FS, Chao MV. Transactivation of trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. Journal of Neuroscience. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamäki T, Hendolin P, Kankaanpää A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Männistö PT, Castrén E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–62. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A, Lesch KP, Rios M, Castrén E. Antidepressant Drugs Transactivate TrkB Neurotrophin Receptors in the Adult Rodent Brain Independently of BDNF and Monoamine Transporter Blockade. PLoS One. 2011;6:e20567. doi: 10.1371/journal.pone.0020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. Journal of Neuroscience. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Bhattacharyya A, Rua LA, Alberta JA, Stephens RM, Kaplan DR, Stiles CD. Differential utilization of trk autophosphorylation sites. Journal of Biological Chemistry. 1996;271:20175–20181. doi: 10.1074/jbc.271.33.20175. [DOI] [PubMed] [Google Scholar]
- Seiler M, Schwab ME. Specific retrograde transport of nerve growth-factor (ngf) from neocortex to nucleus basalis in the rat. Brain Research. 1984;300:33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology. 2006;51:474–486. doi: 10.1016/j.neuropharm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Van Dam D, Abwamowski D, StaufenBiel M, De Deyn PP. Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology. 2005;180:177–190. doi: 10.1007/s00213-004-2132-z. [DOI] [PubMed] [Google Scholar]
- Webster N, Pirrung M. Small molecule activators of the trk receptors for neuroprotection. BMC Neuroscience. 2008;9:S1. doi: 10.1186/1471-2202-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: Loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Widmer HR, Knusel B, Hefti F. BDNF protection of basal forebrain cholinergic neurons after axotomy: Complete protection of p75NGFR-positive cells. Neuroreport. 1993;4:363–366. doi: 10.1097/00001756-199304000-00005. [DOI] [PubMed] [Google Scholar]
- Yano K, Koda K, Ago Y, Kobayashi H, Kawasaki T, Takuma K, Matsuda T. Galantamine improves apomorphine-induced deficits in prepulse inhibition via muscarinic ACh receptors in mice. British Journal of Pharmacology. 2009;156:173–180. doi: 10.1111/j.1476-5381.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


