Abstract
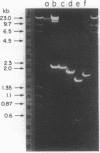
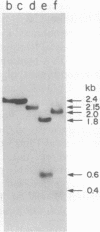
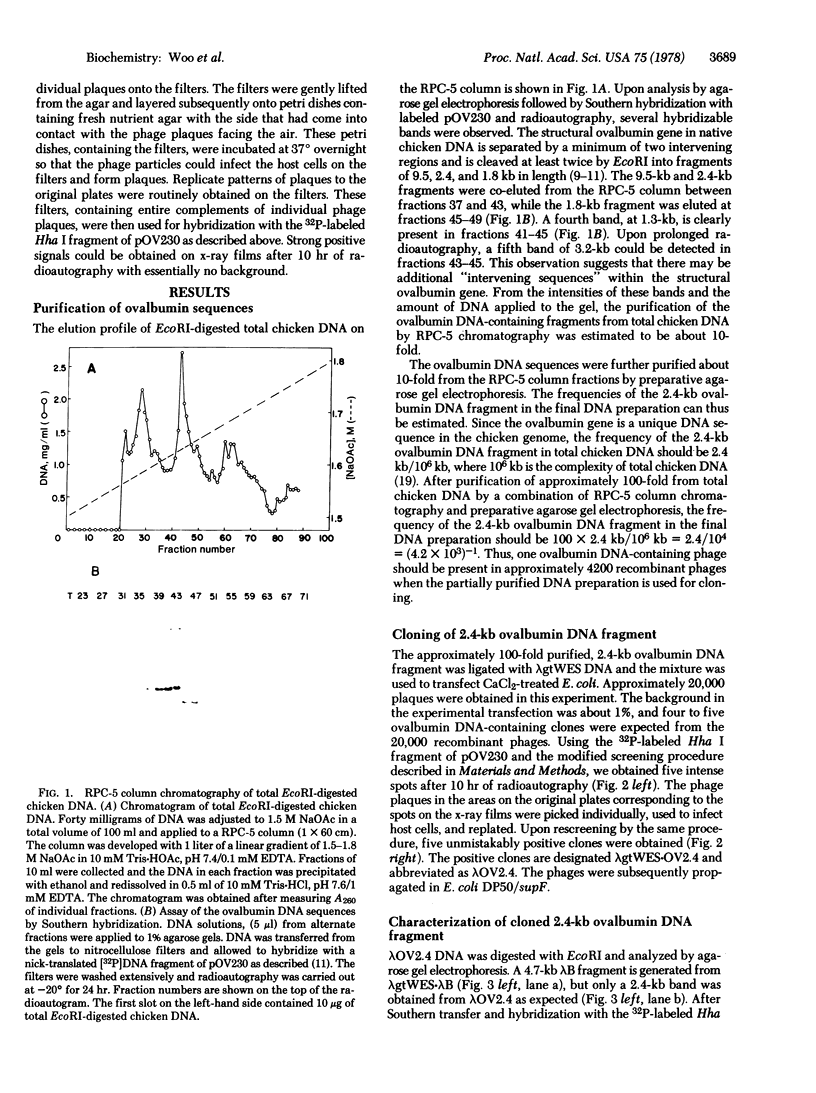
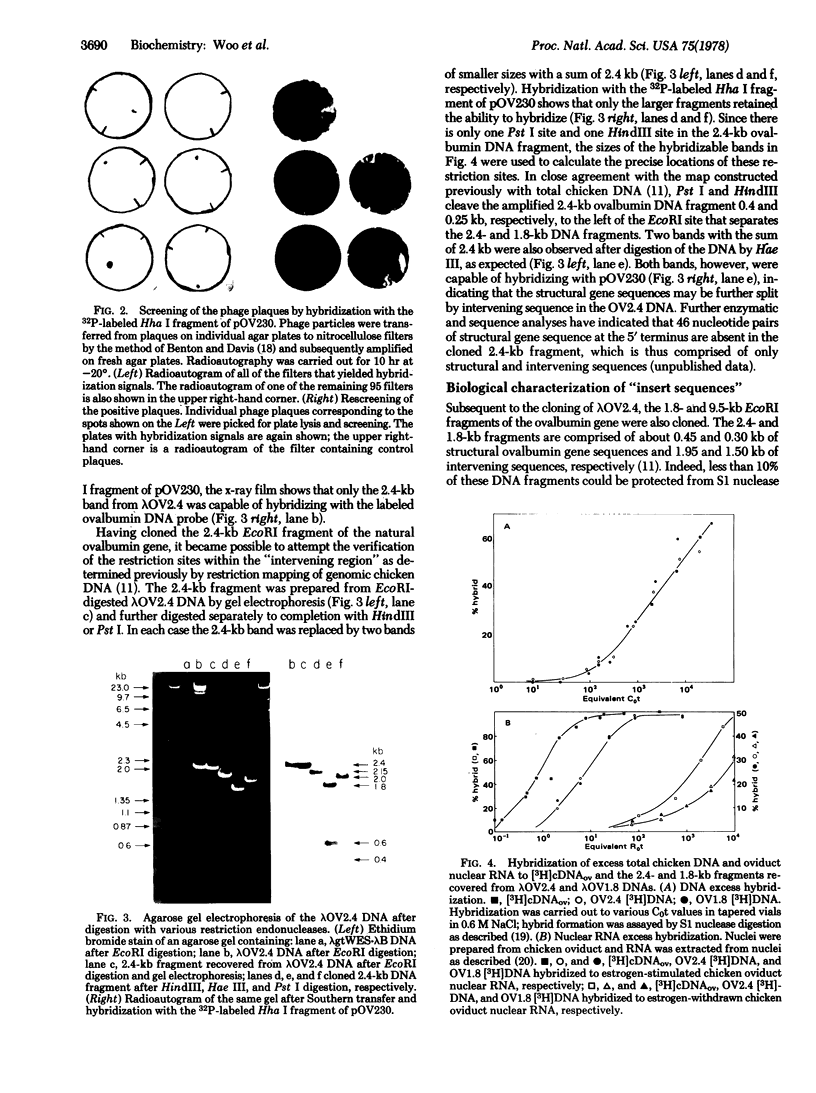
The structural ovalbumin DNA sequences are not contiguous and are separated by multiple “intervening regions” in native chicken DNA. EcoRI, a restriction endonuclease that does not cleave the structural ovalbumin DNA sequences, digests the natural ovalbumin gene into three distinct fragments of 2.4, 1.8, and 9.5 kilobase pairs in length by cleaving within these “intervening regions.” The 2.4-kilobase pair fragment contains only about 450 nucleotide pairs of coding sequence, with the rest being intervening sequences. This DNA fragment was cloned in bacteria by using the certified EK2 vector λgtWES·λB after enrichment from total EcoRI-digested chicken DNA by a combination of RPC-5 column chromatography and preparative agarose gel electrophoresis. Five out of approximately 20,000 recombinant phage plaques were capable of hybridizing with a 32P-labeled Hha I fragment of a recombinant plasmid pOV230 containing the entire structural ovalbumin gene. DNA amplified in these recombinant phages, λgtWES·OV2.4, was shown to contain the same restriction endonuclease cleavage sites as in the 2.4-kilobase pair EcoRI fragment previously determined by restriction mapping of total genomic chicken DNA. The intervening sequences were allowed to hybridize with excess total chicken DNA and oviduct nuclear RNA after nick-translation. They were found to be unique chicken DNA sequences, and appeared to be transcribed in their entireties during gene expression. Like the structural gene sequences, the expression of the intervening sequences is also inducible by steroid hormones.
Keywords: gene splicing, recombinant phage screening, intervening sequence expression, restriction mapping
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Wells R. D. Preparative fractionation of DNA restriction fragments by reversed phase column chromatography. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3117–3121. doi: 10.1073/pnas.73.9.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Means A. R., Mitchell W. M., O'Malley B. W. Synthesis of (3H)DNA complementary to ovalbumin messenger RNA: evidence for limited copies of the ovalbumin gene in chick oviduct. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3776–3780. doi: 10.1073/pnas.70.12.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Woo S. L., Dugaiczyk A., Catterall J. F., O'Malley B. W. The ovalbumin gene: structural sequences in native chicken DNA are not contiguous. Proc Natl Acad Sci U S A. 1978 May;75(5):2205–2209. doi: 10.1073/pnas.75.5.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Leder P., Tilghman S. M., Tiemeier D. C., Polsky F. I., Seidman J. G., Edgell M. H., Enquist L. W., Leder A., Norman B. The cloning of mouse globin and surrounding gene sequences in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):915–920. doi: 10.1101/sqb.1978.042.01.093. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan D., Palacios R., Stavnezer J., Taylor J. M., Faras A. J., Kiely M. L., Summers N. M., Bishop J. M., Schimke R. T. Synthesis of a deoxyribonucleic acid sequence complementary to ovalbumin messenger ribonucleic acid and quantification of ovalbumin genes. J Biol Chem. 1973 Nov 10;248(21):7530–7539. [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Brack C., Hozumi N., Schuller R. Cloning of an immunoglobulin variable region gene from mouse embryo. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3518–3522. doi: 10.1073/pnas.74.8.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., Schwartz R., Kalimi M., Clark J. H., O'Malley B. W. Effects of estrogen on gene expression in chick oviduct: nuclear receptor levels and initiation of transcription. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4228–4232. doi: 10.1073/pnas.72.11.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. L., O'Malley B. W. Hormone inducible messenger RNA. Life Sci. 1975 Oct 10;17(7):1039–1047. doi: 10.1016/0024-3205(75)90322-7. [DOI] [PubMed] [Google Scholar]