Abstract
This paper reports an in vitro system for studying generalized genetic recombination. The system uses extracts from Escherichia coli as a source of enzymes and plasmid DNA molecules as substrates. Unit-size plasmid DNA rings are converted into genomes fused at a region of DNA homology at a frequency of about 5-10% over a period of hours. That the fused structures are the result of recombination is supported by two lines of evidence. When two partially homologous plasmids of different sizes are used as substrates for the in vitro system, intermediates containing one plasmid of each size are obtained. Furthermore, fused structures are not formed with high efficiency in extracts from recombination-deficient (Rec A-) cells.
DNA synthesis does not appear to be required for the formation of the recombination intermediates; it is possible to omit DNA precursors from the reaction mixture and, furthermore, to develop the fused structures even in the presence of chaintermininating dideoxynucleoside triphosphates.
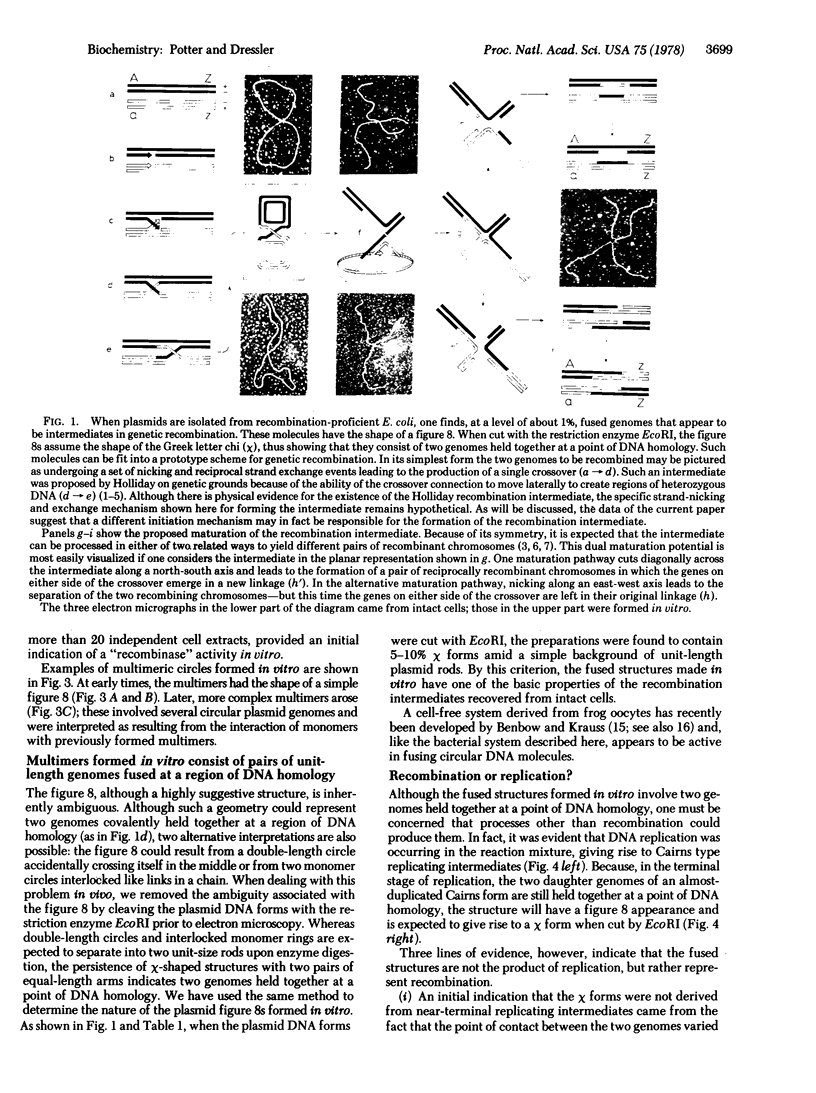
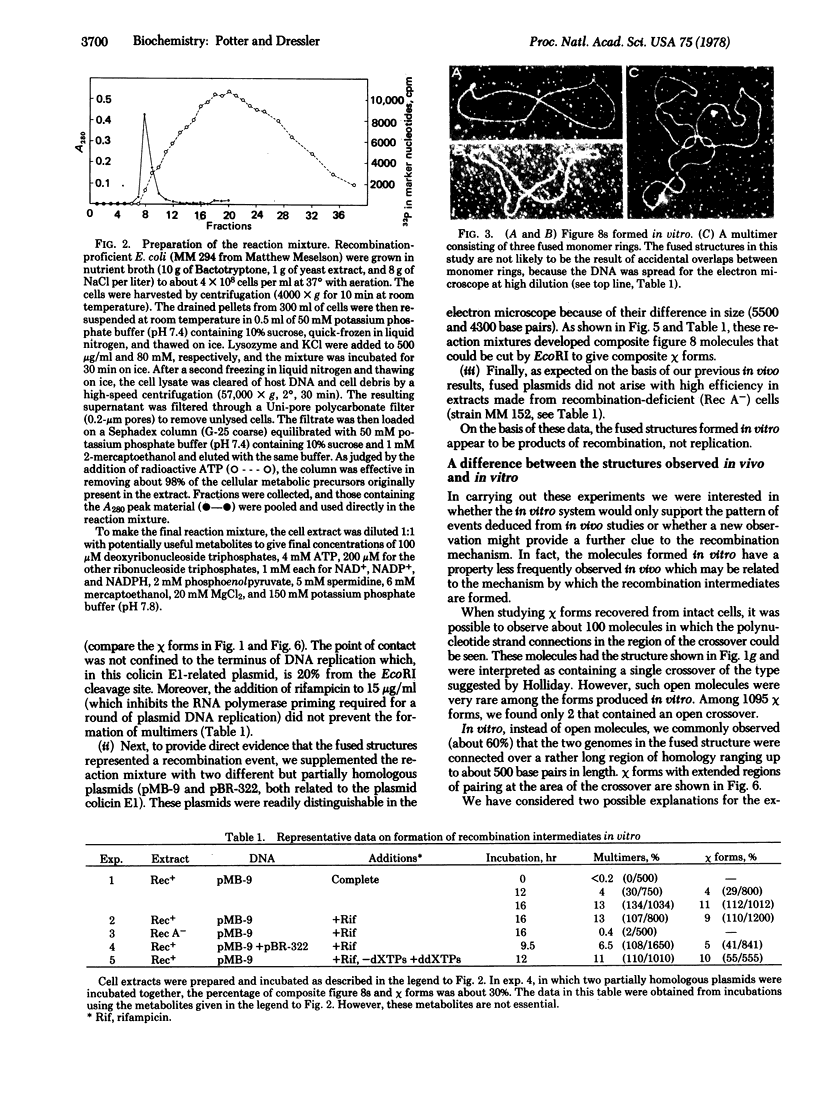
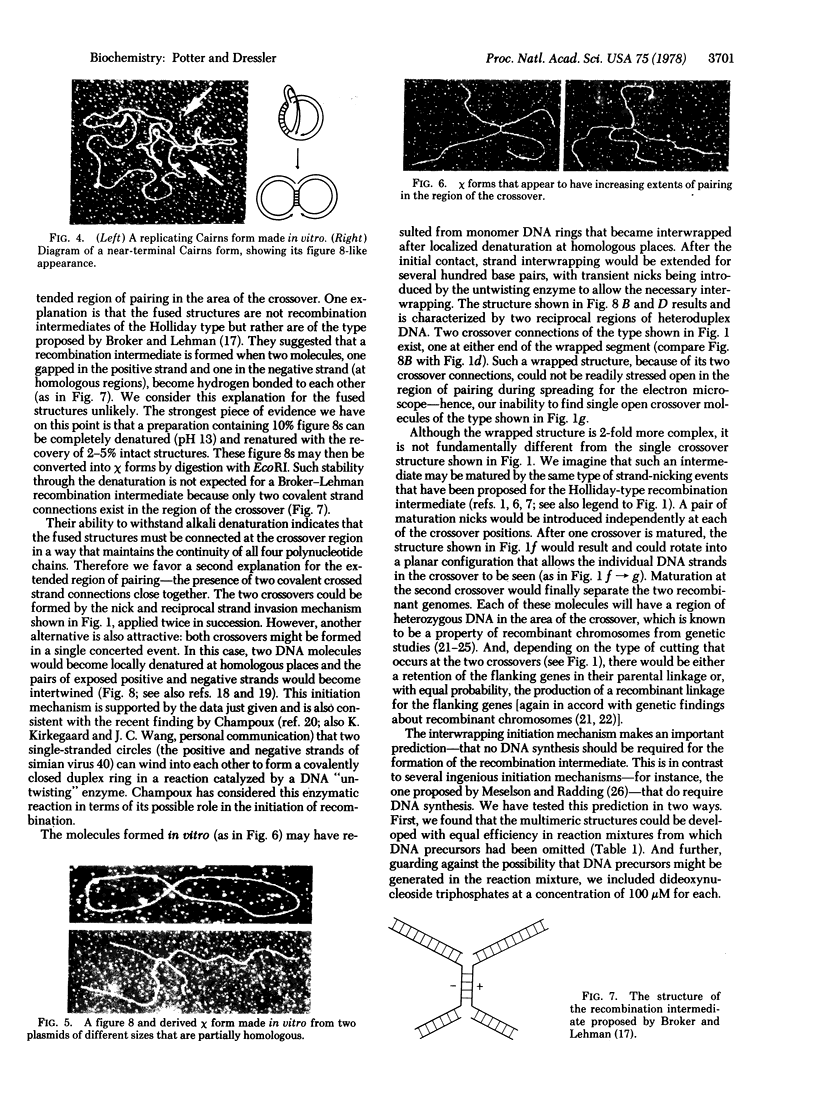
The structures formed in vitro have the basic properties of recombination intermediates previously recovered from intact cells. That is, two genomes are demonstrably fused at a region of homology. However, in one way the molecules formed in vitro have a property less frequently observed in vivo—the fused genomes often appear to be connected over an extended region of homology ranging up to several hundred base pairs in length. This extended region of pairing may indicate the presence of two crossover connections very close together and, as will be discussed, may provide an insight into the mechanism by which the recombination intermediate is formed.
Keywords: reciprocal recombination, plasmid DNA, figure 8 structures, chi forms, initiation of recombination
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Krauss M. R. Recombinant DNA formation in a cell-free system from Xenopus laevis eggs. Cell. 1977 Sep;12(1):191–204. doi: 10.1016/0092-8674(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. Recombinant DNA molecules of bacteriophage phi chi174. Proc Natl Acad Sci U S A. 1975 Jan;72(1):235–239. doi: 10.1073/pnas.72.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Renaturation of complementary single-stranded DNA circles: complete rewinding facilitated by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5328–5332. doi: 10.1073/pnas.74.12.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. A., Lieb M. Heat-inducible lambda phage. V. Induction of prophages with mutations in genes O, P, and R. Genetics. 1967 Nov;57(3):549–560. doi: 10.1093/genetics/57.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger J., Warner R. C., Tessma I. Role of circular dimer DNA in the primary recombination mechanism of bacteriophage S13. Nat New Biol. 1973 Mar 7;242(114):9–12. doi: 10.1038/newbio242009a0. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Heteroduplex DNA: a recombinational intermediate in bacteriophage f1. J Mol Biol. 1976 Feb 15;101(1):25–38. doi: 10.1016/0022-2836(76)90064-4. [DOI] [PubMed] [Google Scholar]
- Gandini Attardi D., Mattoccia E., Tocchini-Valentini G. P. Formation of branched DNA structures by Xenopus laevis oocyte extract. Nature. 1977 Dec 22;270(5639):754–756. doi: 10.1038/270754a0. [DOI] [PubMed] [Google Scholar]
- Holliday R. Molecular aspects of genetic exchange and gene conversion. Genetics. 1974 Sep;78(1):273–287. doi: 10.1093/genetics/78.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst D. D., Fogel S., Mortimer R. K. Conversion-associated recombination in yeast (hybrids-meiosis-tetrads-marker loci-models). Proc Natl Acad Sci U S A. 1972 Jan;69(1):101–105. doi: 10.1073/pnas.69.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. Formation of hybrid DNA by rotary diffusion during genetic recombination. J Mol Biol. 1972 Nov 28;71(3):795–798. doi: 10.1016/s0022-2836(72)80040-8. [DOI] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: electron microscopic observation of recombination intermediates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3000–3004. doi: 10.1073/pnas.73.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: the maturation of recombination intermediates. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4168–4172. doi: 10.1073/pnas.74.10.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Alberts B. Genetic recombination: the nature of a crossed strand-exchange between two homologous DNA molecules. J Mol Biol. 1972 Nov 28;71(3):789–793. doi: 10.1016/s0022-2836(72)80039-1. [DOI] [PubMed] [Google Scholar]
- Sobell H. M. Molecular mechanism for genetic recombination. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2483–2487. doi: 10.1073/pnas.69.9.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. J., Camien M. N., Warner R. C. Kinetics of branch migration in double-stranded DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2299–2303. doi: 10.1073/pnas.73.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M. S., Inman R. B. Visualization of a novel junction in bacteriophage lambda DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3024–3028. doi: 10.1073/pnas.72.8.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Fox M. S. On the molecular basis of high negative interference. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1544–1548. doi: 10.1073/pnas.71.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]








