Abstract
Fluorescamine, which can label surface components of cells grown as monolayers in culture, has been used to probe alterations in chicken embryo fibroblasts infected with a temperature-sensitive mutant of Rous sarcoma virus, Prague A, LA24. The fluorescence of bound fluorescamine on cells at the permissive temperature (35 degrees) was found to be about 1/3 that of cells cultured at the nonpermissive temperature (41 degrees). During development of the transformed phenotype, i.e., after transfer of the cells from 41 degrees to 35 degrees, the decrease in surface fluorescence was observed to be an early event occurring within the first 4-8 hr after temperature shift. This alteration took place on a time scale similar to that of changes in 2-deoxyglucose transport and an increased rate of DNA synthesis, but before any major morphological changes. The change was related to cell transformation rather than to growth differences of the cells at the two temperatures. Further, it was found that fluorescamine was not monitoring the loss of LETS glycoprotein from the surface or the loss of any other surface components that could be detected by lactoperoxidase-catalyzed iodination of surface proteins. When fluorescamine-labeled components were resolved by polyacrylamide gel electrophoresis, significant differences were seen between components from cells cultured at 35 degrees compared with those from cells cultured at 41 degrees. Based on these results, possible mechanisms accounting for the fluorescence differences are suggested.
Full text
PDF
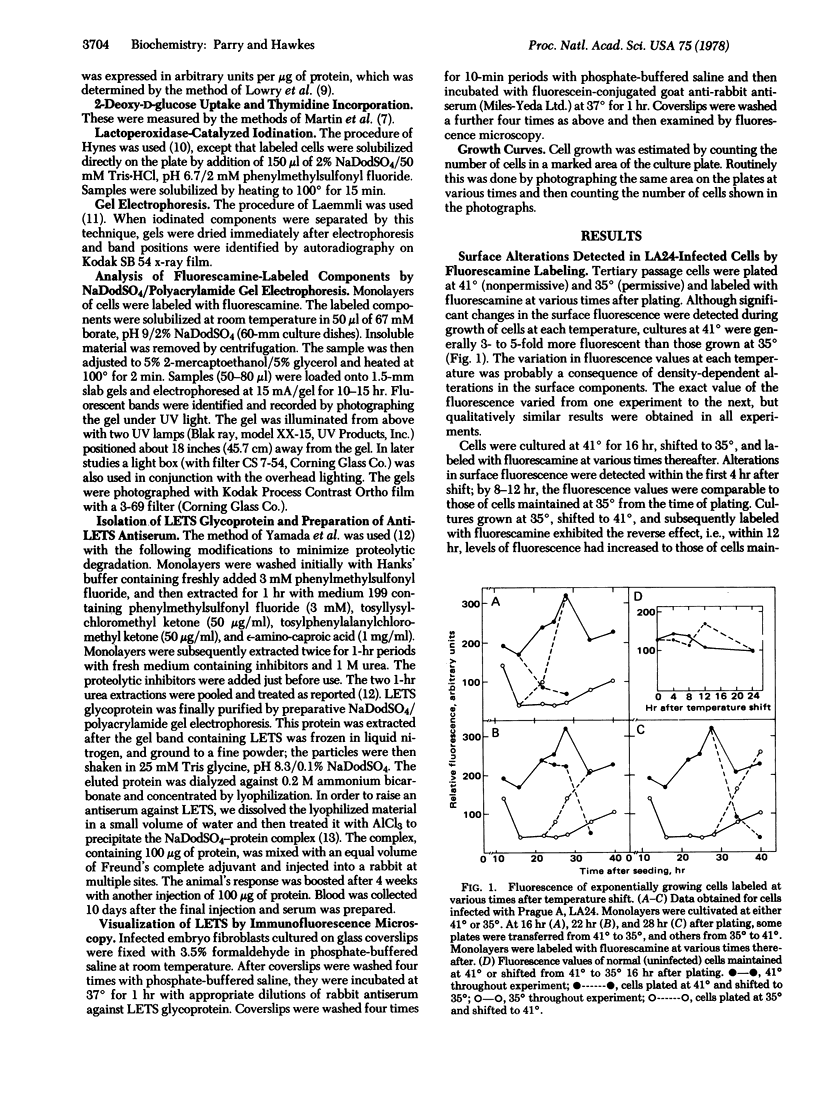
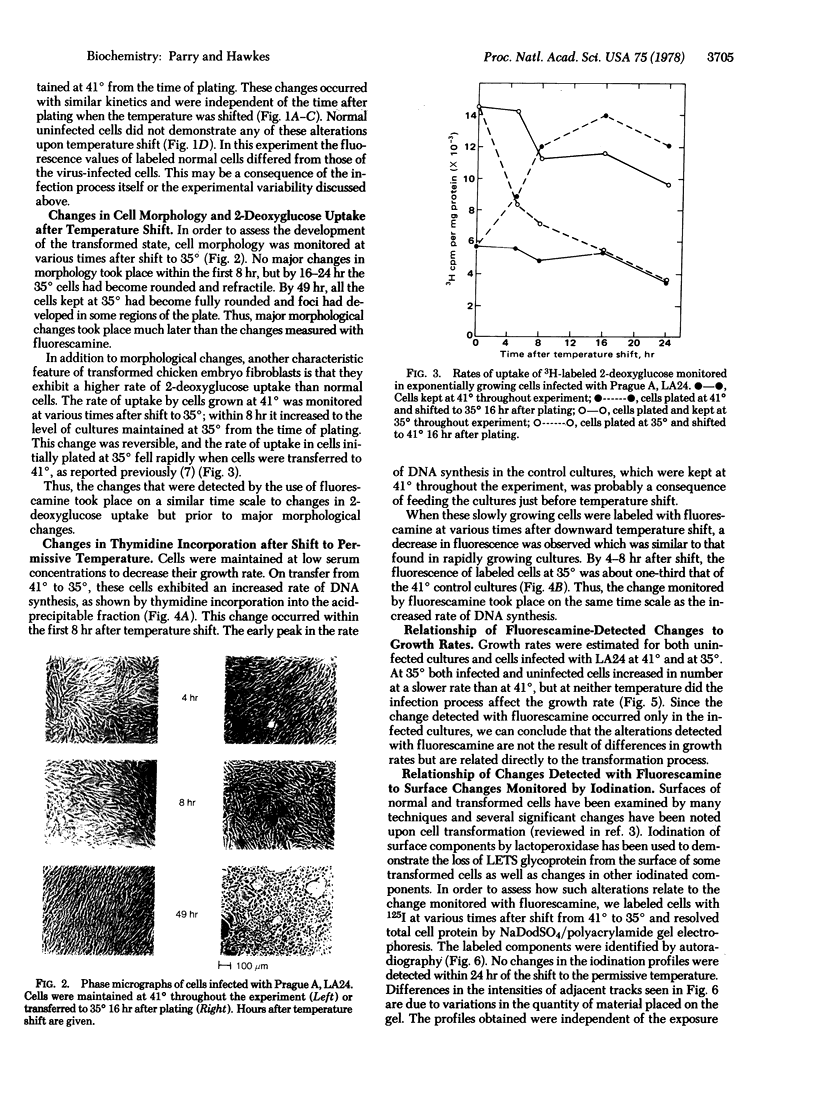

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell M. J., Hatie C., Tischler A. N., Calvin M. Preferential inhibition of the growth of virus-transformed cells in culture by rifazone-82, a new rifamycin derivative. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2520–2524. doi: 10.1073/pnas.71.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes S. P., Bartholomew J. C. Quantitative determination of transformed cells in a mixed population by stimultaneous fluorescence analysis of cell surface and DNA an individual cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1626–1630. doi: 10.1073/pnas.74.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes S. P., Meehan T. D., Bissell M. J. The use of fluorescamine as a probe for labeling the outer surface of the plasma membrane. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1226–1233. doi: 10.1016/0006-291x(76)90328-4. [DOI] [PubMed] [Google Scholar]
- Hawkes S. P. Surface differences of normal and transformed cells as detected by a new, fluorescent labeling technique. Prog Clin Biol Res. 1976;9:149–158. [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Wyke J. A. Alterations in surface proteins in chicken cells transformed by temperature-sensitive mutants of Rous sarcoma virus. Virology. 1975 Apr;64(2):492–504. doi: 10.1016/0042-6822(75)90126-9. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]