Abstract
Although there is a growing literature describing the role of macrophages in breast cancer, the role of macrophages in inflammatory breast cancer (IBC) is unclear. The aim of present study was to isolate and characterize tumor associated macrophages of IBC and non-IBC patients and define their role in IBC. Tumor infiltrating monocytes/macrophages (CD14+ and CD68+) were measured by immunohistochem-istry using specific monoclonal antibodies. Blood drained from axillary vein tributaries was collected during breast cancer surgery and the percentage of CD14+ in the total isolated leukocytes was assessed by flow cytometric analysis. CD14+ cells were separated from total leukocytes by immuno-magnetic beads technique and were cultured overnight. Media conditioned by CD14+ were collected and subjected to cytokine profiling using cytokine antibody array. Wound healing and invasion assays were used to test whether cytokines highly secreted by tumor drained macrophages induce motility and invasion of breast cancer cells. We found that macrophages highly infiltrate into carcinoma tissues of IBC patients. In addition blood collected from axillary tributaries of IBC patients is highly enriched with CD14+ cells as compared to blood collected from non-IBC patients. Cytokine profiling of CD14+ cells isolated from IBC patients revealed a significant increase in secretion of tumor necrosis factor-α; monocyte chemoat-tractant protein-1/CC-chemokine ligand 2; interleukin-8 and interleukin-10 as compared to CD14+ cells isolated from non-IBC patients. Tumor necrosis factor-a, interleukin-8 and interleukin-10 significantly increased motility and invasion of IBC cells in vitro. In conclusion, macrophages isolated from the tumor microenvironment of IBC patients secrete chemotactic cytokines that may augment dissemination and metastasis of IBC carcinoma cells.
Keywords: Inflammatory breast cancer, Macrophages, Cytokines, Invasion, Motility
1. Introduction
Inflammatory breast cancer (IBC) is the most aggressive and lethal form of breast cancer. Women often present with IBC at a young age (Nouh et al, 2011), are more likely to have metastatic disease at the time of diagnosis (Wedam et al, 2006) and have a shorter survival as compared to women with non-IBC (Chang et al, 1998). IBC is characterized by invasion into dermal lymphatic vessels where IBC cells form tumor emboli (Van Laere et al., 2006). Spreading of tumor emboli within lymphatic and blood vessels leads to distant metastasis and multi-organ failure in IBC patients (Tsoi et al., 2010). Dissemination of carcinoma cells can be regulated by cues from the inflammatory cells within the tumor microenvironment. Macrophages, which are the major inflammatory cells that infiltrate into breast tumors (Mukhtar et al, 2011; Pollard, 2008), contribute to high levels of growth factors, hormones, and cytokines (Aaltomaa et al., 1992; Georgiannos et al, 2003) and are designated as tumor associated macrophages (TAMs). Within the tumor microenvironment macrophages polarize into hetero-geneous subpopulations depending on the type of external stimuli they receive (Cassetta et al., 2011). Among TAM subpopulations are: (1) ‘classical activated macrophages’ (M1), which are activated by pro-inflammatory agents such as interferon-γ (INF-γ) and tumor necrosis factor-α (TNF-α) (Cassetta et al., 2011); (2) ‘alternatively activated macrophages’(M2) developed in response to IL-4 and IL-13 (Gordon and Martinez, 2010; Mantovani and Sica, 2010); and 3) ‘regulatory macrophages’ that express anti-inflammatory cytokines and increase tumor growth, invasion and metastasis (Mosser and Edwards, 2008). Classical activation induces the secretion of pro-inflammatory mediators by the macrophages and recruitment of T-cells as in an early inflammatory response (Ojalvo et al., 2009). M2 macrophages exhibit anti-parasite, immunosuppressive, wound healing and tissue remodeling properties (Gordon, 2003; Martinez et al., 2009). Indeed TAMs can regulate multiple mechanisms associated with dissemination of carcinoma cells. For instance, TAMs secrete proteolytic enzymes such as matrix metalloproteinases-2 and 9 (MMP-2 and MMP-9) that can degrade components of the basement membrane, thereby facilitating tumor cell intravasation and spreading in blood and lymphatic vessels (Hagemann et al., 2005; Mantovani et al, 2006). Increases in expression of MMPs and their inhibitors in TAMs have been found to correlate with distant metastasis of invasive ductal carcinomas (Gonzalez et al., 2007). The cysteine protease cathepsin B (Ibrahim et al., 2006) is expressed by TAMs in a transgenic mouse model for mammary carcinoma (Vasiljeva et al, 2006) and co-expressed with interleukin-10 (IL-10) in late stage lung cancer (Daurkin et al., 2011). We have previously shown that high levels of cathepsin B within the IBC microenvironment are associated with lymphatic metastasis (Nouh et al., 2011). Furthermore, TAMs secrete cytokines that control physiological mechanisms associated with tumor progression, i.e.,interleukin-8 (IL-8),which induces angiogenesis; tumor necrosis factor-alpha (TNF-α), which stimulates tumor growth and invasion (Dirkx et al, 2006), as well as immunosuppressive cytokines, i.e., monocyte chemoat-tractant protein-1 (MCP-1) or CC-chemokine ligand 2 (CCL-2) and IL-10(Daurkin et al., 2011). In fact TAMs play crucial roles in the dissemination of breast cancer cells. This is evident from intravital imaging which has provided new insights into how subpopulations of TAMs patrol inside blood vessels in the tumor microenvironment and at the tumor margins (Egeblad et al, 2008). Thus macrophages are considered to be potential diagnostic and therapeutic targets (Mukhtar et al., 2011). Therapeutic strategies include targeting macrophage recruitment to the tumor site by CCL-2 neutralizing antibodies (Balkwill and Mantovani, 2010); or altering macrophage development by targeting macrophage colony stimulating factor-1 receptor (c-Fms) using the tyrosine kinase inhibitor imatinib (Dewar et al., 2005).
Although macrophages have been identified as major cellular components of the non-IBC microenvironment their role in IBC is not well understood (Kleer et al, 2000). Herein, we show that IBC is characterized by high infiltration and venous drainage of macrophages that secrete cytokines different from those secreted by macrophages from non-IBC patients. Moreover, we identified major cytokines that may contribute to IBC invasion and motility and can be therapeutically targeted.
2. Materials and methods
2.1. Patients
For the purpose of patient enrollment in this study, we obtained Institutional Review Board (IRB) approval from the ethics committee of Ain-Shams University, Cairo, Egypt and the National Cancer Institute (NCI), Cairo University, Giza, Egypt. Patients were enrolled from outpatient breast clinics of Ain Shams University hospitals and NCI Cairo University during the period of January 2010-January 2012. All patients signed consent form including approval for publication of the study results before participation. Inclusion criteria of breast cancer patients were dependent upon a combination of clinical, mammographic, ultrasound and pathological diagnoses as we described before (Nouh et al., 2011). Clinical diagnosis of IBC was applied, according to the American Joint Committee on Cancer (AJCC) T4d designation for IBC (for review see (Dawood et al., 2010), i.e., when a patient presented with a diffuse erythema, peau d’orange and edema of the breast. For IBC patients, pathological confirmation of the clinical diagnosis was dependent upon examination of both skin and core biopsies. Non-IBC patients of stage II–III were also included as a comparison group. Based on the criteria described here, we enrolled 66 patients; 39 were diagnosed as non-IBC and 27 as IBC.
2.2. Localization of tumor associated macrophages in breast tumor microenvironment
Cancer tissues excised from modified radical mastectomies were divided into 2 halves: one fixed in 10% neutral buffered formalin and processed into paraffin blocks for routine diagnosis and immunohistochemistry (IHC) and one snap frozen in liquid nitrogen for molecular and biochemical studies. Pathological data [tumor size, tumor grade (Genestie et al., 1998), presence of lymphovascular invasion, and tumor emboli (Bonnier et al., 1995; Gong, 2008) were assessed, reviewed and tabulated for statistical analysis. For IHC staining of macrophages within paraffin embedded tissue, 5 µm sections were first deparaffinized and rehydrated followed by antigen retrieval. Tissue sections were incubated for 1 h at room temperature with the primary antibodies: monoclonal mouse anti-human CD14 (1:50) antibody (CBL453) from Chemicon (Temecula, CA, USA) and monoclonal mouse anti-human CD68 (1:50) antibody (M0814) from (DakoCytomation, Denmark). Immunostaining for each marker was achieved as we described before (Al-Raawi et al, 2011; Nouh et al, 2011) using EnVision+ Dual Link System-HRP (DAB+) from (DakoCytomation, Denmark). Negative control slides were run in parallel in which the primary antibodies were replaced with PBS. Nuclei were counterstained with hematoxylin and specimens were rinsed in phosphate buffered saline (PBS) and mounted using Permount® for microscopic examination. Two independent readers (MAN. and M.M.M.) assessed immunostaining for CD14+ and CD68+ using light microscopy (Olympus, CX41, Japan). Discordant results were resolved by consultation with a third reader (H.I.). Scoring of immunostaining was done by counting cells positive for CD14 and CD68 (cytoplasmic and membranous staining) in paraffin embedded carcinoma tissues of non-IBC (n = 39) and IBC(n = 27) patients: “0”, no immunostaining was observed; “+”, less than 10% of cells showed positive staining; “++”, 10–50% cells showed positive staining; and “+++”, more than 50% cells showed positive staining (Nouh et al., 2011).
2.3. Blood sample collection and isolation of tumor associated monocytes/macrophages
During modified radical mastectomy, 15–20 ml blood that had drained from the tumor microenvironment through axillary tributaries was collected by the surgeon in heparinized tubes. Collected blood was transferred directly to the laboratory for isolation of leukocytes as we have described (El-Shinawi et al., 2010). Briefly, blood was diluted with an equal amount of PBS, pH 7.2, at room temperature. Mononuclear cells were separated by Histopaque-1077 (Sigma, St. Louis, MO, USA) density gradient cen-trifugation at 2000 rpm. The buffy coat layer containing mononuclear cells was separated and washed twice in PBS. Cells were suspended in RPMI 1640 medium containing 5% heat inactivated FBS at density of 5 × 106 cells/ml. To determine the percentage of TAMs in the total isolated leukocytes, 1 × 105 cells/ml were double stained with fluorochrome-labeled monoclonal antibodies (APC-CD14 and PerCP-CD3) and the percentage of cells staining for CD14+/CD3- in the isolated leukocytes was assessed using FACS Calibur flow cytometer as we described previously (El-Shinawi et al, 2010). We purified TAMs (i.e., CD14+ cells) from the mononuclear cells using “Human Monocyte Negative Selection Enrichment kit without CD16 Depletion” (StemCell Technologies, Vancouver, Canada). Steps of monocyte isolation were followed as described in the kit guidelines. Purity of isolated cells was confirmed by flow cytometric analysis (Subimerb et al., 2010) and found to contain 90–95% CD14+. Purified CD14+ were seeded overnight at concentration of 1 × 106 cells/ml in RPMI-1640 media containing 3% FBS. Media conditioned by CD14+ secretions were collected, aliquoted and stored at −80 °C for cytokine profiling and further studies. Adherent CD14+ were washed with PBS and collected in RIPA lysis buffer and stored at −80 °C for further investigation.
2.4. Cytokine profiling of TAMs drained from axillary tributaries
Media conditioned by CD14+ were subjected to profiling using RayBio™ human cytokine antibody array 3 that simultaneously detects 42 cytokines per patient sample. Culture media without CD14+ secretions were run in parallel as negative control. Experimental steps were conducted according to the manufacturer’s instructions as described (Mohamed, 2012). Signal intensity values representing detected cytokines were subtracted from the background and normalized to positive controls on the same membrane using ImageJ software (National Institutes of Health, MD, USA) as we described before (Mohamed, 2012; Sameni et al., 1995). Signal intensity values of each cytokine assessed in non-IBC (n = 39) and IBC (n = 27) are presented as mean ± SD. Significant differences in levels of secretion of cytokines/chemokines/growth factors between non-IBC versus IBC were assessed using Student’s t-test.
2.5. Three dimensional (3D) culture ofSUM149 IBC cell line in media conditioned by CD14+ cells
We investigated whether media conditioned by CD14+ cells isolated from IBC patients may alter morphology and motility of SUM149 IBC cells (a kind gift of Dr. Stephen Ethier, Medical University of South Carolina, Charleston, SC, USA) derived from primary inflammatory ductal carcinoma (Forozan et al, 1999). First, we concentrated media conditioned by CD14+ cells 1:5, using Amicon Ultracell 10K filters (Millipore, Billerica, MA). Concentrated conditioned media of CD14+ cells isolated from axillary tributaries of IBC patients (n = 10) was re-diluted with SUM149 culture media (Ham’s F12 and 5% FBS) to equal protein content as we described before (Mohamed et al., 2010). To prepare 3D models, sterile glass coverslips were coated with 50 µl Cultrex® Basement Membrane Extract (BME) (Trevigen, Inc., MD, USA) and incubated at 37 °C in CO2 incubator for 15min in 12 well culture plates to solidify. SUM149 cells were used at density of 1 × 104 per coverslip and mixed with 2% BME before overlaying onto each coated cover-slip and incubated for 30–40 min at 37 °C to allow cell attachment. Ham’s F12 and 5% FBS was added and cells were incubated at 37 °C for 24h until they formed emboli-like structures as we described before (Mohamed, 2012; Sameni et al., 2003). After 24h of incubation the medium in 10 wells was replaced by CD14+ cell secretions isolated from IBC patients (n = 10). Control SUM149 cells (2 wells) were seeded in fresh Ham’s F12 and 5% FBS. Forty eight hours later coverslips were examined by phase contrast and morphological changes were recorded using Zeiss Axiovert microscope (Carl Zeiss, AG, Germany).
2.6. Invasion assay
To test whether the major identified cytokines secreted by TAMs possess chemotactic properties and induce invasion, we seeded SUM149 cells (Forozan et al., 1999) in serum free media at a density of 3×103 cells per well in the upper chambers of BD Bio-Coat Matrigel™ Invasion Chambers (Becton Dickinson Labware, Franklin Lakes, NJ, USA). To the lower chambers were added recombinant cytokines (TNF-α, MCP-1/CCL2, IL-10 or IL-8) that had been found to be highly secreted by IBC-CD14+ cells as compared to non-IBC-CD14+ cells. We used cytokine concentrations of 200ng/ml in Ham’sF12 and 1% FBS based on our previous study (Mohamed, 2012). After 24 h incubation, the experiment was stopped and cells were fixed and stained (Mohamed et al., 2008). Briefly, noninvasive cells that remained in the Matrigel or were attached to the upper side of the filter were removed with cotton swabs. Cells on the lower side of the membranes were stained using a Diff-Quik staining kit (Dade-Behring, Inc., NJ, USA) and counted using light microscopy. The mean number of invasive cells was quantified by counting in 5 randomly selected microscopic fields. Data represent the mean number of cells that had invaded in response to cytokines divided by the mean number of cells that had invaded through control membranes and multiplied by 100 (Astanehe et al., 2009).
2.7. Wound healing assay
To investigate whether cytokines highly secreted by TAMs may enhance IBC cell motility, we performed wound-healing assays in duplicate for each candidate cytokine (Liang et al., 2007). SUM 149 cells were cultured in Ham’s F12 media containing 5% FBS in 35 mm Petri dishes previously divided by bottom marker line. Once cells reach 90% confluence media was aspirated and cells were washed twice with PBS and seeded in starvation media of Ham’s F12 and 1% FBS. After overnight culture, media were discarded and cells were washed twice with PBS. Using a 200 µl pipette tip three separate wounds were created across the monolayer cultures perpendicular to the bottom line. Cells were grown in Ham’s F12 and 1% FBS media in the absence (control) and the presence of recombinant TNF-α, MCP-1/CCL2,IL-10 or IL-8 (200ng/ml). Cell migration and wound area closure in response to cytokines were determined at different time intervals (2, 4, 8 and 12h) using ImageJ software (National Institutes of Health, MD, USA) and compared with control cultures grown in Ham’s F12 and 1% FBS media (Liang et al., 2007; Valster et al., 2005).
2.8. Statistical analysis
Statistical analysis was performed with statistical package for social sciences software (SPSS) version 18.0, by using Student’s t-test and Fisher’s least significance difference test (LSD).
3. Results
3.1. Patient clinical and pathological characteristics
Clinical and pathological characterization of IBC and non-IBC patients is shown in Table 1. Women with IBC were more likely to present with 4 or more positive lymph nodes than women with non-IBC. All IBC patients showed positive tumor emboli in comparison to 11% of non-IBC patients.
Table 1.
Clinical and pathological characterization of non-IBC versus IBC patients.
| Characteristic | Non-IBC (N = 39) | IBC(N = 27) | p value |
|---|---|---|---|
| Age | 0.08a | ||
| Range | 33–72 | 29–65 | |
| Mean | 52 ± 1.9 | 42 ± 1.8 | |
| NA | 2 | 0 | |
| Tumor size† | 0.05b | ||
| Mean ± SD | 4.2 ± 2.6 | 4.9 ± 3.6 | |
| <4 | 22(61%) | 7 (34%) | |
| >4 | 14 (39%) | 14(66%) | |
| NA | 3 | 1 | |
| Number of lymph nodes | 0.003*b | ||
| <4 | |||
| >4 | 20(54%) | 4 (16%) | |
| NA | 17(46%) | 21(84%) | |
| 2 | 2 | ||
| ER | 0.4b | ||
| Negative | 23 (59%) | 19(70%) | |
| Positive | 16 (51%) | 8 (30%) | |
| NA | 0 | 0 | |
| PR | 1.0b | ||
| Negative | 25(67%) | 17 (63%) | |
| Positive | 14(33%) | 10(37%) | |
| NA | 0 | 0 | |
| Her-2 | 0.7b | ||
| Negative | 32(79%) | 21(77%) | |
| Positive | 7(21%) | 6 (23%) | |
| NA | 0 | 0 | |
| Tumor grade | 0.4b | ||
| G1 | 2(6%) | 0(0%) | |
| G2 | 27(75%) | 21(77%) | |
| G3 | 7(19%) | 5(19%) | |
| G4 | 0(0%) | 1(4%) | |
| NA | 3 | 0 | |
| Tumor emboli | 0.0001*b | ||
| Negative | 35(89%) | 0(0%) | |
| Positive | 4(11%) | 27(100%) | |
| NA | 0 (0%) | 0(0%) |
Data are reported as mean ± SD.
NA: Data not available.
Significant p value calculated by
Student-t test or
Fisher’s exact test.
n = 22 (five IBC patients did not have a tumor mass).
3.2. Inflammatory breast carcinoma tissues characterized by high infiltration ofCD14+ cells
We evaluated the level of infiltration of monocytes/macrophages in non-IBC and IBC paraffin embedded breast carcinoma tissues using monoclonal antibodies specific for CD14+ (monocyte differentiation marker) and CD68+ (macrophage differentiation marker). IHC staining results were scored for the positivity and intensity of CD14+ and CD68+ staining. Our results revealed a statistically significant increase in the number of CD14+ cells that had infiltrated into the carcinoma tissues of IBC patients as compared to those of non-IBC patients (Table 2 and Fig. 1). On the other hand we did not detect statistically significant differences in the number of CD68+ cells that infiltrated into the tumors of non-IBC versus IBC patients (data not shown).
Table 2.
Scoring of CD14+ cell infiltration into non-IBC versus IBC tissues.
| CD14+ (Score**) | Non–IBC n(%) | IBC n (%) |
|---|---|---|
| + | 11(28.2%) | 5(18.5%) |
| ++ | 18(46.2%) | 6(22.2%) |
| +++ | 10(25.6%) | 16(59.3%) |
| Chi-square test | p =0.021* |
Significant p value calculated by Chi-square test.
“+”, Less than 10% of cells showed positive staining; “++”, 10–50% cells showed positive staining; and “+++”, more than 50% cells showed positive staining.
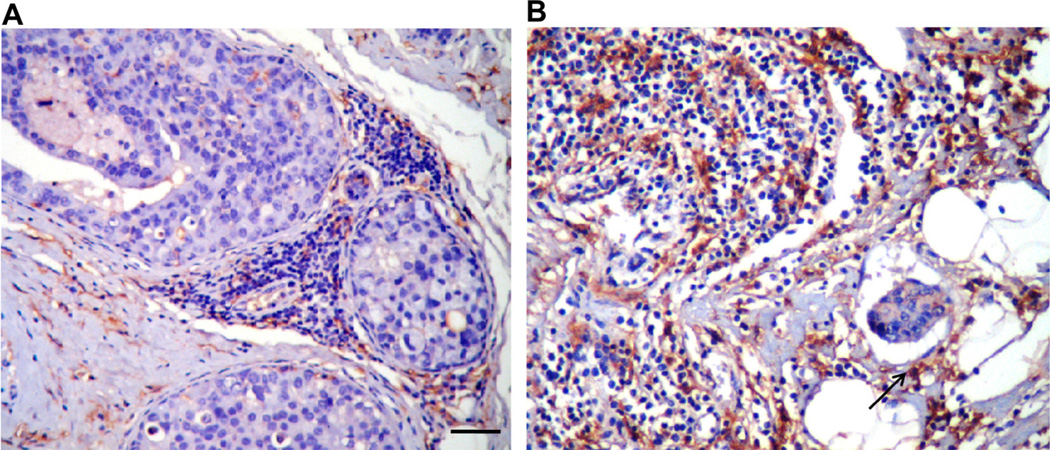
Fig. 1.
Increase inCD14+ cell infiltration in the tumor microenvironment of IBC versus non-IBC. Immunostaining of tumor associated macrophages [CD14+ (brown color)] in paraffin embedded tissue sections. (A) Non-IBC tissues (invasive ductal carcinoma). (B) IBC lymphatic emboli, showing the high number of CD14+ cells in the IBC microenvironment that include aggregates of CD14+ cells (black arrow) adjacent to tumor emboli. Scale bar= 100 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3. Increase in percentage ofTAMs (CD14+ cells) collected from IBC versus non-IBC patients
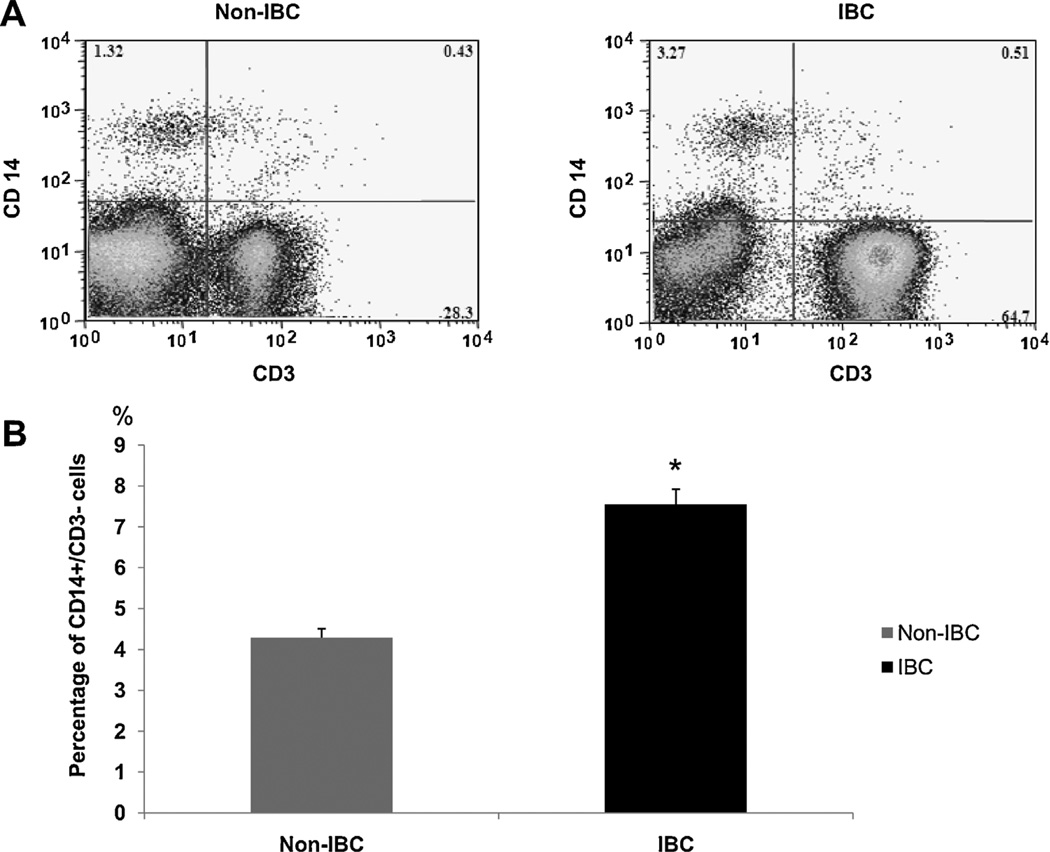
Blood drained from the tumor microenvironment through axillary tributaries of non-IBC and IBC patients was collected during modified radical mastectomy. Immunophenotyping of the total leukocytes collected from axillary tributaries of non-IBC and IBC patient’s was performed by immunofluorescent staining and multi-color flow cytometry and results expressed as a percentage of the total leukocytes as we described before (El-Shinawi et al, 2010). We detected a significant increase in the percentage ofCD14+/CD3- cells present in blood drained from tumor microenvironment of IBC patients as compared to non-IBC patients (Fig. 2A).
Fig. 2.
Analysis of CD14+ cells collected from axillary tributaries of non-IBC and IBC patients. (A) Representative of dual-parameter staining density plots of CD3 (X-axis) and CD14 (Y-axis) cells in the total mononuclear cells isolated from the axilliary tributaries of non-IBC and IBC patients. The values on the X and Yaxis are an arbitrary scale representing the increasing intensity of signal. Numbers shown on graph represent the percentage of stained cells. The proportion of CD14+/CD3− cells is indicated in the upper left corner, the upper right corner represents CD14+/CD3+ cells, the lower left corner represents CD14-/CD3– cells and the lower right corner represents CD14-/CD3+ cells. (B) Bars represent cumulative data for mean ± SD of the percentage of CD14+/CD3– cells in non-IBC (n = 39) and IBC (n = 27) patients; *represents p≤ 0.05 as determined by Student’s t-test.
3.4. Distinct cytokine profile of TAMs (CD14+ cells) drained from axillary tributaries of IBC patients
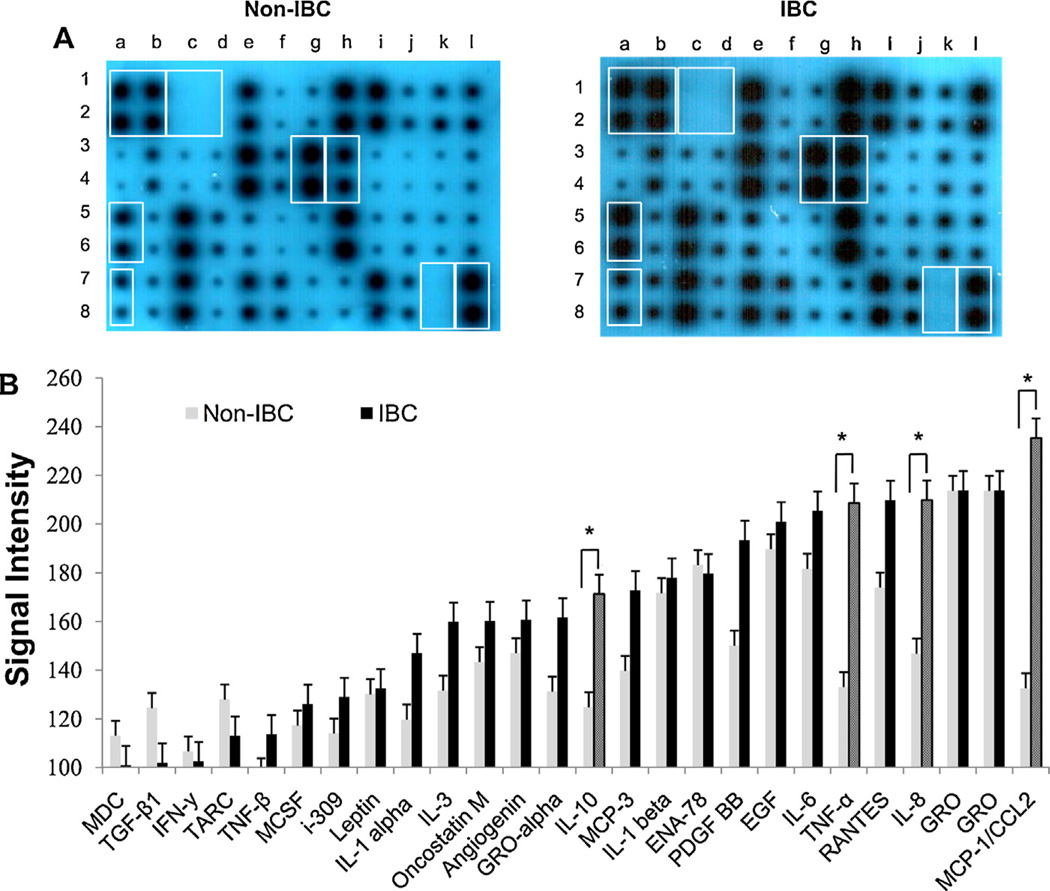
Tumor associated CD14+ drained from axillary tributaries were isolated from total leukocytes as described in “materials and methods” and seeded at concentration of 1 x 106 cells/ml overnight in culture media before profiling for cytokines. We detected statistically significant differences in some cytokines secreted byCD14+ cells isolated from axillary tributaries of IBC patients as compared to non-IBC patients (Fig. 3A). We found that CD14+ cells isolated from of IBC patients secrete high levels ofTNF-α (p = 0.002); MCP-1/CCL2 (p = 0.003); IL-10 (p = 0.013); and IL-8 (p = 0.039) as compared to CD14+ cells isolated from axillary tributaries of non-IBC patients (Fig. 3A). We did not detect any cytokine in the culture media used as negative control (data not shown).
Fig. 3.
Comparison between cytokine profiles of CD14+ cells isolated from non-IBC versus IBC patients. (A) RayBio Human Cytokine Antibody Array 3 as one representative example of anon-IBC patient and an IBC patient. Boxes indicate positive (lane a & b spots 1,2 and lane l spots 7,8) and negative controls (lane c & d spots 1,2 and lane k spots 7,8) and localization of cytokines that show statistically significant differences between non-IBC patients (n = 39) and IBC patients (n = 27). Increases in MCP-1/CCL2 (lane a spot 5, 6), TNF-α (lane a spot 7, 8); IL-8 (laneg spot 3,4) and IL 10 (laneh spot 3, 4) were detected in IBC versus non-IBC patients. (B) Bars represents mean ± SD of signal intensity value of each cytokine secreted by CD14+ cells isolated from non-IBC (n=39) and IBC(n = 27) breast cancer patients as detected by RayBio™ human cytokine antibody array 3 and intensity value quantified by ImageJ software; *represents significant p≤ 0.05 as determined by Student’s t-test.
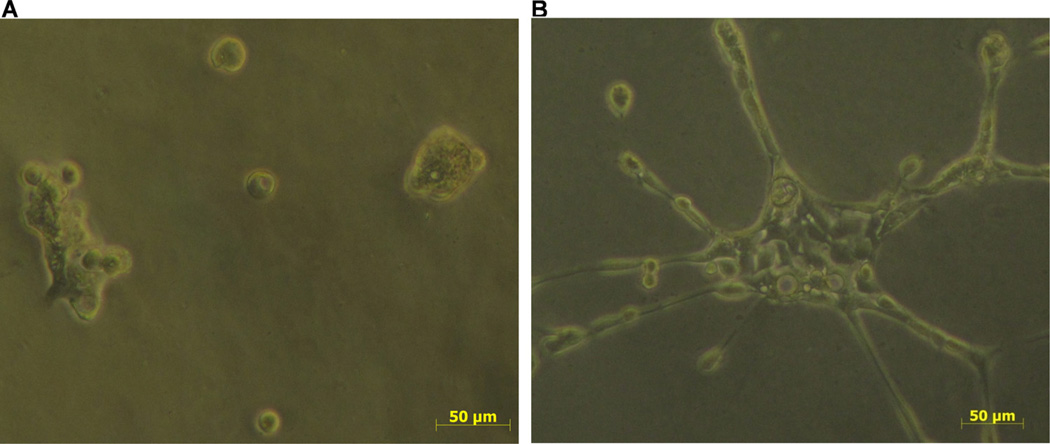
3.5. Media conditioned by CD14+ cells disrupt emboli-like structures and induce motility ofSUM149 cells
SUM149 cells seeded in Ham’s F12 and 5% FBS form emboli-like structure (Fig. 4A). On the other hand SUM149 cells seeded in media conditioned by CD14+ cells isolated from IBC patients form branched-like structures that exhibit migratory properties (Fig. 4B). These results suggest that secretions of CD14+ cells isolated from IBC patients contribute to migratory properties of IBC cells.
Fig. 4.
Media conditioned by CD14+ cells isolated from IBC patients induce migration of SUM149 cells. (A) Tumor emboli of SUM149 cells seeded in Ham’s F12 and 5% FBS culture media. (B) Increase in migration and formation of branched structures by SUM149 cells seeded in media conditioned by CD14+ cells. Images were recorded using phase contrast.
3.6. Cytokines secreted by tumor-drained CD14+ cells increase motility and invasion ofSUM149 IBC cells
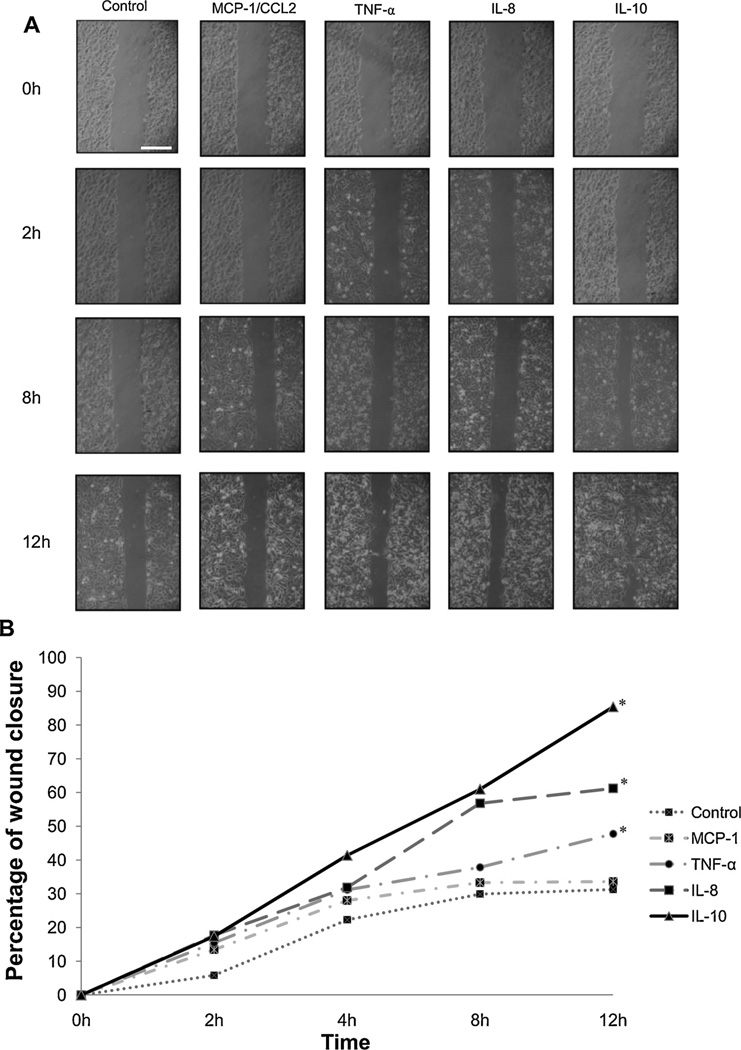
IBC tumor drained macrophages are characterized by secretion of TNF-α,MCP-1/CCL2,IL-10 and IL-8. Therefore we tested whether these cytokines may induce cell migration using a wound-healing assay (Hoffmeyer et al., 2005; Liang et al., 2007). We compared the migration of SUM149 cells in response to TNF-α,MCP-1/CCL2, IL-10 or IL-8 by capturing phase contrast images of wound area closure at different time intervals. Wound closure in the presence of the cytokines was measured with ImageJ software at different time intervals and compared with wound closure of control. The percentage of wound closure area over time was calculated as percentage of the original wound size by dividing the non-closure area at desire time by the initial wound area at 0h and multiply by 100 (Valster et al., 2005). The percentage wound closure by SUM149 cells seeded in media containing MCP-1/CCL2, TNF-α, IL-8 or IL-10 was recorded at 0, 2, 8 and 12 h (Fig. 5A). At 12 h wound closure of control SUM149 cells was 31%, whereas wound closure ofMCP-1/CCL2, TNF-α, IL-8 and IL-10 treated cultures was 33%, 47%, 61% and 87%, respectively (Fig. 5B). Statistical analysis using Fisher’s least significant difference (LSD) test revealed a significant (p ≤0.00) increase in motility of SUM149 cells incubated with TNF-α, IL-8 orIL-10.
Fig. 5.
Cytokines stimulate motility of SUM149 IBC cells. (A) Cells were seeded in media containing MCP-1/CCL2, TNF-α, IL-8 or IL 10 and cell motility was measured by a wound healing assay as described in Section 2. Area of wound closure was imaged at the indicated times and measured using ImageJ. Scale bar= 100 µm. (B) Graph illustrates the percentage of wound closure. TNF-α, IL-8 and IL-10 significantly enhance motility of SUM149 cells. Results are expressed as mean ± S.D of three independent experiments; *represents p≤ 0.00 as determined by Fisher’s least significant difference (LSD).
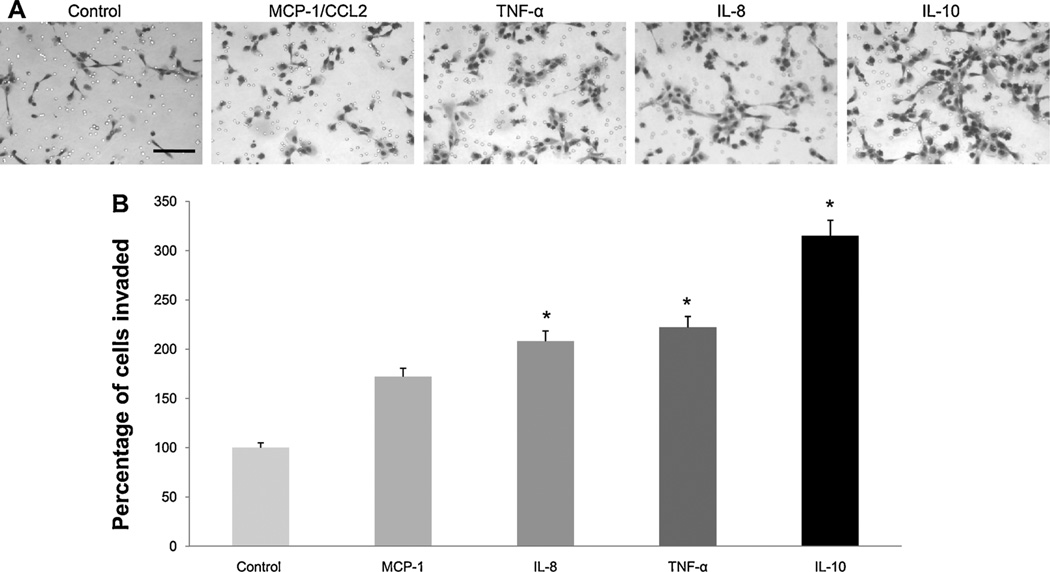
To test whether cytokines highly secreted by IBC tumor drained macrophages alter the invasion of IBC cells, we utilized BD Matrigel invasion chambers in which filters are coated with reconstituted basement membrane. Our results revealed that TNF-α, MCP-1/CCL2, IL-10 and IL-8 possess chemotactic properties and induce invasion of SUM149 cells through basement membrane (Fig. 6A). Only IL-8, TNF-α and IL-10 significantly (p = 0.001, p = 0.02 and p = 0.000, respectively) increased invasion (Fig. 6B).
Fig. 6.
Cytokines stimulate invasion of SUM 149 IBC cells. (A) Images are representative of SUM 149 cells that invaded through the rBM-coated filters in response to either control culture media, MCP-1/CCL2, TNF-α, IL-8 or IL 10. Results are representative of three independent experiments; scale bar=150 µm. (B) Quantification of cells that invaded was performed by counting the average number of nuclei in 5 fields of view per filter (using ImageJ) and is expressed as percentage of cells invaded as compared to control. Results are representative of three independent experiments and are expressed as mean ± SD; * represents p≤ 0.05 as determined by Student’s t-test.
4. Discussion
The tumor microenvironment is characterized by infiltration of monocytes/macrophages that play distinct roles in tumor progression and metastasis (Cassetta et al., 2011). The notion that macrophages are “obligate partners for tumor cell migration, invasion, and metastasis” (Condeelis and Pollard, 2006) and thatTAMs induce an invasive phenotype (Mantovani et al., 2006, Sica et al., 2006) is evident from several studies. Macrophages have been found to attract carcinoma cells to intravasate into blood vessels and spread through the circulation. Using intravital imaging, Wyck-off and colleagues showed that macrophages lining blood vessels release EGF that induces invasion and motility of metastatic adenocarcinoma cells expressing EGFR (Wyckoff et al., 2004). Moreover, large numbers of macrophages are present at the invasive margins of tumors and tumor cell intravasation is associated with “perivascular macrophages” (Wyckoff et al, 2007). In addition, cancer patients are characterized by infiltration with different sub-populations of macrophages (Mosser and Edwards, 2008) which were found to be associated with cancer dissemination and metastasis (Subimerb et al., 2010) and immune suppression (Daurkin et al, 2011). In this regard macrophages have been considered as “mediators” of solid tumor metastasis (DeNardo et al., 2008); however, secretory molecules that control tumor progression need to be examined.
In vitro studies showed that molecules secreted by monocytes/macrophages may regulate breast cancer invasion and motility. For instance, culturing weakly invasive breast cancer cell lines (MCF-7, SKBR-3) with macrophages enhances their invasion via a TNF-α/MMP-dependent mechanism (Hagemann et al., 2004). Our previous studies showed changes in morphology and an increase in invasiveness and degradation of extracellular matrix proteins when the SUM149 IBC cell line was cultured with human U937 monocytes or in media conditioned by human monocytes. Invasiveness and motility of SUM149cells were associated with an increase in expression of the cysteine protease cathepsin B and caveolin-1 (Mohamed et al., 2008). In addition, we found that IL-8 secreted by the U937 cells induced the expression of fibronectin and IBC motility via the phosphatidylinositol-3 kinas (PI3k/Akt) signaling pathway (Mohamed, 2012). Thus we concluded that monocytes/macrophages may contribute to IBC pathogenesis by enhancing dissemination and spreading of carcinoma cells to lymph nodes and distant organs via both lymphatic and hematogenous routes. In the present study we found a statistically significant increase in macrophage infiltration in IBC versus non-IBC tissues. In addition, TAMs was localized around IBC tumor emboli. These results suggest that in IBCTAMs may contribute to tumor emboli formation by stimulating IBC cell invasion, motility and intravasa-tion into lymphatic or blood vessels. These results support those of Wyckoff and colleagues (Wyckoff et al., 2007) who demonstrated breast cancer cell intravasation in association with macrophages in murine mammary models.
To identify major molecules that may participate in the cross talk between TAMs and IBC cells we profiled cytokines secreted by TAMs. We used an inter-operative method to collect TAMs from venous circulation of the breast (El-Shinawi et al., 2010). Our method has clinical significance since we were able to isolate TAMs directly before passing through the reticuloendothelial system and before becoming diluted in circulation (Carroll, 2004). We found that TAMs drained from the axillary tributaries of IBC patients characterized by elevated secretion of TNF-α, MCP-1/CCL2, IL-10 and IL-8as compared to TAMs isolated from non-IBC patients. Interestingly the cytokines detected revealed that TAMs isolated from IBC patients exhibit a mixed cytokine pattern of inflammatory macrophages (M1) as indicated by secretion of TNF-α and resident macrophages (M2) as indicated by secretion of IL-10 (Caras et al., 2011). Secretion of TNF-α has been associated with increased proliferation, growth, invasion and metastasis of malignant diseases (Szlosarek et al., 2006). Moreover, TNF-α has been found to induce breast cancer cell motility and invasion via up-regulation of matrix metalloproteinases (Hagemann et al., 2004) and induction of c-Jun N-terminal kinases (JNK) and Nuclear factor kappa beta (NF-κB) signaling pathways (Hagemann et al., 2005). On the other hand, IL-10 has been shown to contribute to non-small cell lung cancer progression and poor prognosis (Zeni et al, 2007). IL-10 is an immunosuppressive cytokine that prevents maturation of dendritc cells and inhibits antigen presentation (Qin et al., 1997).
In breast cancer IL-10 is considered to be a multifunctional cytokine associated with disease progression (Hamidullah et al., 2012). In breast cancer patients there is an increase in serum IL-10 (Kozlowski et al., 2003) and in breast cancer tissue IL-10 is associated with poor prognosis (Llanes-Fernandez et al., 2006). Furthermore, in the breast tumor microenvironment IL-10 induces tumor cell proliferation (Hamidullah et al., 2012). TAMs are the main source of IL-10 in the breast tumor microenvironment and IL-10 secreted by TAMs suppresses differentiation and maturation of T-cells (Hao et al., 2012). Herein, we found that TAMs isolated from IBC secretes high levels of IL-10 as compared to TAMs isolated from non-IBC. IL-10 expressed by TAMs in non-small cell lung cancer correlates with pleural and lymphovascular invasion; lymph node metastasis and poor prognosis (Wang et al., 2011). Studies showed that, human cytomegalovirus (HCMV) infection stimulates the production of IL-10 in macrophages (Nordoy et al., 2003). Our recent results, showed that that IBC carcinoma tissues were significantly more infected with HCMV-DNA compared to non-IBC (El-Shinawi et al., 2013). Thus over-expression of IL-10 by IBC TAMs, detected in the present study may be due to HCMV infection, since 80% of IBC carcinoma tissues are positive HCMV-DNA (El-Shinawi et al., 2013).
Increases in expression of MCP-1/CCL2 and IL-8 by TAMs of IBC patients may contribute to metastatic and angiogenic properties of IBC (Giordano and Hortobagyi, 2003). MCP-1/CCL2 has been found to be highly expressed within the breast tumor microenvironment and suggested to play a role in malignant transformation and breast cancer metastasis (Soria and Ben-Baruch, 2008). In MMTV-PyMT mouse models CCL2 secreted by carcinoma cells and TAMs facilitates trans-endothelial migration and lung metastasis of cancer cells, a mechanism involving interaction between cancer cells and TAMs expressing C-C chemokine receptor type-2 (CCR2) within the tumor microenvironment (Qian et al., 2011). Furthermore, macrophage-derived IL-8 found to play potential role in angiogenesis of inflammatory diseases (Koch et al., 1992) and endometrial cancer (Fujimoto et al., 2002).
Since IBC is characterized by highly invasive and metastatic properties, we tested whether recombinant TNF-α, CCL-2/MCP-1, IL-10 or IL-8 alter the motility and invasion of IBC cells. Our results revealed that IL-10 was the most effective at inducing motility and invasion of IBC cells. We propose that enhanced motility and invasion of SUM149 cells in response to IL-10 may be due to stimulation of Signal Transducer and Activator of Transcription (STAT3) signaling pathways which subsequently induce cell motility (Hamidullah et al., 2012). In the present study we showed for the first time that macrophages are major cellular components of the IBC microenvironment and they secrete cytokines that affect motility and invasion of IBC cells. Cytokines secreted by IBC-TAMs can be therapeutically targeted in IBC patients.
Acknowledgements
This work was conducted at Cancer Biology Research Laboratory (CBRL), Department of Zoology, Faculty of Science, Cairo University, Egypt. We acknowledge the contribution of Dr. Sayed F. Abdelwhab and Dr. Maha Sobhy, the Egyptian Company for Blood Transfusion Services (Egyblood)-VACSERA, Giza, Egypt for their help in some flow cytometric experiments. We thank Ms. Amal Youns, postgraduate student at CBRL, for her help in the preparation of clinical-pathological data. We also thank Ms. Haba Basioni and Mr. Atef Abdelmonen, Assistant Lecturers (Department of Zoology, Faculty of Science, Cairo University, Egypt), for their help with statistical analysis. We thank Prof. Hoda Ismail (Department of Pathology, National Cancer Institute, Cairo University, Giza, Egypt) for her assistance in reviewing and scoring of pathology slides. This work was supported by Avon-Foundation Grants # 02-2007-049 (M.M.M., B.F.S.) and # 02-2009-085b (R.J.S., M.M.M.); and National Institutes of Health R03 TW008624 (B.F.S,M.M.M.).
Abbreviations
- CCL-2
CC-chemokines ligand 2
- CCR2
C-C chemokine receptor type 2
- c-Fms
macrophage colony stimulating factor-1 receptor
- HCMV
human cytomegalovirus
- IBC
inflammatory breast cancer
- IL-4
interleukin-4
- IL-8
interleukin- 8
- IL-10
interleukin-10
- IL 13
interleukin-13
- INF-γ
interferon-γ
- M1
classical activated macrophages
- M2
alternatively activated macrophages
- MCP-1
monocyte chemoattractant protein-1
- MMP-2
matrix metalloproteinases-2
- MMP-9
matrix metalloproteinases-9
- NF-κB
Nuclear factor kappa beta
- PI3K
phosphatidylinositol-3 kinas
- STAT3
signal transducer and activator of transcription
- TAMs
tumor associated macrophages
- TNF-α
tumor necrosis factor-alpha
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Author’s contribution
EAG, MES, MAN and MMM carried out the experiments. MMM, BFS, RJS, MAN and MES made substantial contributions to concept and design of experiments as well as to the drafting and/or revising of the manuscript. All authors have read and approved the manuscript.
References
- Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28:859–864. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- Al-Raawi D, Abu-El-Zahab H, El-Shinawi M, Mohamed MM. Membrane type-1 matrix metalloproteinase (MT1-MMP) correlates with the expression and activation of matrix metalloproteinase-2 (MMP-2) in inflammatory breast cancer. IntJ Clin Exp Med. 2011;4:265–275. [PMC free article] [PubMed] [Google Scholar]
- Astanehe A, Finkbeiner MR, Hojabrpour P, To K, Fotovati A, Shadeo A, et al. The transcriptional induction of PIK3CA in tumor cells is dependent on the oncoprotein Y-box binding protein-1. Oncogene. 2009;28:2406–2418. doi: 10.1038/onc.2009.81. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 2010;87:401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- Bonnier P, Charpin C, Lejeune C, Romain S, Tubiana N, Beedassy B, et al. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? IntJ Cancer. 1995;62:382–385. doi: 10.1002/ijc.2910620404. [DOI] [PubMed] [Google Scholar]
- Caras I, Tucureanu C, Lerescu L, Pitica R, Melinceanu L, Neagu S, et al. Influence of tumor cell culture supernatants on macrophage functional polarization: in vitro models of macrophage-tumor environment interaction. Tumori. 2011;97:647–654. doi: 10.1177/030089161109700518. [DOI] [PubMed] [Google Scholar]
- Carroll RG. Arresting metastases during excisional cancer surgery. Lancet Oncol. 2004;5:147–148. doi: 10.1016/S1470-2045(04)01409-3. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. Sci World J. 2011;11:2391–2402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD. Inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program of the National Cancer Institute, 1975–1992. Cancer. 1998;82:2366–2372. [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM, et al. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–6409. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2010;22(3):515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imat-inib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators ofangiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning diskconfocal microscopy. Dis Model Mech. 2008;1:155–167. doi: 10.1242/dmm.000596. [discussion 65] [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shinawi M, Abdelwahab SF, Sobhy M, Nouh MA, Sloane BF, Mohamed MM. Capturing and characterizing immune cells from breast tumor microenvironment: an innovative surgical approach. Ann Surg Oncol. 2010;17:2677–2684. doi: 10.1245/s10434-010-1029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shinawi M, Mohamed HT, El-Ghonaimy EA, Tantawy M, Younis A, Schneider RJ, et al. Human cytomegalovirus infection enhances NF-kappaB/p65 signaling in inflammatory breast cancer patients. PLoS ONE. 2013;8:e55755. doi: 10.1371/journal.pone.0055755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forozan F, Veldman R, Ammerman CA, Parsa NZ, Kallioniemi A, Kallioniemi OP, et al. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br J Cancer. 1999;81:1328–1334. doi: 10.1038/sj.bjc.6695007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T. Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann Oncol. 2002;13:430–434. doi: 10.1093/annonc/mdf078. [DOI] [PubMed] [Google Scholar]
- Genestie C, Zafrani B, Asselain B, Fourquet A, Rozan S, Validire P, et al. Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res. 1998;18:571–576. [PubMed] [Google Scholar]
- Georgiannos SN, Renaut A, Goode AW, Sheaff M. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery. 2003;134:827–834. doi: 10.1016/s0039-6060(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Giordano SH, Hortobagyi GN. Inflammatory breast cancer: clinical progress and the main problems that must be addressed. Breast Cancer Res. 2003;5:284–288. doi: 10.1186/bcr608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y. Pathologic aspects of inflammatory breast cancer: Part 2. Biologic insights into its aggressive phenotype. Semin Oncol. 2008;35:33–40. doi: 10.1053/j.seminoncol.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez LO, Pidal I, Junquera S, Corte MD, Vazquez J, Rodriguez JC, et al. Over-expression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer. 2007;97:957–963. doi: 10.1038/sj.bjc.6603963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa Band JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer MR, Wall KM, Dharmawardhane SF. In vitro analysis of the invasive phenotype of SUM 149, an inflammatory breast cancer cell line. Cancer Cell Int. 2005;5:11. doi: 10.1186/1475-2867-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SA, Abdelwahab SF, Mohamed MM, Osman AM, Fathy E, Al-Badry KS, et al. T cells are depleted in HCV-Induced hepatocellular carcinoma patients: possible role of apoptosis and p53. Egypt J Immunol. 2006;13:11–22. [PubMed] [Google Scholar]
- Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Llanes-Fernandez L, Alvarez-Goyanes RI, Arango-Prado Mdel C, Alcocer-Gonzalez JM, Mojarrieta JC, Perez XE, et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast. 2006;15:482–489. doi: 10.1016/j.breast.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. CurrOpin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mohamed MM. Monocytes conditioned media stimulate fibronectin expression and spreading of inflammatory breast cancer cells in three-dimensional culture: A mechanism mediated by IL-8 signaling pathway. Cell Commun Signal. 2012;10:3. doi: 10.1186/1478-811X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MM, Cavallo-Medved D, Rudy D, Anbalagan A, Moin K, Sloane BF. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cell Physiol Biochem. 2010;25:315–324. doi: 10.1159/000276564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MM, Cavallo-Medved D, Sloane BF. Human monocytes augment invasiveness and proteolytic activity of inflammatory breast cancer. Biol Chem. 2008;389:1117–1121. doi: 10.1515/BC.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar RA, Nseyo O, Campbell MJ, Esserman LJ. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn. 2011;11:91–100. doi: 10.1586/erm.10.97. [DOI] [PubMed] [Google Scholar]
- Nordoy I, Rollag H, Lien E, Sindre H, Degre M, Aukrust P, et al. Cytomegalovirus infection induces production of human interleukin-10 in macrophages. Eur J Clin Microbiol Infect Dis. 2003;22:737–741. doi: 10.1007/s10096-003-1028-x. [DOI] [PubMed] [Google Scholar]
- Nouh MA, Mohamed MM, El-Shinawi M, Shaalan MA, Cavallo-Medved D, Khaled HM, et al. Cathepsin B: a potential prognostic marker for inflammatory breast cancer. J Transl Med. 2011;9:1. doi: 10.1186/1479-5876-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Noffz G, Mohaupt M, Blankenstein T. Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocyte-macrophage colony-stimulating factor gene-modified tumor cells. J Immunol. 1997;159:770–776. [PubMed] [Google Scholar]
- Sameni M, Dosescu J, Moin K, Sloane BF. Functional imaging of proteolysis: stromal and inflammatory cells increase tumor proteolysis. Mol Imag. 2003;2:159–175. doi: 10.1162/15353500200303136. [DOI] [PubMed] [Google Scholar]
- Sameni M, Elliott E, Ziegler G, Fortgens PH, Dennison C, Sloane BF. Cathepsin B and D are localized at the surface of human breast cancer cells. Pathol Oncol Res. 1995;1:43–53. doi: 10.1007/BF02893583. [DOI] [PubMed] [Google Scholar]
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, et al. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471–479. doi: 10.1111/j.1365-2249.2010.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Tsoi DT, Rowsell C, McGregor C, Kelly CM, Verma S, Pritchard KI. Disseminated tumor embolism from breast cancer leading to multiorgan failure. J Clin Oncol. 2010;28:e180–e183. doi: 10.1200/JCO.2009.25.1009. [DOI] [PubMed] [Google Scholar]
- Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Van Laere SJ, Van den Eynden GG, Van der Auwera I, Vandenberghe M, van Dam P, Van Marck EA, et al. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243–255. doi: 10.1007/s10549-005-9015-9. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- Wang R, Lu M, Zhang J, Chen S, Luo X, Qin Y, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. 2011;30:62. doi: 10.1186/1756-9966-30-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. EurRespirJ. 2007;30:627–632. doi: 10.1183/09031936.00129306. [DOI] [PubMed] [Google Scholar]