Abstract
Background
Interaction with heparan sulfate (HS) proteoglycans is supposed to provide chemokines with the capacity to immobilize on cell surface and extracellular matrix for accomplishing both tissue homing and signalling of attracted cells. However, the consequences of the exclusive invalidation of such interaction on the roles played by endogenous chemokines in vivo, remains unascertained.
Methods and results
We engineered a mouse carrying a Cxcl12 gene (Cxcl12Gagtm) mutations that preclude interactions with HS structures while they do not affect CXCR4-dependent cell-signalling of CXCL12 isoforms (α,β,γ). Cxcl12Gagtm/Gagtm mice develop normally, express normal levels of total and isoform-specific Cxcl12 mRNA and show increased counting of circulating CD34+ haematopoietic precursor cells. Following induced acute ischemia, a marked impaired capacity to support revascularization was observed in Cxcl12Gagtm/Gagtm animals associated to a reduced number of infiltrating cells in the ischemic tissue despite the massive expression of CXCL12 isoforms. Importantly, exogenous administration of CXCL12γ, which binds HS with the highest affinity ever reported for a cytokine, fully restores vascular growth, while HS-binding CXCL12γ, mutants failed to promote revascularization in Cxcl12Gagtm/Gagtm animals.
Conclusions
These findings prove the role played by HS-interactions in the functions of CXCL12 both in homeostasis and physiopathological settings and document originally the paradigm of chemokine-immobilisation in vivo.
Keywords: Animals; Chemokine CXCL12; biosynthesis; genetics; Heparin; analogs & derivatives; metabolism; Hindlimb; blood supply; Homeostasis; Ischemia; genetics; metabolism; Mice; Models, Animal; Muscle, Skeletal; blood supply; Neovascularization, Physiologic; genetics; Protein Isoforms; genetics; Proteoglycans; metabolism; RNA, Messenger; Transcription, Genetic
Keywords: Angiogenesis, Ischemia, Chemokines, Proteoglycans, CXCL12
INTRODUCTION
Chemokines control the migration of a large array of cells, thus regulating function and homeostasis of a number of tissues. Glycosaminoglycans (GAG), the glycanic moiety of proteoglycans, are considered as the critical biological structure that determines the immobilisation of chemokines on the extracellular matrix and cell surfaces. Immobilisation provides chemokines with a robust anchoring against blood flow and, by restraining their diffusion, facilitates both the formation of local gradients and the synchronous coordination of motility and cell adhesion [1,2,3].
Among GAG, chemokines bind preferentially to heparan sulfate (HS) through interactions either of canonical BBXB and BBBXXB (B, basic; X, any residue) or discontinuous cationic protein epitopes, with the negatively charged sulfated residues of HS [4]. Although chemokine binding to GAGs has been well described from a biochemical point of view, the functional aspects of these interactions remain poorly understood and in vivo investigations to address the importance of chemokine-GAG interactions are limited. In some cases the experimental approach, like the administration of sulfated glycans competing exclusively with HS [5] or the genetic interference selectively inhibiting endothelial HS biosynthesis [6], have broad effects and would affect, beyond chemokines, the interaction of GAG with many other factors. In other cases, the overlapping or spatial proximity of chemokine domains involved in receptor-mediated cell signalling and GAG-attachment has obscured interpretation and significance of the results obtained by exogenous administration of GAG-binding mutant chemokines with reduced agonist capacity [7].
In this regard, CXCL12 is unique among chemokines for the spatial separation between the receptor- and HS-binding sites, localized on the opposite sides of the molecule [8], thus permitting the evaluation of the contribution of each domain to their biological functions.
In mice three isoforms (CXCL12α, CXCL12β and CXCL12γ), that show an exceptional degree of interspecies conservation exceeding 95% homology for their amino-acid sequences, are generated by alternative splicing [9,10]. CXCL12β and CXCL12γ contain the entire aminoacid sequence (68Aa) of CXCL12α which encompasses the CXCR4-binding domain and the protein core BBXB motif (K24H25L26K27) critically required for the HS-binding. CXCL12β (72Aa) and CXCL12γ (98Aa) differs from CXCL12α by the addition of the last four and thirty Aa, respectively encoding one and four additional BBXB motifs. While CXCL12α and CXCL12β display affinities for HS or heparin in the same range of magnitude (KD, 93 and 25nM, respectively), that of CXCL12γ is 1.5 nM and constitutes the strongest HS-affinity ever measured for a cytokine [11,12]. This firm interaction with HS relies on the cooperativeness of the core and the high cationic C-ter domain of the chemokine, where the four overlapping BBXB motifs of CXCL12γ mostly accounts for the slow off-rates from immobilized HS [12].
We reported previously that neutralisation of the K24H25L26K27 cationic charge (K24S and K27S substitutions) is sufficient to drastically reduce binding to HS and complexing of CXCL12α either with immobilized-heparin or HS [11,12,13,14]. Similarly, O’Boyle et al recently reported that combined K24S and K27S in CXCL12β, are sufficient to impede HS-binding of this isoform on the apical surface of endothelial cells and promote a dramatic blood and delayed clearance as compared to the WT counterpart [15]. To abrogate HS-binding of CXCL12γ, the combined mutation of K24H25L26K27 and the C-ter four overlapping BBXB motifs is therefore required [12,13]. Of note, while all these HS-binding CXCL12 mutants show a preserved global structure defined by Nuclear Magnetic Resonance [11,12] and are full CXCR4 agonists in vitro [11,10,12,13, 15], they have only a marginal capacity to attract leukocytes or endothelial progenitor cells in vivo and are unable to immobilise and localise properly on GAG structures [13,15]. Importantly, CXCL12 HS-binding mutants induce homologous CXCR4 desensitisation thus neutralising the chemoattractant properties of CXCL12 WT molecules [15]. CXCL12 binds both CXCR4 and CXCR7 [16,17,18,19], is the only cognate ligand of CXCR4, and plays essential and non-redundant roles in organogenesis [20,21]. CXCL12 is widely expressed by mesothelial, epithelial, endothelial and oteoblasts as well as bone marrow (BM) stromal cells [22,23,24]. It acts in post-natal life as a key chemoattractant for haematopoietic progenitor cells (HPC) and leukocytes [24,16,17] and regulates the BM homing and retention/egress of haemotopoietic cells [25,26] as well as the basal trafficking and transendothelial migration of leukocytes [27,28,29]. Aside from homeostasis, CXCL12 is involved both in tumorigenesis and cancer metastasis [30] and is a pathogenic factor in systemic disease like rheumatoid arthritis where it shows proangiogenic and inflammatory effects [31]. In addition, CXCL12 plays a role of paramount importance as an essential and non-redundant factor involved in tissue remodelling, in particular in vascular regeneration [32].
Overall, the structural characteristics and pleiotropic roles played by CXCL12 make it ideally suited for investigating how the interactions with GAG/HS modulate the biological role of a chemokine in vivo. To this aim, we engineered a transgenic mouse expressing a Cxcl12 gene where the critical sequences encoding CXCL12 domains involved in HS-binding are selectively invalidated while preserving intact the CXCR4-agonist potency and efficiency of the mutant proteins. The mutant mice show enhanced serum levels of free CXCL12 and an increased number of circulating leukocytes and CD34+ haematopoietic cells [5]. Strikingly, and in keeping with the essential role played by CXCL12 in tissue repair, these mice display a dramatically reduced capacity to regenerate vascular growth following acute ischemia that is recovered by expression of WT CXCL12 demonstrating the importance of GAG binding for proper in vivo function. This is the first description to our knowledge of an animal model where the HS-binding capacities of an endogenous chemokine were genetically invalidated and the consequences of the selectively impaired function investigated both in homeostasis and physiopathological settings.
METHODS
Experiments were conducted according to the French veterinary guidelines and those formulated by the European Community for experimental animal use (L358-86/609EEC).
Chemokines: cDNA expression vectors and synthesis
Tissues were obtained by dissection of Cxcl12Gagtm/Gagtm mice or WT littermates previously sacrificed by cervical dislocation. Bone marrow was recovered from right tibiae by the flushing procedure using 5ml PBS. Total RNAs were purified using the RNAeasy Kit (Qiagen), and Cxcl12 cDNAs were synthesized using 0.5μg of the corresponding RNAs with random hexamer. The isoform-specific Cxcl12 cDNA corresponding to WT and Cxcl12Gagtm/Gagtm mice were generated as described previously [13] using the Cxcl12 primers: forward common, 5′-cacccatggacgccaaggtcgtcgcc-3′ and the reverse, 5′-ttacttgtttaaagctttctccaggta-3′, 5′-tcacatcttgagcctcttgtttaaagc-3′and 5′-ctagtttttccttttctgggcagcc-3′ for Cxcl12α, Cxcl12β and Cxcl12γ.
The coding CXCL12γm2 DNA was derived by mutagenesis from the WT sequence. All of them were subcloned in a CMV-promoter driven pcDNA3 expression vector (InVitrogen). HEK293 cells were transfected with CXCL12-expression vectors to generate CXCL12-containing cell supernatants. Chemically synthesized chemokines were generated and evaluated for their purity and concentration as previously described[11].
Cxcl12 knock-in and Cxcl12Gagtm mouse generation
A 14.5 kb genomic DNA used to construct the targeting vector was first subcloned from a positively identified C57BL/6 BAC clone (Dr. A. Lu. iTl, Fresh Meadows, New York, USA). The AAG > TCG and AAA > TCT (aa: K > S) mutations within exon 2, as well as the stop codon TAG insertion in exon 4, that selectively prevents translation of CXCL12γ last 30Aa, were generated by 3-step PCR mutagenesis. The PCR fragments carrying mutations were then used to replace the WT sequence using conventional subcloning methods. The long homology arm extends ~6.6 kb 5′ to the first set mutations in exon 2. The LoxP/FRT-Neo cassette was inserted ~2.2 kb downstream of the first set mutations, and ~2.3 kb upstream of the second set mutations (stop codon insertion in exon 4) in intron 3–4. The short homology arm extends 3.3 kb 3′ to the second set mutations. The targeting vector (backbone, a 2.4 kb pSP72 based vector) was validated by restriction analysis and sequencing after each modification. Ten micrograms of the targeting vector was linearized by Cla I digestion and then transfected by electroporation of BA1 (C57BL/6 x 129/SvEv) hybrid embryonic stem cells. After selection with G418 antibiotic, surviving clones were expanded for PCR analysis to identify recombinant ES clones and a secondary confirmation of positive clones identified by PCR was performed by Southern Blotting analysis. Targeted BA1 (C57BL/6 x 129/SvEv) hybrid embryonic stem cells were microinjected into C57BL/6 blastocysts. Resulting chimeras with a high percentage agouti coat colour were mated to wild-type C57BL/6 mice to generate F1 Cxcl12Gagtm/wt offspring. Tail DNA was analyzed by PCR to confirm the integration of the desired sequences. The obtained Cxcl12Gagtm/wt animals, mice were crossed with transgenic 129sv PGK-Cre for LoxP/FRT-Neo cassette excision. Offspring that do not inherit PGK-CRE and delete LoxP/FRT-Neo cassette were selected and the colony was amplified. Identification of genotypes of mice encoding Cxcl12Gagtm upon excision of Neo-cassette was performed by PCR with forward, 5′tgccagcataaagacactccg3′ and reverse 5′cagcccttgaagtaatcactgc 3′ primers.
Characterisation of Cxcl12 mRNA and protein expression in normal and ischemic tissues
For quantitative real time PC, cerebellum, brain, thymus, heart and skeletal muscle tissues were obtained by dissection of Cxcl12Gagtm/Gagtm mice or WT littermates. Bone marrow was recovered from right tibiae by the flushing procedure using 5ml PBS. Total RNAs were purified using the Rneasy Kit (Qiagen) according to the manufacturer instructions, and cDNAs were synthesized using 0.5μg of the corresponding RNAs with random hexamer. Quantitative real time-PCR was performed in a Mix3005Tm QPCR System with a MxPro QPCR Software 3.00 (Stratagene, La Jolla, CA) and SYBR Green detection system, using the forward common primer 5′tgcccttcagattgttgcac3′ and the reverse primers 5′ccacggatgtcagccttcc3′, 5′cttgagcctcttgtttaaagctt3′, or 5′gctagcttacaaagcgccagagcagagcgcactgcg3′ for Cxcl12α, Cxcl12β or Cxcl12γ, respectively. Mouse HPRT and GAPDH genes were used as controls. Quantitative analysis was then performed using the Livak method.
For evaluating CXCL12 accumulation and localisation in muscle, frozen sections (7 μm) were incubated with CXCL12 mAb K15C (10μg/ml) antibodies, FITC-Griffonia Simplicifolia Agglutinin isolectin B4 (1:100, Sigma) and rabbit anti-mouse alpha smooth muscle actin (1:100, Abcam) at room temperature. Finally, sections were incubated with DAPI (1:10,000, Sigma). Alexa fluor 594 (Invitrogen, 1:100) and Cy5-labeled secondary antibodies (1:200, Jackson Immunoresearch) were then used to reveal CXCL12 and alpha actin-positive stainings, respectively
Quantification of CXCL12 in mice blood serum was carried out using the DuoSet ELISA Development kit for mouse CXCL12 (R&D Systems, MN, USA). Blood was collected by cardiac puncture.
Cell surface binding potential and chemotaxis assay
Evaluation of synthetic CXCL12 chemokines adsorption on cell surfaces of CHO GAG-expressing cells and chemotaxis assay (human CD4+ T lymphocytes or human endothelial progenitor cells (EPC), were performed as described previously[11,13]. EPC were isolated from human umbilical cord blood and differentiated ex vivo, as previously described[33].
Peripheral blood analysis
WT and Cxcl12Gagtm/Gagtm mice (n=10) were killed and blood collected as described above and transferred to EDTA pre-treated tubes (Sarstedt). Then, 20μl of sample were collected using the disposable diluting pipette system of the BD Unopette procedure (BD, NJ, USA). Cells were stained using the Gr1-FITC, CD4-PB and CD34-PE antibodies or the matching control isotypes and analyzed in a BD FACS Canto Flow Cytometer (BD Bioscience). Eythrocytes and Thrombocytes were quantified by flow cytometry (Siemens ADVIA 120).
Experimental model of surgically-induced hindlimb ischemia
Induction of ischemia and plasmids electrotransfert
Cxcl12Gagtm/Gagtm mice and their wild-type C57BL/6 littermate underwent surgical ligation of the proximal part of the right femoral artery, above the origin of the circumflexa femoris lateralis. 50μg of expression plasmids encoding for CXCL12α, CXCL12γ or CXCL12γm2 were then injected into both tibial anterior and gastrocnemius muscles of the anesthetized mouse, as previously described[34]. Transcutaneous electric pulses (8 square-wave electric pulses of 200 V/cm, 20 ms each, at 2 Hz) were delivered by a PS-15 electropulsator (Jouan) using two stainless steel plate electrodes placed 4.2 to 5.3 mm apart, at each side of the leg. The left leg was not ligated neither electrotransfered and was used as an internal control. In additional set of experiments, C57BL/6 mice with surgically-induced hindlimb ischemia and treated with expression plasmids also received intravenous injection of 1.105 human EPC.
Analysis of neovascularization
Post-ischemic neovascularisation was evaluated by three different methods, as previously described[34].
Microangiography
Mice were anesthetized (pentobarbital) and longitudinal laparotomy was performed to introduce a polyethylene catheter into the abdominal aorta and inject contrast medium (Barium sulphate, 1 g/mL). Angiography of hindlimbs was then performed and images (two per animals) were acquired using a high-definition digital X-ray transducer. Images were assembled to obtain a complete view of the hindlimbs.
Capillary and arteriole density analysis
Frozen tissue sections (7 μm) from calf muscle were incubated with rabbit polyclonal antibody directed against total fibronectin (dilution 1:50, Abcys) to identify capillaries and rabbit anti-mouse alpha smooth muscle actin (dilution 1/100, abcam) to identify arterioles. The capillary-to-myocytes ratio was determined in both ischemic and non ischemic legs.
Laser Doppler Perfusion Imaging
Briefly, excess hairs were removed by depilatory cream from the limb, and mice were placed on a heating plate at 37°C to minimize temperature variation. Nevertheless, to account for variables, including ambient light, temperature, and experimental procedures, perfusion was calculated in the foot and expressed as a ratio of ischemic to non ischemic legs.
Analysis of cell infiltration in the ischemic tissue
To evaluate the number of infiltrating CD45.1-positive cells, 107 mononuclear cells isolated from the bone marrow of CD45.1 mice were intravenously injected to Cxcl12Gagtm/Gagtm mice and their wild-type C57BL/6 littermate, 1 day after femoral artery ligation. Five days after the injection, the ischemic gastrocnemius muscles were harvested, weighed, minced and digested in 450 U/ml Collagenase I, 125 U/ml Collagenase XI, 60 U/ml DNAse I and 60 U/ml hyaluronidase (Sigma Aldrich) for 1 hour at 37°C. After Ficoll separation, infiltrating cells were stained with CD45.1-PerCPCy5.5, CD34-APC and CXCR4-PE and analyzed using a FACS Aria (BD).
To measure the number of infiltrating EPC, the ischemic gastrocnemius muscles were digested as described above. The number of cells being positive for β2-microglobuline anti-human FITC (Biolegend, 1:200) and DAPI was then evaluated on a LSRII Flow Cytometer (Becton Dickinson). In a third set of experiment, 10-week wild-type C57BL/6 mice underwent medullar aplasia by total body irradiation (9.5 gray). Bone marrow cells were then isolated from femurs and tibias of GFP C57BL/6 mice and intravenously injected in irradiated animals. After 8 weeks, mice underwent surgical ligation of the proximal part of the right femoral artery, as described above. The gastrocnemius muscles were then harvested 2 days after ischemia. Infiltrating circulating BM-derived GFP-positive cells were detected by fluorescent microscopy and their endothelial cell phenotype revealed by costaining with rhodamine Griffonia Simplicifolia Agglutinin isolectin B4 (1:100, Sigma) and their inflammatory phenotype by co-staining with rat anti-mouse Mac-3 (BD pharmingen, 1:300) and donkey anti-rat RITC (Jackson ImmunoReaserch, 1:200).
Statistical Analysis
Statistical analysis were performed using StatView. Kruskall Wallis analysis of variance was used to compare each parameter. Bonferroni-corrected Mann-Whitney tests were then performed to identify which group differences account for the significant overall Kruskall Wallis. Results were expressed as mean ± SEM. A value of p<0.05 was considered significant.
RESULTS
We have engineered mice encoding a Cxcl12 gene carrying mutations (Figure 1A and S1) that drastically reduce CXCL12/HS interactions as deduced from biochemical and biophysical measurements and functional assays in vitro and in vivo [11,12,13,15]. K24S and K25S point mutations were introduced in the critical HS-binding domain (encoded in the second exon) shared by the three CXCL12 isoforms (Figure S2(A)). Furthermore, a nonsense amber mutation was introduced in the fourth exon to prevent the translation of the distinctive, thirty-last Aa of the highly cationic C-ter domain of CXCL12γ (Figure S1(A)). We opted for this strategy to avoid unpredictable consequences in the expression of a heavily CXCL12γ mutated protein carrying multiple substitutions in the overlapping C-ter BBXB motifs required for abrogating HS-binding of this isoform.
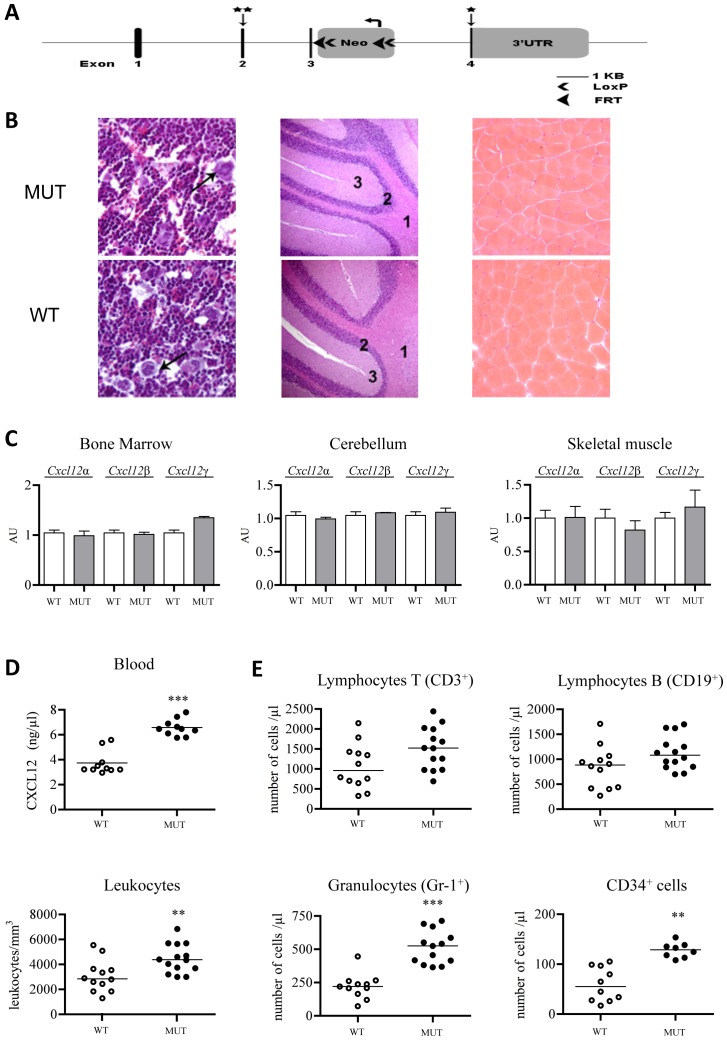
Figure 1. Characterization of Cxcl12Gagtm/Gagtm mice.
(A) Schematic representation of Cxcl12 genomic locus, targeting vector and recombined Cxcl12 locus (Cxcl12Gagtm). Exons 1-4 are represented by darked boxes. Mutations incorporating residue substitutions (2nd exon) and stop codon (4th exon) are indicated by vertical arrows and stars. The LoxP-FRT-Neo cassette is inserted in an opposite orientation regarding to the target gene in intron 3-4. Long (LA) and short (SA) homology arm positions and length are indicated. (B) Hematoxylin and eosin staining of bone marrow (left panels), cerebellum (middle panels) and skeletal muscle (right panels) tissue sections from Cxcl12Gagtm/Gagtm (MUT) and wild-type littermate (WT) animals. Arrows show megakaryocytes; 1 display white substance; 2, granular layer; 3, molecular layer. (C) Real-time PCR analysis of Cxcl12. Relative RNA expression levels of Cxcl12 isoforms normalized to HPRT in cerebellum, bone marrow and skeletal muscle tissues. Cxcl12α, Cxcl12β and Cxcl12γ ARN levels on MUT animals are expressed relative to the WT animals level, arbitrary set to 1 (n=12 per group). (D). CXCL12 levels in blood were measured by ELISA in MUT and WT animals. (E) Cell populations in peripheral blood collected from MUT and WT animals. Results are mean±SEM. **p<0.01, ***p<0.001 versus WT.
The relative amounts of each mRNA isoform in Cxcl12Gagtm/Gagtm were comparable to that of WT littermates (Figure 1C and S2(C)) while serum CXCL12 levels were markedly increased in mutant animals, likely as a consequence of the mutant chemokines to immobilise on HS structures (Figure 1D). Expression and functional analysis of Cxcl12Gagtm/Gagtm cDNA isoforms confirmed their preserved CXCR4-agonist capacities (Figure S3(D)).
Cxcl12Gagtm/Gagtm mice were both viable and fertile. Extensive macroscopic and histologic analysis did not reveal any detectable anatomic abnormalities in the Cxcl12Gagtm/Gagtm mice. Micro photos corresponding to tissue preparations of WT and Cxcl12Gagtm/Gagtm BM and cerebellum, which development is affected in Cxcl12 and Cxcr4 knock-out animals, as well as skeletal muscle, are shown in Figure 1B.
No significant differences in the composition of blood, BM and CD34+ HPC were observed between WT and mutant animals (data not shown). Interestingly, whereas the number of BM haematopoietic cell subpopulations was similar in WT and Cxcl12Gagtm/Gagtm mice, mutant animals displayed higher numbers of circulating total leukocytes, granulocytes and CD34+ cells (Figure 1E) and no significant differences for both T and B lymphocytes. The number of erythrocytes (10.72±0.33/mm3 versus 10.32±0.31/mm3, n=6) and that of platelets (944±132/mm3 versus 1029±197/mm3, n=6) in WT and Cxcl12Gagtm/Gagtm mice, respectively, were similar.
CXCL12 regulates both the tissue homing and survival of circulating tissue-specific progenitors, HPC and BM-stromal stem cells [32,35], triggers inflammatory cells infiltration and is required for the peri-endothelial retention of circulating, BM-derived myeloid cells[36,32,35]. These findings prompted us to assess the role of CXCL12/GAG interactions in the regenerative process following acute regional ischemia.
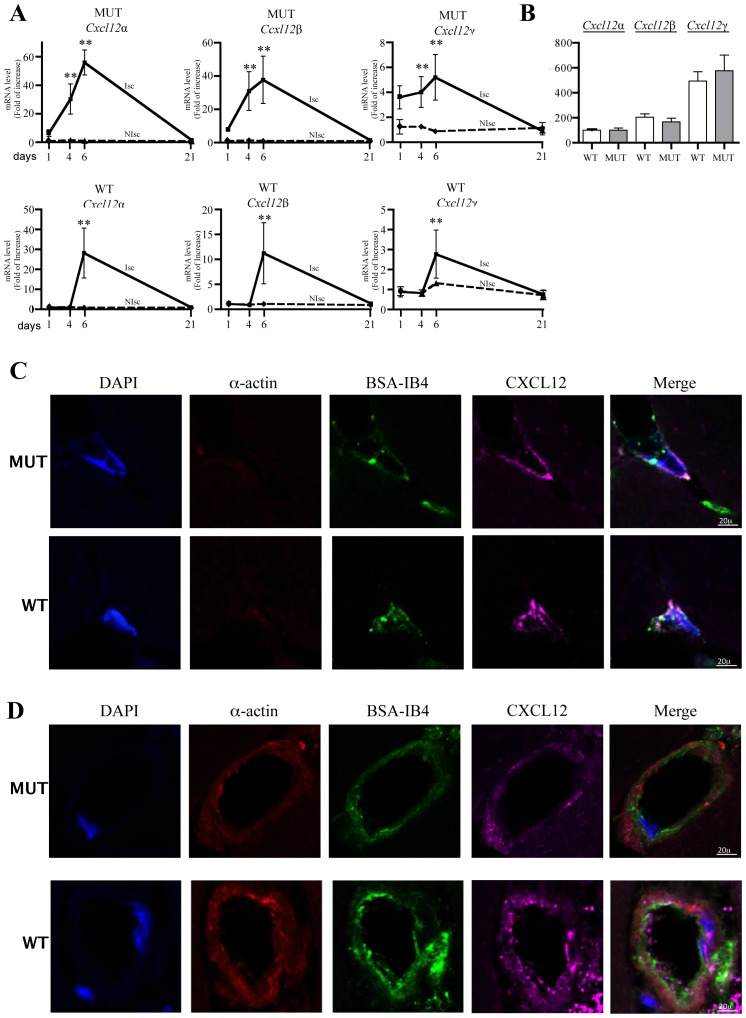
We observed that in both WT and Cxcl12Gagtm/Gagtm mice, hindlimb-ischemia induced massive transcription and expression of Cxcl12 isoforms (Figure 2A). Kinetic analysis of mRNA levels showed that Cxcl12 isoforms expression enhanced by day 4 post-ischemia and returned to basal levels by day 21 (Figure 2A). The expression ratio between the Cxcl12 isoforms varies between different tissues (Figure S2(D)). Of interest, CXCL12γ was the most abundant isoform in both the normal and ischemic muscle (Figure 2B). We also determined that CXCL12 was expressed in ischemic capillary structure and, in both WT and Cxcl12Gagtm/Gagtm mice, is co-localized with isolectin B4 staining, a specific marker of endothelial cells (Figure 2C). In addition, CXCL12 expression was also detected in number of arterioles, as revealed by the co-staining between CXCL12 and alpha-smooth muscle actin (Figure 2D).
Figure 2. Expression of CXCL12 isoforms in ischemic tissue.
(A) Quantitative evaluation and relative levels of CXCL12α, CXCL12β and CXCL12γ mRNA contents 1, 4, 6 and 21 days after ischemia in ischemic and non ischemic legs of Cxcl12Gagtm/Gagtm (MUT) and wild-type littermate (WT) animals. Values are mean ± SEM. n=10/group, representative of 2 independent experiments. 28 possible comparisons for Bonferroni correction, a value of p<0.0018 was considered significant, **p<0.0003 versus N Isch at day 1. (B) mRNA levels of each Cxcl12 isoform were normalized to that of Cxcl12α in skeletal muscle of non ischemic tissue. 6 possible comparisons for Bonferroni correction, a value of p<0.008 was considered significant, n=10 per group. (C–D) Histological sections from ischemic skeletal muscles of WT and MUT animals showing CXCL12 expression in capillary (c) and arteriolar (d) structures. Red staining, alpha smooth muscle actin (alpha smooth muscle cells); green staining, Griffonia Simplicifolia Agglutinin isolectin B4 (endothelial cells); purple staining, CXCL12. Nuclei were stained with DAPI (blue staining). Bars: 10μm. One representative experiment out of six is shown.
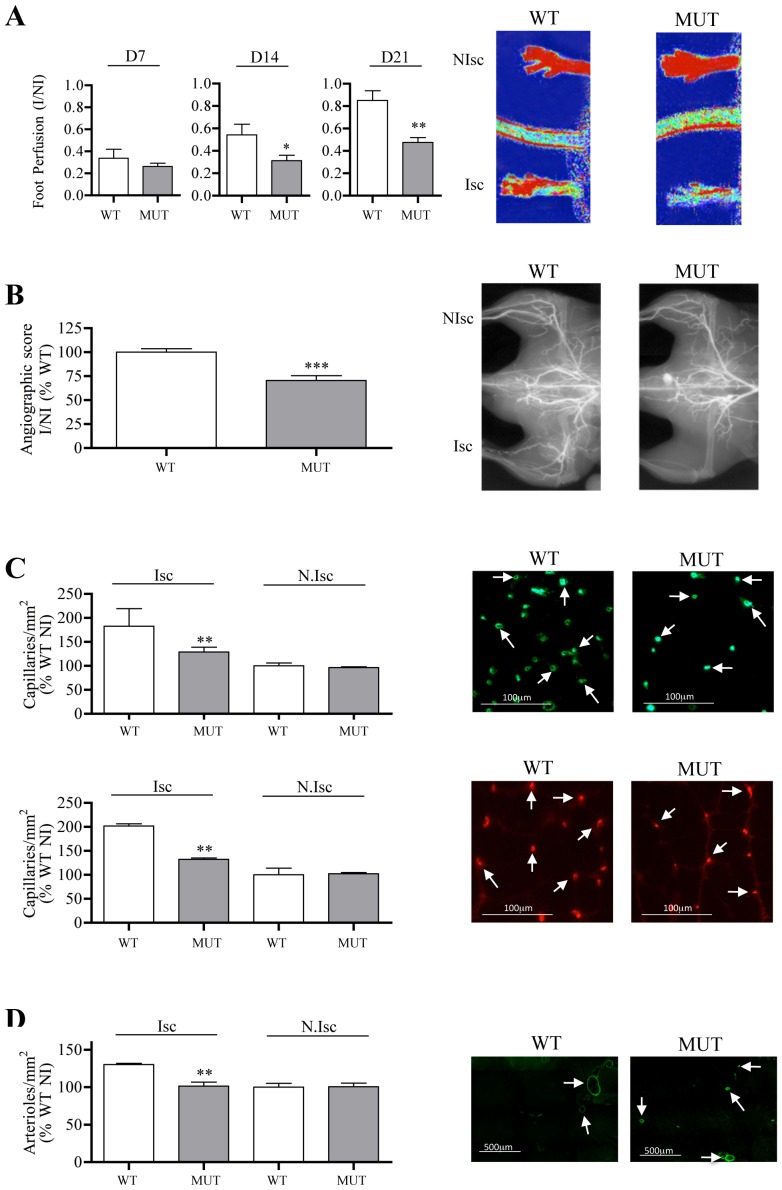
It is of note that Cxcl12Gagtm/Gagtm mice displayed a markedly impaired capacity to support efficient post-ischemic revascularization in a model of hindlimb ischemia. Indeed, laser Doppler imaging showed a reduced paw perfusion in ischemic hind limb of Cxcl12Gagtm/Gagtm as compared to WT animals, as early as day 14 after the onset of ischemia (Figure 3A). Angiographic scores obtained by microangiographic analysis revealed that vessel density was hampered by 30% in ischemic muscle of Cxcl12Gagtm/Gagtm mice in reference to that of WT animals (Figure 3B). Furthermore, analysis of post-ischemic frozen calf muscle samples showed that regeneration of both capillary and arteriole vessels were reduced in Cxcl12Gagtm/Gagtm animals by up to 44% and 55%, respectively as compared to WT animals (Figure 3C and 3D).
Figure 3. Postischemic revascularization in Cxcl12Gagtm/Gagtm animals.
Quantitative evaluation and representative photomicrographs of foot perfusion at different time points (A), and at day 21, for angiographic score (B, vessels in white), capillary density (C, capillaries in green, arrows show fibronectin–labelled capillaries), arteriole density (D, arterioles in green, arrows show α-actin –labelled arterioles) in Cxcl12Gagtm/Gagtm (MUT) and wild-type littermate (WT) animals. Results are shown in ischemic (Isc) and non-ischemic (N.Isc) legs. D indicates day. Values are mean ± SEM. n = 15 per group, representative of 3 independent experiments. A) 15 possible comparisons for Bonferroni correction, a value of p<0.003 was considered significant, *p<0.03 and **p<0.0006 versus WT. B) 1 possible comparisons for Bonferroni correction, a value of p<0.05 was considered significant, ***p<0.001 versus WT. n = 15 per group. C–D) 6 possible comparisons for Bonferroni correction, a value of p<0.008 was considered significant, **p<0.0016 versus Isc WT. n = 15 per group.
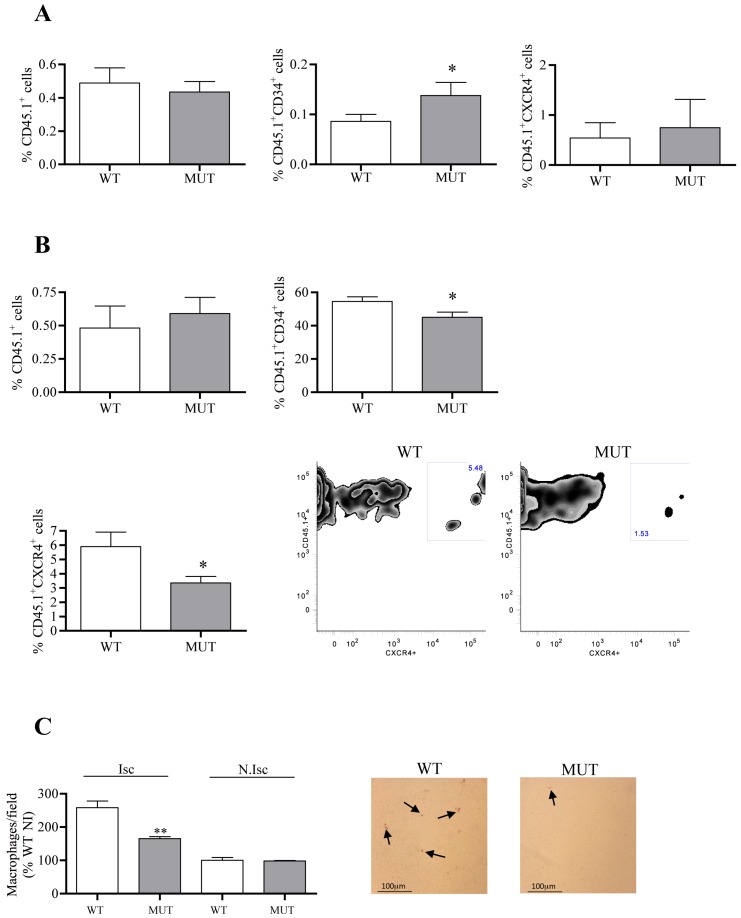
As previously mentioned, CXCL12/CXCR4 interactions are involved in the recruitment and/or the retention of circulating cells in the ischemic tissue. To gain further insights into the cellular and molecular mechanisms associated with the reduction of post-ischemic vessel growth in Cxcl12Gagtm/Gagtm mice, we analyzed the ability of circulating cells to home to ischemic tissues in Cxcl12Gagtm/Gagtm animals. CD45.1-positive cells were intravenously injected one day after the onset of ischemia, and their number and fate were analysed in the blood and in the ischemic tissue, 6 days after ischemia, a time point associated with a marked upregulation of transcription and expression of Cxcl12 isoforms. The number of CD45.1+, CD45.1+/CD34+ and CD45.1+/CXCR4+ was higher in the blood of Cxcl12Gagtm/Gagtm animals compared to that of WT mice. In contrast, the percentage of CD45.1+/CD34+ and CD45.1+/CXCR4+ was decreased in the ischemic muscle of Cxcl12Gagtm/Gagtm animals compared to that of WT mice (Figure 4A, B). As a consequence, the post-ischemic inflammatory response, a key component of ischemic tissue remodelling is affected in our experimental conditions and we showed that the number of Mac3-positive cells was reduced in ischemic leg of Cxcl12Gagtm/Gagtm mice compared to WT littermates (Figure 4C).
Figure 4. Quantification of infiltrating circulating cells in Cxcl12Gagtm/Gagtm animals.
Percentage of CD45.1+, CD45.1+/CD34+ and CD45.1+/CXCR4+ in blood (A) and ischemic muscle (B), 6 days after the onset of ischemia. Representative FACS dot plots obtained from WT and MUT digested muscle at 6 days after ischemia. Cells were gated on CXCR4 expressing CD45.1-positive cells. n = 7 per group. A–B) 1 possible comparisons for Bonferroni correction, a value of p<0.05 was considered significant. *p<0.05 versus WT. (C) Quantitative evaluation and representative photomicrographs of Mac3-positive cells (macrophages in brown, arrows show Mac-3 labelled macrophages) in Cxcl12Gagtm/Gagtm (MUT) and wild-type littermate (WT) animals. Results are shown in ischemic (Isc) and non-ischemic (N.Isc) legs. Values are mean ± SEM. n = 15 per group, representative of 3 independent experiments. 6 possible comparisons for Bonferroni correction, a value of p<0.008 was considered significant, **p<0.0016 versus Isc WT.
The predominant expression of CXCL12γ in muscle and in particular its massive accumulation in ischemic tissues, suggests that this isoform might play a critical role in neovascularisation through an HS-binding dependent mechanism. Thus, if the angiogenic default observed in Cxcl12Gagtm/Gagtm was due to the selective invalidation of HS-binding capacity of Cxcl12 products, exogenous administration of wild type CXCL12 proteins with full HS-binding activity, should restore vascular regeneration in ischemic tissues with the highest efficiency as compared.
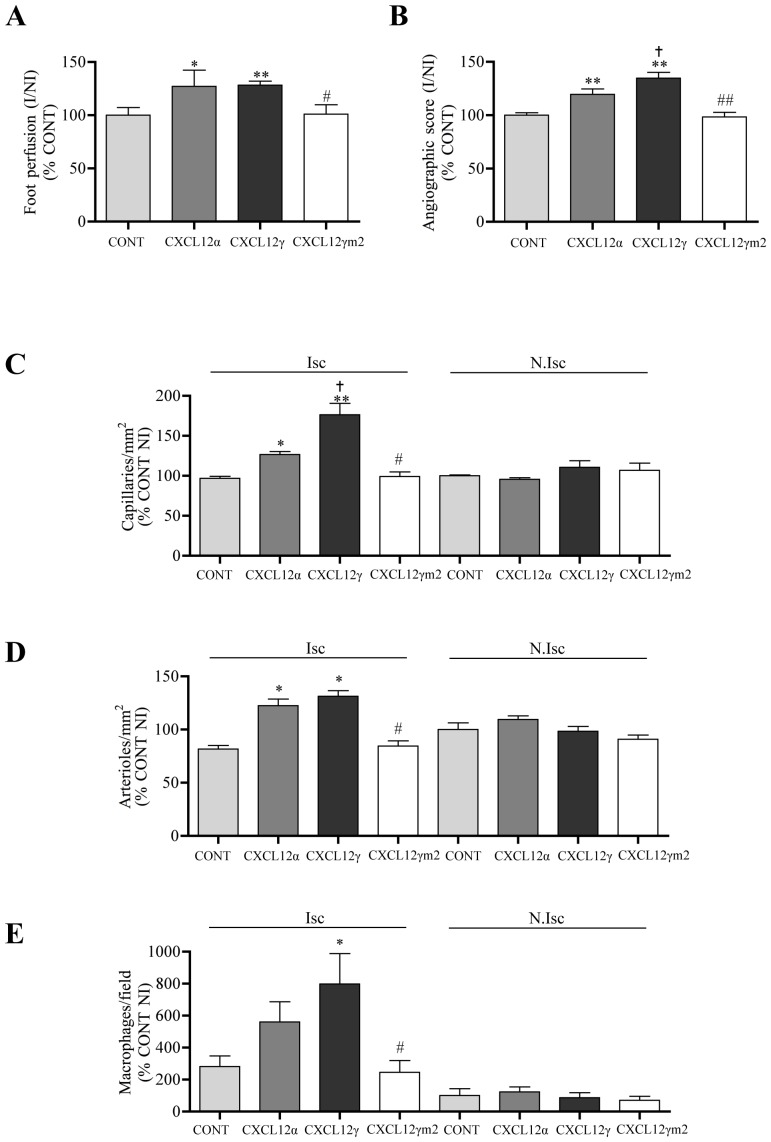
To assess this hypothesis we first compared the capacity of both WT CXCL12α and CXCL12γ, which differ notably for their affinity capacity to bind cell surface in a GAG-dependent manner, to induce post-ischemic vascular regeneration in control animals. Electrotransfered expression of DNA plasmids encoding for CXCL12α or CXCL12γ (Figure 5A), revealed a dramatically increased potency of CXCL12γ to promote neovascularisation as evaluated by tissue perfusion, angiographic score, capillary density and attraction of inflammatory macrophages in the ischemic tissue (Figure 5B, 5C, 5D and 5E). To bring direct and formal evidence that the superior efficiency of CXCL12γ was actually related to the interaction of the chemokine with HS, we evaluated the pro-angiogenic capacities of CXCL12γ and mutant CXCL12γm2 which carries the K24S and K27S substitutions combined with the neutralisation of nine of the seventeen positively charged residues encompassed in the C-ter domain of this isoform [12,13] (Figure S3(A)). CXCL12γm2 displays no detectable HS-binding to cell surface HS whereas it activates CXCR4 with preserved efficiency and even with increased potency as compared to the WT control (Figure S3 (B,C)) and as we reported previously[13]. Our findings unambiguously proved that while CXCL12α and CXCL12γ showed pro-angiogenic efficiency correlating with their affinities for HS, CXCL12γm2 failed to induce post-ischemic vessel growth beyond baseline levels (Figure 5B, C and E).
Figure 5. Effect of CXCL12 isoforms on post-ischemic revascularization.
Quantitative evaluation of foot perfusion (A), microangiography (B), capillary density (C), and arteriolar density (D) and macrophages infiltration (E), 21 days after ischemia in C57BL/6 mice transfected with empty DNA plasmid (CONT) or DNA expression vectors encoding CXCL12α, CXCL12γ or CXCL12γm2. Values are mean ± SEM. n = 15 per group, representative of 3 independent experiments. A–B) 6 possible comparisons for Bonferroni correction, a value of p<0.008 was considered significant, *p<0.008, **p<0.0016 versus PBS, † p<0.008 versus CXCL12α, #p<0.008, ##p<0.0016 versus CXCL12γ. C–E) 28 possible comparisons for Bonferroni correction, a value of p<0.0017 was considered significant, *p<0.0017 versus Isc CONT, †p<0.0017 versus Isc CXCL12α, #p<0.0017 versus Isc CXCL12γ. Isc indicates ischemic leg and N.Isc, non ischemic leg.
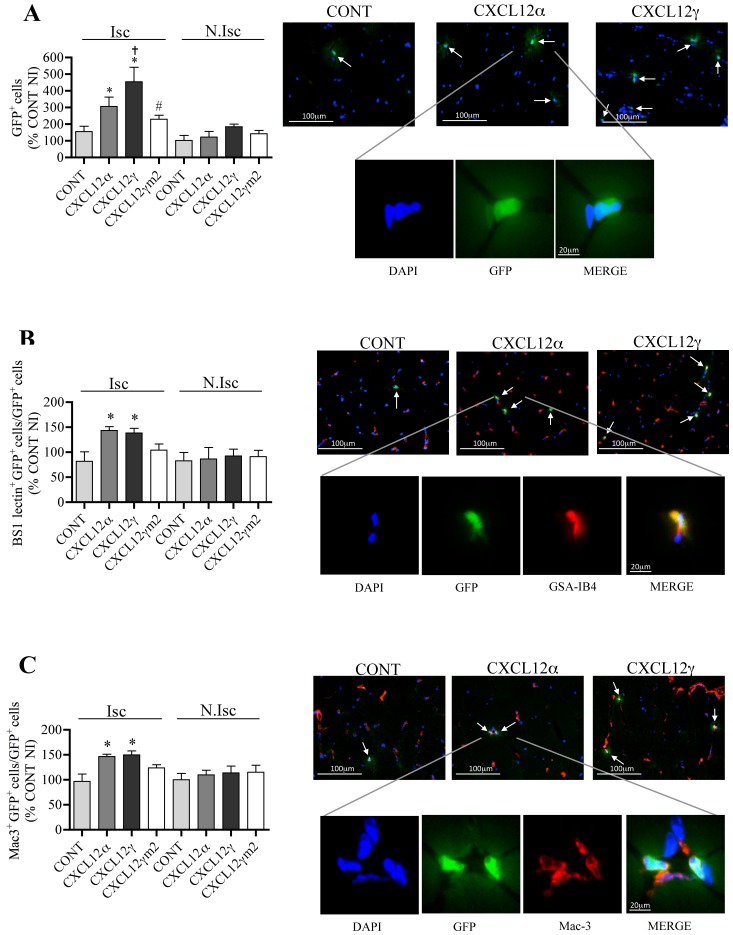
To further investigate the mechanism of the increased pro-angiogenic effect of CXCL12γ, we compared the capacity of each isoform to regulate both the number of cells infiltrating the ischemic tissue and their fate. To this aim, hindlimb ischemia was induced in mice that has been lethally irradiated and reconstituted with BM-derived cells isolated from GFP+ mice. Electrotransfer of CXCL12γ plasmid enhanced by 300% and 150% the number of GFP+ cells infiltrating the ischemic muscle compared to mice treated with empty plasmid or CXCL12α, respectively (Figure 6A). The failure of CXCL12γm2 to induce a significant effect further proved the involvement of an HS-dependent mechanism in the robust neovascularisation induced by CXCL12γ. Of note, the percentage of GFP+/BS1-lectin+ cells of the endothelial lineage, and that of GFP+/Mac3+ revealing the macrophage phenotype, were similar in mice treated with CXCL12γ or CXCL12α. Further experiments using human endothelial progenitor cells (EPC) labelled with CFSE revealed that CXCL12γ, but not CXCL12γm2, increased the number of EPC infiltrating ischemic hindlimb muscle (day 4 pos-ischemia) by 150% and subsequently vessel growth by roughly 50% (day 14 post-ischemia) when compared to mice treated with CXCL12α (Figure S4).
Figure 6. CXCL12γ controls BM-derived cells infiltration in ischemic tissues.
Quantitative evaluation and representative photomicrographs of the percentage of GFP-expressing cells (A), GFP-expressing and BS1-labelled cells (B) and GFP-expressing and Mac3-labelled cells (C) in the ischemic leg of irradiated wild-type mice reconstituted with bone marrow of WT GFP+ animals and treated with empty DNA plasmid (CONT), or DNA expression vectors encoding for CXCL12α, CXCL12γ or HS-binding mutant CXCL12γm2. Value quantification are represented in histograms. Values are mean ± SEM. n = 10 per group, representative of 2 independent experiments. A–C) 28 possible comparisons for Bonferroni correction, a value of p<0.0017 was considered significant, *p<0.0017 versus Isc CONT, †p<0.0017 versus Isc CXCL12α, #p<0.0017 versus Isc CXCL12γ. Isc indicates ischemic leg and N.Isc, non ischemic leg.
Collectively, these results suggest that CXCL12γ regulates the accumulation of BM-derived cells in ischemic tissues with the highest efficiency, in comparison to CXCL12α, and in a strictly HS-dependent manner, while it does not differ from CXCL12α regarding induction of cell differentiation. The increased number of EPC homing to the injured muscle early after ischemia permits to ascribe its prominent pro-angiogenic effect to a primary attraction of endothelial cell precursors.
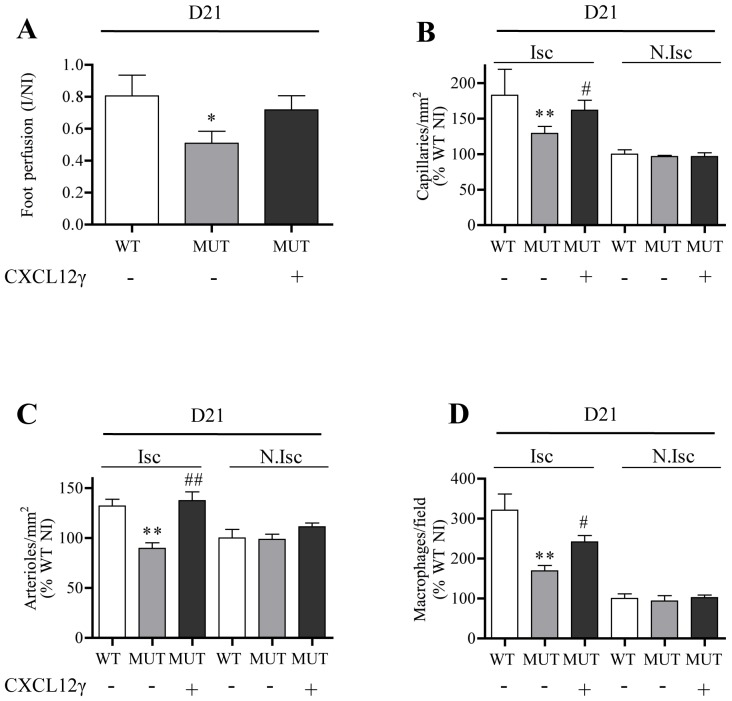
Finally, to demonstrate that the deficient neoangiogenesis observed in Cxcl12Gagtm/Gagtm animals was related to the expression of CXCL12 HS-binding mutants and their inability to promote efficient vascular reparation, we electrotransfered a plasmid encoding WT CXCL12γ plasmid in the ischemic hindlimb. Expression of this isoform virtually normalized vascular regeneration in ischemic tissues as proved by the significant increase in both vessel density and infiltration of inflammatory macrophages (Figure 7). This finding conclusively links the default of tissue reparation observed in these animals to the reduced capacity of mutant CXCL12 proteins to form complexes with HS proteoglycans.
Figure 7. CXCL12γ restores the defective angiogenic phenotype in Cxcl12Gagtm/Gagtm animals.
Quantitative evaluation of foot perfusion (A), capillary density (B), arteriole density (C) and Mac3-positive cells (D) in Cxcl12Gagtm/Gagtm (MUT) and wild-type littermate (WT) animals treated with or without DNA expression vector encoding CXCL12γ. Results are shown in ischemic (Isc) and non-ischemic (N.Isc) legs. Values are mean ± SEM. n = 10 per group. A) 3 possible comparisons for Bonferroni correction, a value of p<0.016 was considered significant *p<0.016 versus WT. B–D) 15 possible comparisons for Bonferroni correction, a value of p<0.003 was considered significant. **p<0.0006 versus Isc WT, #p<0.003, ##p<0.0006 versus Isc MUT.
DISCUSSION
The animal model we have engineered allows the selective characterization of proteoglycan-chemokine interactions in vivo, separately from interactions of a defined chemokine to its specific receptor. Cxcl12Gagtm/Gagtm mice exhibit normal expression of isoform-specific Cxcl12 mRNAs encoding for CXCR4-agonist chemokines. No detectable developmental anomalies were observed in Cxcl12Gagtm/Gagtm animals. This is in sharp contrast to Cxcl12- and Cxcr4-deficient mice, which show in both cases abnormal haematopoiesis and multiple organogenesis default associated to perinatal mortality[20,21,37], and to Cxcr7- knock-out mice, which show disrupted cardiac development[38]. Manifold reasons could explain the lack of detectable anatomic anomalies in the mutant animals. First, the preserved expression levels of total and isoform specific Cxcl12 mRNA in Cxcl12Gagtm/Gagtm mice tissues. Second, the intact cell signaling capacities of mutant chemokines leading to orientated cell migration, as previously shown for mutated CCL19 and CCL21 [1]. Third, the oligosaccharidic HS structure recognized by CXCL12, which remains unknown, could not feature during embryogenesis the high affinity CXCL12 binding site, thus explaining the lack of observed effects. In this line, it has been proved a binding specificity switch of brain HS proteoglycans from FGF-2 to FGF-1 [39], demonstrating that indeed the binding capacity of embryonic HS for a given protein is not necessarily present, and is acquired during development.
The Cxcl12Gagtm/Gagtm mice nevertheless display a subtle anomaly revealing an increased number of circulating white blood cells, including CD34+ HPC. This phenomenon could be explained by a reduced attraction/retention in lymphoid tissues and is probably related to impaired HS-dependent tissue adhesiveness of the CXCL12 mutant proteins [15,5]. Given the crucial role played by CXCL12 and CXCR4 in both the homing of haematopoietic cells to the BM[25,26], the inability of the HS-binding CXCL12 mutants to adhere to the cell surface and the surrounding extracellular matrix of CXCL12-producing stromal cells, forming CXCL12-BM niches [40] could facilitate their egress from the BM. In this regard, the accumulation of circulating CD34+ cells, which normally reside in BM[41], strongly argues in favour of reduced retention of these cells in BM. The BM egress of the mature leukocytes could also be due to the same mechanism. Alternatively, the increased number of these cells in the periphery could be ascribed to the gradient generated by the blood accumulation of free, HS-binding disabled CXCL12 proteins and/or their failure to firmly immobilize on the apical surface of sinus and post-capillary endothelial cells [13,15] that would inhibit the return of leukocytes to the BM [42].
Whereas basal coronary flow were impaired in Cxcr4(+/−) mice, paralleled by reduced angiogenesis and myocardial vessel density, we did not detect changes in the basal number of capillary and arterioles in skeletal muscle of Cxcl12Gagtm/Gagtm animals [43]. In contrast, Cxcl12Gagtm/Gagtm animals show a marked default in angiogenesis and neovascularisation of ischemic tissues, revealing a biological role for CXCL12/GAG interactions in tissue repair after ischemia. It has been previously reported that recruitment of CXCR4-positive progenitor cells to regenerating tissues is mediated by hypoxic gradients via HIF-1-induced expression of CXCL12 [32]. Furthermore, CXCL12 attracts EPC, induces angiogenesis and increases the number of newly formed vessels and blood flow in models of hindlimb ischemia when overexpressed, either alone [44] or combined with cell therapy [45]. Recruitment and retention of BM-derived circulating inflammatory cells mediated by CXCL12 participates to vascular remodelling and arteriole growth [36]. In addition, CXCL12 seems to have a direct effect on migration of smooth muscle cells and smooth muscle progenitor cells [46].
However, all previous studies focussed on deciphering the capacity of CXCL12α to promote angiogenesis, without dissecting the respective contribution of receptor activation and GAG-binding. Furthermore, only the therapeutic capacity of exogenous WT chemokines was investigated, while the role played by endogenous CXCL12/GAG interactions in the spontaneous post-ischemic recovery remained unknown.
The defective post-ischemic neovascularisation observed in Cxcl12Gagtm/Gagtm animals thus brings a new light to the original evidence that, beyond CXCL12-induced receptor cell signalling, the endogenous CXCL12/GAG interactions play a critical role in tissue regeneration and in particular in vascular growth. Indeed, the efficiency of CXCL12γ to rescue the default in Cxcl12Gagtm/Gagtm animals contrasts with the lack of effect of CXCL12γm2 and reveals the key role of this mechanism in the reparative process. This finding is in keeping with the efficiency of CXCL12γ and the inability of CXCL12γm2 to promote homing of BM-derived circulating inflammatory cells and of circulating EPC into ischemic tissues, and modulate vessel growth in this setting.
Bound to HS-structures on the apical surface of endothelial cells [47], CXCL12 expressed by autocrine or juxtacrine mechanisms could be determinant for vascular targeting of circulating progenitor and inflammatory cells and induction, in an integrin- and CXCR4-dependent manner, of both firm adhesion and transendothelial migration of infiltrating BM-derived cells [48]. Among CXCL12 isoforms, CXCL12γ binds to microvascular endothelial cell surface with the highest efficiency [13,47] and is ideally suited to accomplish this role. Abundantly expressed and induced in injured tissues by hypoxia, CXCL12γ could delimit a static field of chemokine keeping cells motile (haptokinesis) in a restrained tissue compartment. This may contribute to the reparative effect of EPC by enhancing cell-adhesiveness and survival[49]. It could be hypothesized that, released progressively upon cleavage of its C-ter domain the pool of immobilized CXCL12γ could provide a gradient of active, diffusible chemokines that would promote long-range chemotaxis toward the injured tissue. An analogous mechanism contributes to the regulation by CCL21 of lymphocyte T cell homing in secondary lymphoid organs [1].
Regarding the potential of CXCL12 in regenerative medicine, the continuous delivery or administration in an immobilized form of CXCL12α is required to provide therapeutic effect in post-ischemic treatment[50]. CXCL12γ could overcome this limitation thus activating efficiently the pro-angiogenic mechanisms.
Collectively, our findings unravel the contribution of endogenous CXCL12/HS complexes to the biological functions of this chemokine. The prevalence of CXCL12γ expression in hypoxic conditions and its superior capacity to promote neovascularisation further highlights the importance of HS-binding mechanisms in the tissue reparative activity of CXCL12. The findings of this work pave the way for investigating the contribution of CXCL12/HS interactions to other homeostatic and physio-pathological functions played by this unique chemokine and could have broad relevance eventually for other chemokine systems.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported by grants from Agence Nationale de la Recherche (ANR; Chemoglycan, NT05-4_41967; Chemrepair 2010-BLAN112702), INSERM, Institut Pasteur PTR-395, Mizutani Fundation (Japan) and by institutional funding from INSERM. J.S.S is a recipient of a Contrat d’Interface from Assistance Publique-Hôpitaux de Paris and supported by “Fondation pour la Recherche Médicale”. P.R and P.G are recipients of ANR fellowships. A.Récalde is a recipient of fellowships from Fondation pour la Recherche Médicale and from Région Ile de France. The Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURES
None
References
- 1.Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 3.Lortat-Jacob H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol. 2009;19:543–548. doi: 10.1016/j.sbi.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Lortat-Jacob H, Grosdidier A, Imberty A. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci U S A. 2002;99:1229–1234. doi: 10.1073/pnas.032497699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of sdf-1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood. 2002;99:44–51. doi: 10.1182/blood.v99.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Bao X, Moseman EA, Saito H, Petryniak B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, von Andrian UH, Fukuda M. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laguri C, Arenzana-Seisdedos F, Lortat-Jacob H. Relationships between glycosaminoglycan and receptor binding sites in chemokines-the cxcl12 example. Carbohydr Res. 2008;343:2018–2023. doi: 10.1016/j.carres.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: A cloning strategy for secreted proteins and type i membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 10.Gleichmann M, Gillen C, Czardybon M, Bosse F, Greiner-Petter R, Auer J, Muller HW. Cloning and characterization of sdf-1gamma, a novel sdf-1 chemokine transcript with developmentally regulated expression in the nervous system. Eur J Neurosci. 2000;12:1857–1866. doi: 10.1046/j.1460-9568.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 11.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 12.Laguri C, Sadir R, Rueda P, Baleux F, Gans P. The novel cxcl12c isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and cxcr4. PLoS ONE. 2007;2:e111. doi: 10.1371/journal.pone.0001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rueda P, Balabanian K, Lagane B, Staropoli I, Chow K, Levoye A, Laguri C, Sadir R, Delaunay T, Izquierdo E, Pablos JL, Lendinez E, Caruz A, Franco D, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. The cxcl12gamma chemokine displays unprecedented structural and functional properties that make it a paradigm of chemoattractant proteins. PLoS One. 2008;3:e2543. doi: 10.1371/journal.pone.0002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadir R, Baleux F, Grosdidier A, Imberty A, Lortat-Jacob H. Characterization of the stromal cell-derived factor-1alpha-heparin complex. J Biol Chem. 2001;276:8288–8296. doi: 10.1074/jbc.M008110200. [DOI] [PubMed] [Google Scholar]
- 15.O’Boyle G, Mellor P, Kirby JA, Ali S. Anti-inflammatory therapy by intravenous delivery of non-heparan sulfate-binding cxcl12. Faseb J. 2009;23:3906–3916. doi: 10.1096/fj.09-134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The cxc chemokine sdf-1 is the ligand for lestr/fusin and prevents infection by t-cell-line-adapted hiv-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 17.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant sdf-1 is a ligand for lestr/fusin and blocks hiv-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 18.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine sdf-1/cxcl12 binds to and signals through the orphan receptor rdc1 in t lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 19.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for sdf-1 and i-tac involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of b-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the cxc chemokine pbsf/sdf-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 21.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired b-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in cxcr4- and sdf-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coulomb-L’Hermin A, Amara A, Schiff C, Durand-Gasselin I, Foussat A, Delaunay T, Chaouat G, Capron F, Ledee N, Galanaud P, Arenzana-Seisdedos F, Emilie D. Stromal cell-derived factor 1 (sdf-1) and antenatal human b cell lymphopoiesis: Expression of sdf-1 by mesothelial cells and biliary ductal plate epithelial cells. Proc Natl Acad Sci U S A. 1999;96:8585–8590. doi: 10.1073/pnas.96.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, Delaunay T, Virelizier JL, Arenzana-Seisdedos F. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine sdf-1 is a chemoattractant for human cd34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of cd34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-csf induces stem cell mobilization by decreasing bone marrow sdf-1 and up-regulating cxcr4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 26.Broxmeyer HE, Kohli L, Kim CH, Lee Y, Mantel C, Cooper S, Hangoc G, Shaheen M, Li X, Clapp DW. Stromal cell-derived factor-1/cxcl12 directly enhances survival/antiapoptosis of myeloid progenitor cells through cxcr4 and g(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73:630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 27.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine sdf-1 stimulates integrin-mediated arrest of cd34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for b cell entry to lymph nodes and peyer’s patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scimone ML, Felbinger TW, Mazo IB, Stein JV, Von Andrian UH, Weninger W. Cxcl12 mediates ccr7-independent homing of central memory cells, but not naive t cells, in peripheral lymph nodes. J Exp Med. 2004;199:1113–1120. doi: 10.1084/jem.20031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (sdf-1/cxcl12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 31.Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, Garcia-Lazaro FJ. Synoviocyte-derived cxcl12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170:2147–2152. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- 32.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through hif-1 induction of sdf-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 33.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, Lere-Dean C, Contreres JO, Sulpice E, Levy BI, Plouet J, Tobelem G, Le Ricousse-Roussanne S. Psgl-1-mediated activation of ephb4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S, Clergue M, Duriez M, Merval R, Levy B, Tedgui A, Amigorena S, Mallat Z. Lactadherin promotes vegf-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 35.Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in nod/scid mice. Blood. 2002;99:792–799. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- 36.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Vegf-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor cxcr4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 38.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez AC, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second cxcl12/sdf-1 receptor, cxcr7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurcombe V, Ford MD, Wildschut JA, Bartlett PF. Developmental regulation of neural response to fgf-1 and fgf-2 by heparan sulfate proteoglycan. Science. 1993;260:103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by cxcl12-cxcr4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: State of the art. Curr Opin Organ Transplant. 2008;13:53–58. doi: 10.1097/MOT.0b013e3282f42473. [DOI] [PubMed] [Google Scholar]
- 42.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via cxcr2 and cxcr4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 43.Liehn EA, Tuchscheerer N, Kanzler I, Drechsler M, Fraemohs L, Schuh A, Koenen RR, Zander S, Soehnlein O, Hristov M, Grigorescu G, Urs AO, Leabu M, Bucur I, Merx MW, Zernecke A, Ehling J, Gremse F, Lammers T, Kiessling F, Bernhagen J, Schober A, Weber C. Double-edged role of the cxcl12/cxcr4 axis in experimental myocardial infarction. J Am Coll Cardiol. 2011;58:2415–2423. doi: 10.1016/j.jacc.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: Next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 46.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. Sdf-1alpha/cxcr4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 47.Santiago B, Izquierdo E, Rueda P, Del Rey MJ, Criado G, Usategui A, Arenzana-Seisdedos F, Pablos JL. Cxcl12gamma isoform is expressed on endothelial and dendritic cells in rheumatoid arthritis synovium and regulates t cell activation. Arthritis Rheum. 2012;64:409–417. doi: 10.1002/art.33345. [DOI] [PubMed] [Google Scholar]
- 48.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended lfa-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 49.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segers VF, Revin V, Wu W, Qiu H, Yan Z, Lee RT, Sandrasagra A. Protease-resistant stromal cell-derived factor-1 for the treatment of experimental peripheral artery disease. Circulation. 2011;123:1306–1315. doi: 10.1161/CIRCULATIONAHA.110.991786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.