Abstract
Background
There have been few studies of the natural history of Mycoplasma genitalium in women. We investigated patterns of clearance and recurrence of untreated M. genitalium infection in a cohort of female sex workers in Uganda.
Methods
Women diagnosed as having M. genitalium infection at enrollment were retested for the infection at 3-month intervals. Clearance of infection was defined as testing negative after having a previous positive result: persistence was defined as testing positive after a preceding positive test result, and recurrence as testing positive after a preceding negative test result. Adjusted hazard ratios for M. genitalium clearance were estimated using Cox proportional hazards regression.
Results
Among 119 participants infected with M. genitalium at enrollment (prevalence, 14%), 55% had spontaneously cleared the infection within 3 months; 83%, within 6; and 93%, within 12 months. The overall clearance rate was 25.7/100 person-years (pyr; 95% confidence interval, 21.4–31.0). HIV-positive women cleared M. genitalium infection more slowly than did HIV-negative women (20.6/100 pyr vs. 31.3/100 pyr, P = 0.03). The clearance rate was slower among HIV-positive women with CD4 counts less than 350/mL3 than among those with higher CD4 counts (9.88/100 pyr vs. 29.5/100 pyr, P < 0.001). After clearing the infection, M. genitalium infection recurred in 39% women.
Conclusions
M. genitalium is likely to persist and recur in the female genital tract. Because of the urogenital tract morbidity caused by the infection and the observed association with HIV acquisition, further research is needed to define screening modalities, especially in populations at high risk for HIV, and to optimize effective and affordable treatment options.
In the past decade, Mycoplasma genitalium infection has gained increasing attention as an emerging sexually transmitted infection (STI). In men, the infection is now recognized as one of the main causes of acute nonchlamydial nongonococcal urethritis, and in women, the evidence for an association with urethritis, cervicitis, pelvic inflammatory disease (PID), and infertility is growing, although not yet conclusive.1–3
Clinical studies suggest that M. genitalium may result in persistent infections if not treated or inappropriately treated. In men, the pathogen causes chronic nonchlamydial nongonococcal urethritis.4–11 In women however, the natural history of the infection is not well documented. A community-based prospective cohort study among female university students in the UK reported that 7 (26%) of 27 women with M. genitalium infection at baseline remained positive after 12 to 21 months (median, 16 months).12 Similar results were seen in a cohort of Kenyan female sex workers where 17% and 9% of untreated M. genitalium infections persisted after 3 and 12 months, respectively.13
We recently published data on M. genitalium from a cohort of women at high risk for HIV in Kampala, Uganda.14,15 The prevalence of M. genitalium infection at enrollment was 14% (18% in HIV-positive vs. 12% in HIV-negative women, P < 0.01). The aims of the current study are to gain further insights into the natural history of M. genitalium and to describe rates of clearance of the infection and associated factors in our cohort.
MATERIALS AND METHODS
Study Population and Study Procedures
Between April 2008 and May 2009, a cohort of 1027 women at high risk for HIV infection was recruited from red light areas within southern Kampala. Details of the recruitment process and study procedures have been published previously.16 Briefly, self-reporting sex workers and/or women employed in entertainment facilities were invited to visit the Good Health for Women Project clinic for screening and enrollment into the cohort and scheduled to return at 3-month interval for follow-up visits. At every visit, women were interviewed about their sociodemographic characteristics, sexual risk behavior, alcohol use, intravaginal practices, reproductive health history, and symptoms of respiratory tract infections (RTIs)/STIs. Blood was tested for HIV, human herpes virus type 2 (HSV2), and syphilis. A speculum examination was performed at each visit. Two endocervical specimens were collected, one for the diagnosis of gonococcal and chlamydial infection and one for later diagnosis of M. genitalium. One high vaginal specimen was collected and inoculated for culture of Trichomonas vaginalis and another to prepare a slide for the detection of bacterial vaginosis and of Candida infection. Women with symptomatic STIs were immediately treated syndromically. Women with asymptomatic STIs were treated as soon as laboratory results became available.
The endocervical specimens for future M. genitalium studies were transported within 12 hours of collection to the Medical Research Council/Ugandan Virus Research Institute laboratories in Entebbe, where they were stored at −20°C until shipment to South Africa.
In 2010, all stored endocervical samples collected at the enrollment visit were tested for M. genitalium retrospectively, to study the baseline prevalence, risk factors, and clinical characteristics.14,15 For the current work, we tested stored samples of women who were infected with M. genitalium at enrollment from all scheduled visits every 3 months within the first follow-up year.
Treatment of laboratory-diagnosed M. genitalium infection could not be provided during the study because there was a delay of at least 2 years between sample taking and testing for M. genitalium.
Laboratory Testing
Testing for M. genitalium was performed using a commercially available Real-TM polymerase chain reaction (PCR) assay (Sacace Biotechnologies, Como, Italy) at the Centre for HIV and Sexually Transmitted Infections, National Institute for Communicable Diseases, National Health Laboratory Service, in Johannesburg. Further details of this PCR test have been reported previously.14 All other laboratory test procedures were performed at the central laboratories of the Medical Research Council/Ugandan Virus Research Institute Uganda Unit in Entebbe: serum specimens were tested for antibodies against HIV-1 (Abbott determined HIV-1/2 with confirmation by 2 independent enzyme-linked immunosorbent assay tests: Vironostika Uniform II plus O, Murex HIV 1.2.O) and HSV2 (immunoglobulin G enzyme-linked immunosorbent assay test; Kalon Biologicals Ltd, Guildford, UK) and for syphilis (Rapid plasma reagin (RPR) Biotec and Treponema pallidum hemagglutination assay (TPHA) Biotec). Neisseria gonorrhoeae and Chlamydia trachomatis were diagnosed on endocervical specimens using the Amplicor PCR test (Roche Diagnostic Systems Inc, Branchburg, NJ), and T. vaginalis was detected using a commercially available culture kit (InPouch TV; BioMed Diagostics, White City, OR). Microscopy on a gram-stained vaginal smear was performed to diagnose bacterial vaginosis (using Nugent criteria) and Candida infection.
Clinical Definitions
Clearance of infection was defined as testing negative for M. genitalium after having a previous positive result, and time of clearance was estimated as the midpoint between the last positive and first negative visit. An infection was defined as persistent if the preceding test result was positive and was defined as recurrent if the preceding test result was negative. Time of recurrence was estimated as the midpoint between the last negative and the first subsequent positive visits. Because genotyping was not performed, it was not possible to assess whether a so-called recurrent infection was a new infection (reinfection) or a persistent infection.
Statistical Analysis
Data were double entered in Access and analyzed using STATA 11.0 (Stata Inc, College Station, TX). Cox proportional hazards regression with time-dependent exposures was used to estimate hazard ratios (HRs) and adjusted HR for M. genitalium clearance. Time was calculated as time since enrollment for each woman. Syphilis was categorized as no infection (RPR−TPHA−), past infection (RPR−TPHA+), or recent infection (RPR+TPHA+). CD4 counts were categorized as less than 350 cells/mm3 or 350 cells/mm3 and above, which is the cutoff point for eligibility for antiretroviral therapy in Uganda. For visits with missing CD4 count data (10/194 visits), CD4 count at the most recent visit was used. Factors associated with M. genitalium clearance in univariable analysis (P < 0.10) were included in a multivariable model and retained if they remained independently associated with M. genitalium clearance.
Ethical Considerations
Informed consent was obtained from all study participants. The study was approved by the Science and Ethics Committee of the Ugandan Virus Research Institute and the Uganda National Committee for Science and Technology.
RESULTS
Characteristics of the Study Population and Follow-Up
One hundred forty-eight (14%) of the 1027 women enrolled in the cohort were tested positive for M. genitalium at baseline and were included in the current study. Of these, 119 women (80%) had at least 1 sample tested for M. genitalium during the first 12 months of follow-up and were included in subsequent analyses. There were 420 follow-up visits in total, with most participants attending all 4 visits (n = 80; 67%) or 3 of the 4 visits (n = 25; 21%).
Baseline Characteristics of Women With M. genitalium Infection
The median age of the 119 women was 26 years (range, 18–40 years). At enrollment, 90% reported transactional sex in the last month and 43% inconsistent condom use with paying partners; 31% of nonpregnant women used hormonal contraception, 54% had ever cleansed the vagina using soap, and 62% had ever inserted substances to lubricate or dry the vagina. On gynecological examination, 10 (8%) women were diagnosed as having both presumptive cervicitis and PID; 17 (14%), with cervicitis only; and 18 (15%), with PID only. All these women were treated according to the national STI guidelines. The prevalence of N. gonorrhoeae was 22%; C. trachomatis, 12%; T. vaginalis, 25%; C. albicans, 6%; and bacterial vaginosis, 65%. Human herpes virus type 2 antibodies were detected in 85%, and 13% were diagnosed as having active syphilis; 45% of the women were HIV positive at enrollment, with 30% of the HIV-positive women having CD4 counts of less than 350/mL3.
The 29 women without any follow-up results were similar in age, risk behavior, prevalence of HIV and other STI/RTI, and CD4 counts (data not shown).
Prevalence of M. genitalium During Follow-Up
Most women (n = 82; 69%) were tested positive for M. genitalium at least once during follow-up, and 8 (7%) women were tested positive at all follow-up visits. Overall, one third (140/420) of samples were tested positive for M. genitalium.
Clearance Rate of M. genitalium
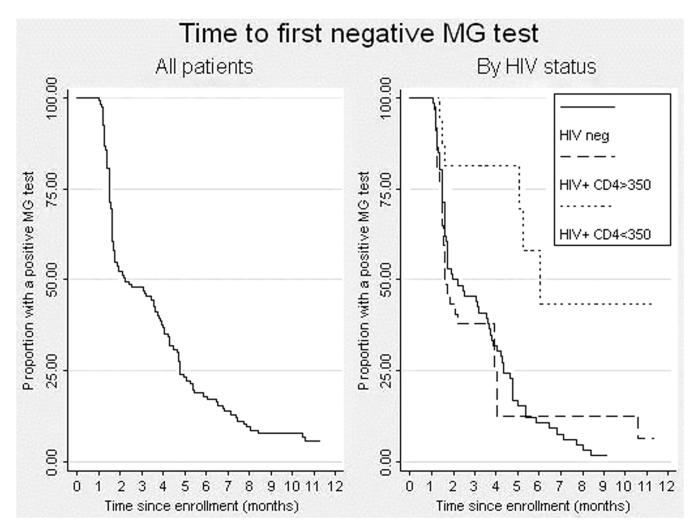
Among the 119 participants infected with M. genitalium at enrollment, 111 (93%) cleared the infection within 12 months of follow-up. More than half of the participants (n = 65; 55%) had cleared M. genitalium infection by the time of their first follow-up visit, which occurred at a median of 3.0 months after enrollment (interquartile range, 2.6–3.2); 33 (28%) additional women had cleared the infection by their second visit; 11 (9%), by the third visit; and 2 (1%), by the fourth follow-up visit (Fig. 1).
Figure 1. Time to first negative M. genitalium test result, in the study population overall and by HIV status and CD4 count.
The median time to clearance was 2.1 months (interquartile range, 1.5–4.8 months; Fig. 1), and the overall clearance rate was 25.7/100 person-years (pyr; 95% confidence interval [CI], 21.4–31.0). HIV-positive women cleared M. genitalium infection more slowly than did HIV-negative women (20.6/100 pyr vs. 31.3/100 pyr, P = 0.03). Among HIV-infected women, the clearance rate was slower among those with CD4 counts below 350/mL3 than that among those with higher CD4 counts (9.9/100 pyr vs. 29.5/100 pyr, P < 0.001; Table 1).
Table 1. Clearance Rates of M. genitalium by HIV Status and CD4 Count.
| Population | No. Women | No. Cleared, n (%) | Clearance Rate/100 pyr (95% CI) |
|---|---|---|---|
| All | 119 | 111 (94) | 25.7 (21.4–31.0) |
| HIV negative at enrollment | 66 | 65 (98) | 31.3 (24.5–39.9) |
| HIV positive at enrollment | 53 | 46 (87) | 20.6 (15.4–27.5) |
| HIV positive with CD4 >350/mL3 | 37 | 36 (97) | 29.5 (21.3–40.8) |
| HIV positive with CD4 <350/mL3 | 16 | 10 (63) | 9.9 (5.3–18.4) |
Factors Associated With Clearance of M. genitalium
Clearance of M. genitalium infection was associated with HIV status (Table 2). HIV-infected women with a CD4 count below 350 cells/mm3 had a significantly slower rate of clearance than did HIV-negative women (adjusted HR, 0.27; 95% CI, 0.14–0.54). The rate of clearance for HIV-infected women with CD4 count above 350 cell/mm3 was similar to that of HIV-negative women (adjusted HR, 0.93; 95% CI, 0.62–1.41). There was no evidence for an association between clearance of M. genitalium and sexual behavior, coinfection with any other STI, or treatment with antibiotics for an STI or any other health condition at our clinic.
Table 2. Risk Factors for Clearance of MG Infection.
| Variable | MG Cleared (n = 111), n (%) | MG Clearance Rate/100 pyr | Crude HR (95% CI) |
|---|---|---|---|
| Follow-up time, mo | P = 0.43 | ||
| 0–3 | 65 (58) | 23.4 | 1 |
| 3–6 | 33 (30) | 33.9 | 2.08 (0.78–5.54) |
| 6–9 | 11(10) | 27.2 | 1.29 (0.36–4.65) |
| 9–12 | 2 (2) | 13.1 | 0.82 (0.06–11.74) |
| Age (y)* | P = 0.87 | ||
| <25 | 57 (60) | 26.7 | 1 |
| 25–34 | 49 (54) | 24.6 | 0.92 (0.63–1.35) |
| 35+ | 5 (55) | 27.8 | 1.13 (0.45–2.83) |
| No. sexual partners last month† | P = 0.96 | ||
| 0–4 | 44 (57) | 26.3 | 1 |
| 5–19 | 28 (58) | 26.3 | 0.96 (0.59–1.54) |
| 20–49 | 21 (55) | 22.6 | 0.82 (0.48–1.39) |
| 50+ | 13 (59) | 28.7 | 1.03 (0.55–1.91) |
| Cannot remember | 5 (56) | 25.8 | 0.88 (0.35–2.24) |
| Condom use with paying clients last month† | P = 0.83 | ||
| Consistent | 55 (60) | 27.2 | 1 |
| Inconsistent | 45 (54) | 25.3 | 0.92 (0.62–1.37) |
| No such clients | 11 (55) | 21.6 | 0.84 (0.43–1.61) |
| Cleansing of vagina in last 3 mo† | P = 0.06 | ||
| Not cleansing, cleansing without soap | 55 (65) | 29.9 | 1 |
| Cleansing using soap | 56 (51) | 22.6 | 0.69 (0.48–1.01) |
| Inserting substances in vagina in last 3 mo† | P = 0.57 | ||
| No | 56 (58) | 26.9 | 1 |
| Yes | 55 (56) | 24.7 | 0.90 (0.61–1.31) |
| Pregnant† | P = 0.43 | ||
| No | 107 (57) | 26.2 | 1 |
| Yes | 4 (50) | 17.5 | 0.69 (0.25–1.87) |
| Use of hormonal contraception in past 3 mo†,‡ | P = 0.37 | ||
| Not using | 69 (54) | 24.3 | 1 |
| Using oral pill | 15 (62) | 28.6 | 1.29 (0.74–2.26) |
| Using injectable | 23 (67) | 31.8 | 1.26 (0.79–2.03) |
| Treatment with antibiotics for presumptive PID and/or VDS†,§ | P = 0.57 | ||
| No | 62 (60) | 27.3 | 1 |
| Yes | 49 (54) | 24.0 | 0.89 (0.61–1.32) |
| Syphilis serology† | P = 0.75 | ||
| RPR−TPHA− | 83 (60) | 26.7 | 1 |
| RPR−TPHA+ | 12 (48) | 21.2 | 0.80 (0.44–1.48) |
| RPR+TPHA+ | 16 (53) | 25.2 | 0.90 (0.53–1.55) |
| HSV2 serology† | P = 0.26 | ||
| Negative | 16 (52) | 21.1 | 1 |
| Positive | 95 (58) | 26.7 | 1.35 (0.79–2.29) |
| N. gonorrhoeae†,∥ | P = 0.63 | ||
| Negative | 93 (58) | 26.4 | 1 |
| Positive | 18 (42) | 23.6 | 0.88 (0.53–1.47) |
| C. trachomatis†,∥ | P = 1.00 | ||
| Negative | 100 (57) | 25.8 | 1 |
| Positive | 11 (71) | 26.9 | 1.00 (0.53–1.87) |
| T. vaginalis † | P = 0.34 | ||
| Negative | 90 (60) | 27.0 | 1 |
| Positive | 21 (47) | 21.4 | 0.80 (0.49–1.29) |
| C. albicans † | P = 0.21 | ||
| Negative | 107 (58) | 26.3 | 1 |
| Positive | 4 (40) | 16.2 | 0.55 (0.20–1.51) |
| Bacterial vaginosis† | P = 0.21 | ||
| Negative | 31 (64) | 29.3 | 1 |
| Intermediate | 14 (74) | 37.1 | 1.32 (0.70–2.49) |
| Positive | 66 (52) | 23.0 | 0.79 (0.52–1.22) |
| HIV status† | P < 0.01 | ||
| Negative | 65 (65) | 31.3 | 1 |
| Positive with CD4 counts ≥350/mL3 | 36 (62) | 29.5 | 0.96 (0.63–1.45) |
| Positive with CD4 counts < 350/mL3 | 10 (28) | 9.9 | 0.28 (0.14–0.56) |
At enrollment.
At last MG-positive visit.
Among not pregnant women.
Pelvic inflammatory disease was treated either with ciprofloxacin 2 × 500mg/d for 3 days or ceftriaxon 1 g single dose plus doxycycline 2 × 100 mg/d for 14 days, and VDS was treated with doxycycline 2 × 100 mg/d for 7 days, ciprofloxacin 500 mg single dose, and metronidazole 2 g single dose (plus clotrimazole in case of thrush).
One missing data.
MG indicates M. genitalium.
Recurrent M. genitalium Infections
Among the 111 women who cleared the M. genitalium infection, further follow-up data were available for 103 women (93%; median follow-up time of 5.9 months after clearance). Of these, a subsequent M. genitalium infection was detected in 40 (39%) women. The median time to recurrence was 3.4 months (interquartile range, 2.8–6.1 months). The rate of recurrence was 6.6/100 pyr (95% CI, 4.8–9.0). Among the women who were HIV positive at baseline (n = 42) or seroconverted before M. genitalium recurrence (n = 4), the recurrence rate was 6.63/100 pyr (95% CI, 4.17–10.52), similar to that among the 57 HIV-negative women (rate, 6.53/100 pyr; 95% CI, 4.30–9.91; P = 0.98). The rate was higher among the HIV-positive women with CD4 count below 350/mL3 than those with CD4 count above 350/mL3 (8.65/100 pyr vs. 6.55/100 pyr), but the difference in recurrence rates was not statistically significant (P = 0.59).
DISCUSSION
M. genitalium infection persisted in nearly half (45%) of the women at month 3, which is higher than in the Kenyan sex workers cohort (17%). In both settings, women were not specifically treated for M. genitalium infection, although they frequently received antibiotics for other STIs or other health conditions at the clinic. The slower clearance may be related to the higher HIV prevalence in our cohort (45% vs. 30% in Kenya).13 M. genitalium infection persisted up to 1 year in 7% of our women, which was in range with the Kenyan study (9%) but which was substantially less than in the cohort of female university students in the UK (26%). Comparison with the latter study is, however, less straightforward because in the UK, the first retesting for M. genitalium happened only after 12 to 21 months (median, 16 months) and samples were only available for 27 (26%) of the women testing positive at enrollment. Although DNA sequence typing of paired samples of some 7 women demonstrated the same strain of M. genitalium, suggesting that these were persistent infections, the observed higher percentage in the UK may have included recurrent or reinfections.12
It is very likely that the proportion of the women clearing the infection is overestimated because it may simply be an effect of a low load of M. genitalium DNA in the specimens. The median M. genitalium load is known to be 100-fold lower than that of C. trachomatis.17 This may have led to situations where positive specimens were close to the limit of detection of the assay and therefore were likely to become negative at the next sampling event. Future planned studies whereby M. genitalium bacterial load will be quantified at consecutive positive visits may provide more accurate results.
Overall, one third of the samples collected at follow-up visits were tested positive for M. genitalium, and 39% of the women who cleared the infection regained positive samples again within 3 to 6 months. Some of these recurrent infections could have been persistent infections after a previous false-negative result. In the Kenyan sex workers cohort, the incidence rate was 23/100 women-years, which was higher than the rate of chlamydial (2.4/100) and gonococcal (2.0/100) infections.13 This high infection rate may have included recurrent or persistent infections besides incident infections. More studies, preferably using genotyping, are required to confirm true incidence rates and differentiate persistent from reinfections.
An interesting finding of our study was that clearance of M. genitalium infection was slower in HIV-positive women with low CD4 counts than in that HIV-positive women with CD4 counts greater than 350/mL3 and in HIV-negative women. This indicates that a failing cell-mediated immune system may facilitate maintenance and survival of M. genitalium infection because in non–immune-compromised individuals, the vaginal and cervical epithelial mucosa respond to M. genitalium infection by recruitment and stimulation of macrophages to eliminate the pathogen.18 Alternatively, the observed longer persistence of mycoplasma infection in the immune-compromised women could be explained by a higher infection rate, whether new or reinfections.
Antibiotic use is frequent in our cohort because participants are regularly treated at our clinic for other STIs or other health conditions. As recommended by the Ugandan STI guidelines, we treated clinical PID with doxycycline 2 × 100 mg/d for 14 days, metronidazole 400 mg for 14 days, and either ciprofloxacin 2 × 500 mg/d for 3 days or ceftriaxon 1 g single dose; vaginal discharge syndrome (VDS) was treated with doxycycline 2 × 100 mg/d for 7 days, ciprofloxacin 500 mg single dose, and metronidazole 2 g single dose (plus clotrimazole in case of thrush). Although all attending women were treated for these conditions at any of the follow-up visits, we did not find evidence that treatment at most recent visit affected clearance of M. genitalium infection. This is not unexpected because earlier studies demonstrated that those antibiotics may fail to eliminate the infection completely.19
Considering the high rate of chronic infections, it seems likely that M. genitalium evades the host immune response and antibiotic action. Studies investigating the interaction between M. genitalium and the host cell found that the organism, which itself has limited biosynthetic capabilities, does not always adhere to the surface of the epithelial cells, but also has the potential to reside and replicate intracellularly over extended periods, resulting in persistent infections.17,20–24 In addition, mycoplasmas have shown the impressive capacity of rapidly changing their surface components, and this antigenic variation allows for immune evasion and persistence of infection.24,25
A recent study in the United States demonstrated that persistent M. genitalium infection elicits chronic inflammatory cytokine secretion in endocervical epithelial cells, even with low organism burdens.26 This may have important implications: persistent M. genitalium infection could possibly enhance HIV shedding and, as such, HIV transmission; furthermore, the chronic inflammation could possibly also enhance the susceptibility of the genital tract to acquisition of other RTI/STIs and therefore, perhaps, partly explain the positive associations between M. genitalium and HIV acquisition. Finally, persistence of M. genitalium infection could affect the burden of urogenital disease such as cervicitis and PID. Longitudinal studies investigating these potential implications of persistent M. genitalium infection are planned in our cohort.
Our study has several limitations: first, the relatively small sample size somehow limits our conclusions and the use of 3-month intervals between testing for M. genitalium makes the estimates less precise than if using shorter intervals. Next, our M. genitalium specimens were stored for approximately 2 years until PCR testing in Johannesburg, which may have decreased the sensitivity of the test.27 Also, endocervical specimens seem to be less sensitive than vaginal specimens for detection of M. genitalium; this could also have had some influence on differentiation of persistence and recurrence as well as on the estimate of duration of persistence.28,29 In the absence of genotyping, it was not possible to differentiate persistent from recurrent infections. It is likely that the observed recurrence rate was overestimated because some of the observed recurrent infections may have had the same genotype as the initial infection and thus may actually have been persistent infections; besides, some of the observed recurrent infection episodes may have been persistent with a false-negative test results in between 2 positive samples. We are currently planning further work on a larger study sample whereby use of DNA genotyping will allow for differentiating between true persistent episodes and reinfections. Finally, considering the high-risk nature of our study population, we are aware that our findings on recurrence rates may not be generalizable to the wider population.
In conclusion, our study adds to the evidence that M. genitalium is likely to persist and recur in the female genital tract. The pathogen may cause lower and upper urogenital tract morbidity, leading to serious sequelae such as tubal infertility and ectopic pregnancy, and is also strongly associated with HIV acquisition. More research is urgently needed to define how best to screen for this emerging STI, especially in populations at high risk for HIV, and to optimize effective and affordable treatment options.
Acknowledgments
The authors thank the study participants for their collaboration and the study team for their dedication to the work. They also thank Professor David Lewis, Head of Department, Centre for HIVand Sexually Transmitted Infections, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa.
Supported by Medical Research Council, UK, and European and Developing Countries Clinical Trials Partnership.
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Jensen J. Mycoplasma genitalium: The etiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venerol. 2004;18:1–11. doi: 10.1111/j.1468-3083.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 2.McGowin C, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLos Pathogens. 2011;7:e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manhart L. Has the time come to systematically test for Mycoplasma genitalium? Sex Transm Dis. 2009;36:607–608. doi: 10.1097/OLQ.0b013e3181b9d825. [DOI] [PubMed] [Google Scholar]
- 4.Hooton TM, Roberts MC, Roberts PL, et al. Prevalence of Mycoplasma genitalium determined by DNA probe in men with urethritis. Lancet. 1988;1:266–268. doi: 10.1016/s0140-6736(88)90350-9. [DOI] [PubMed] [Google Scholar]
- 5.Horner P, Thomas B, Gilroy CB, et al. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995–1003. doi: 10.1086/319594. [DOI] [PubMed] [Google Scholar]
- 6.Maeda S, Tamaki M, Kojima K, et al. Association of Mycoplasma genitalium persistence in the urethra with recurrence of nongonococcal urethritis. Sex Transm Dis. 2001;28:472–476. doi: 10.1097/00007435-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Taylor-Robinson D, Gilroy CB, Hay PE. Occurrence of Mycoplasma genitalium in different populations and its clinical significance. Clin Infect Dis. 1993;17(suppl 1):S66–68. doi: 10.1093/clinids/17.supplement_1.s66. [DOI] [PubMed] [Google Scholar]
- 8.Taylor-Robinson D, Gilroy CB, Thomas BJ, et al. Mycoplasma genitalium in chronic non-gonococcal urethritis. Int J STD AIDS. 2004;15:21–25. doi: 10.1258/095646204322637209. [DOI] [PubMed] [Google Scholar]
- 9.Wikstrom A, Jensen JS. Mycoplasma genitalium: A common cause of persistent urethritis among men treated with doxycycline. Sex Transm Infect. 2006;82:276–279. doi: 10.1136/sti.2005.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3:e3618. doi: 10.1371/journal.pone.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006;12:1149–1152. doi: 10.3201/eid1207.051558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “new chlamydia?” A community-based prospective cohort study. Clin Infect Dis. 2010;51:1160–1166. doi: 10.1086/656739. [DOI] [PubMed] [Google Scholar]
- 13.Cohen C, Nosek M, Astete S, et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34(5):274–279. doi: 10.1097/01.olq.0000237860.61298.54. [DOI] [PubMed] [Google Scholar]
- 14.Vandepitte J, Muller E, Bukenya J, et al. Prevalence and determinants of Mycoplasma genitalium infection among female sex workers in Kampala, Uganda. J Infect Dis. 2012;205:289–596. doi: 10.1093/infdis/jir733. [DOI] [PubMed] [Google Scholar]
- 15.Vandepitte J, Bukenya J, Hughes P, et al. Clinical characteristics associated with Mycoplasma genitalium infection among women at high risk of HIV and other STI in Uganda. Sex Transm Dis. 2012;39:487–491. doi: 10.1097/OLQ.0b013e31824b1cf3. [DOI] [PubMed] [Google Scholar]
- 16.Vandepitte J, Bukenya J, Weiss HA, et al. HIV and other sexually transmitted infections in a cohort of women involved in high-risk sexual behavior in Kampala, Uganda. Sex Transm Dis. 2011;38:316–323. [PMC free article] [PubMed] [Google Scholar]
- 17.Walker J, Fairley C, Bradshaw C, et al. The difference in determinants of Chlamydia trachomatis and Mycoplasma genitalium in a sample of young Australian women. BMC Infect Dis. 2011;11:35. doi: 10.1186/1471-2334-11-35. http://www.biomedcentral.com/1471-2334/11/35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowin CL, Popov VL, Pyles RB. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol. 2009;9:139. doi: 10.1186/1471-2180-9-139. doi:10.1186/1471-2180-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JS, Blom J, Lind K. Intracellular location of Mycoplasma genitalium in cultured Vero cells as demonstrated by electron microscopy. Int J Exp Pathol. 1994;75:91–98. [PMC free article] [PubMed] [Google Scholar]
- 22.Blaylock MW, Musatovova O, Baseman JG, et al. Determination of infectious load of Mycoplasma genitalium in clinical samples of human vaginal cells. J Clin Microbiol. 2004;42:746–752. doi: 10.1128/JCM.42.2.746-752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dallo SF, Baseman JB. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog. 2000;29:301–309. doi: 10.1006/mpat.2000.0395. [DOI] [PubMed] [Google Scholar]
- 24.Baseman JB, Lange M, Criscimagna NL, et al. Interplay between mycoplasmas and host target cells. Microb Pathog. 1995;19:105–116. doi: 10.1006/mpat.1995.0050. [DOI] [PubMed] [Google Scholar]
- 25.Iverson-Cabral SL, Astete SG, Cohen CR, et al. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol Microbiol. 2007;66:55–73. doi: 10.1111/j.1365-2958.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- 26.McGowin CL, Annan RS, Qualye AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842–3849. doi: 10.1128/IAI.00819-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsen K, Jensen J. Mycoplasma genitalium PCR: Does freezing of specimens affect sensitivity? J Clin Microbiol. 2010;48:3624–3627. doi: 10.1128/JCM.00232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wroblewski J, Manhart L, Dickey K, et al. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol. 2006;44:3306–3312. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillis R, Nsuami M, Myers L, et al. Utility of urine, vaginal, cervical, and rectal specimens for detection of Mycoplasma genitalium in Women. J Clin Microbiol. 2011;49:1990–1992. doi: 10.1128/JCM.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]