Abstract
The basal ganglia (BG) are involved in numerous neurobiological processes that operate on the basis of wakefulness, including motor function, learning, emotion and addictive behaviors. We hypothesized that the BG might play an important role in the regulation of wakefulness. To test this prediction, we made cell body-specific lesions in the striatum and globus pallidus (GP) using ibotenic acid. We found that rats with striatal (caudoputamen) lesions exhibited a 14.95% reduction in wakefulness and robust fragmentation of sleep–wake behavior, i.e. an increased number of state transitions and loss of ultra-long wake bouts (> 120 min). These lesions also resulted in a reduction in the diurnal variation of sleep–wakefulness. On the other hand, lesions of the accumbens core resulted in a 26.72% increase in wakefulness and a reduction in non-rapid eye movement (NREM) sleep bout duration. In addition, rats with accumbens core lesions exhibited excessive digging and scratching. GP lesions also produced a robust increase in wakefulness (45.52%), and frequent sleep–wake transitions and a concomitant decrease in NREM sleep bout duration. Lesions of the subthalamic nucleus or the substantia nigra reticular nucleus produced only minor changes in the amount of sleep–wakefulness and did not alter sleep architecture. Finally, power spectral analysis revealed that lesions of the striatum, accumbens and GP all resulted in a shifting of fast theta power to slow delta power, i.e. a slowing of the cortical electroencephalogram. Collectively, our results suggest that the BG, via a cortico-striato-pallidal loop, are important neural circuitry regulating sleep–wake behaviors and cortical activation.
Keywords: globus pallidus, ibotenic acid, sleep–wake cycle, striatum, ultradian oscillation
Introduction
The basal ganglia (BG) are generally considered to comprise four major functional units, including the striatum (caudoputamen), globus pallidus (GP), subthalamic nucleus (STN) and substantia nigra (SN). These structures are collectively involved in a wide range of motor and cognitive behaviors, including planning and other executive functions (Nambu, 2008). While these ‘higher level’ cognitive processes are associated with the vigilance state of wakefulness, the involvement of the BG in the control of wakefulness per se has received very little attention.
The BG are reciprocally connected to the cortex and this circuitry is believed to be responsible for many functions including the processing and computing of cortical signals (Joel & Weiner, 1994; Parent & Hazrati, 1995; Miyachi, 2009). Within the BG, the striatum is the largest nucleus and the primary cortico-recipient zone of the BG, and is comprised of γ-aminobutyric acid (GABA)ergic medium spiny neurons (~ 95%; MSN) and interneurons (~ 5%; Kemp & Powell, 1971; Wilson & Groves, 1980). Excitatory glutamatergic projections from the neocortex and thalamus, and dopaminergic projections from the midbrain ventral tegmental area (A10), SN pars compacta (SNc, A9) and retrorubral area (A8) influence the activity of these MSN (Gerfen et al., 1987; Parent & Hazrati, 1995). The MSN fire synchronously with cortical pyramidal neurons under slow-wave sleep or anesthetized condition, whereas the MSN fire fast, but it is not synchronized with cortical activity during spontaneous wakefulness (O’Donnell & Grace, 1995; Wilson & Kawaguchi, 1996; Kasanetz et al., 2006; Mahon et al., 2006). C-Fos expression in the striatum is also increased during enhanced wakefulness (Sastre et al., 2000). The firing pattern of GP neurons is relatively regular across the sleep–wake cycle, with only slightly higher average firing rates during wake and rapid eye movement (REM) sleep than during non-REM (NREM) sleep (Urbain et al., 2000). STN neurons exhibit burst firing during NREM sleep and random firing during wakefulness, but their firing rate is not altered during the sleep–wake cycle (Urbain et al., 2000). Overall, neuronal activity (either firing rate or firing pattern or both) of the BG neurons is sleep–wake state dependent, but the differences in firing patterns across the BG suggest a complicated regulatory network, with multiple extra-BG input sources, e.g. cortex, thalamus and dopamine systems, and intra-BG inputs, e.g. GP–STN and striatum–GP interactions. It is then via the large projections to the cerebral cortex that we predict the BG may exert its regulatory influence on sleep–wake behavior and cortical activation.
To demonstrate an important regulatory role for BG neurons in sleep–wake behavior, lesion studies are an excellent starting point. Thus far, only one study has examined the effects of cell body-specific lesions of the striatum on sleep–wake behaviors, and in this study it was reported that striatal lesions transiently increased both general locomotor activity and wakefulness (Mena-Segovia et al., 2002). Unfortunately, the neuroanatomic extent of the lesion was not revealed in that study, complicating the interpretation of those findings. Hence, in the present study, we systematically investigated the role of striatum, GP, STN and substantia nigra pars reticulata (SNr) in sleep–wake by ablating each of these structures.
Materials and methods
Animals
Pathogen-free adult male Sprague–Dawley rats (275–300 g; Harlan) were housed in individual cages. The cages were housed inside isolation chambers, which provided ventilation, computer-controlled lighting (12 h light/12 h dark cycle, with lights on at 07.00 h; ~ 200 lux) and an ambient temperature of 22 ± 1°C. Care of the rats in the experiment met the National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School. Every effort was made to minimize the number of animals used.
Surgery
Under chloral hydrate anesthesia (7% in saline, 350 mg/kg), a fine glass pipette (1 mm glass stock, tapering slowly to a 10–20-μm tip) containing 10% ibotenic acid (Sigma, St Louis, MO, USA) was lowered to the predetermined targets, viz. striatum (AP = +0.2 mm, ML = ±2.9 mm, DV = −5.0 mm), nucleus accumbens core (NAc; AP = +1.2 mm, ML = ±1.8 mm, DV = −7.0 mm), GP (AP = −0.92 mm, ML= ±2.8 mm, DV = −0.62 mm), STN (AP = −3.8 mm, ML = ±2.5 mm, DV = −7.52 mm) and SNr (AP = −5.3 mm, ML = ±2.8 mm, DV = −7.5 mm), as per the atlas of Paxinos & Watson (1998). Then, the toxin was injected with nitrogen gas pulses of 20–40 psi using an air compression system previously described (Lu et al., 2000). Control animals, on the other hand, were injected with saline into the BG. After two additional minutes, the pipette was slowly withdrawn and the animals were then implanted with electrodes for recording electroencephalogram (EEG) and electromyogram (EMG) as explained earlier (Lu et al., 2000). In brief, four EEG screw electrodes were implanted into the skull (two in the frontal and two in the parietal bones on each side) and two flexible EMG wire electrodes were placed into the nuchal muscles. The free ends of the leads were soldered into a head socket, which was then attached to the skull using dental cement. Incisions were closed with wound clips and animals were allowed to recover for 5 days.
EEG/EMG recording and sleep scoring
After 5 days of post-operative recovery, the animals were connected via flexible recording cables to a commutator, which in turn was connected to a Grass polygraph (Model 8, MA, USA), and EEG/EMG signals were recorded continuously for 48 h from the beginning of the light period on post-lesion days 6 and 13. Cortical EEG and EMG signals were amplified, filtered (EEG, 0.5–40 Hz; EMG, 40–200 Hz), digitized at a sampling rate of 250 Hz and recorded by using SleepSign software (Kissei Comtec, Nagano, Japan). The behavior of the animals was recorded simultaneously with time-lock video recordings. When complete, EEG/EMG recording data were automatically scored offline by 12-s epochs as wake, REM and NREM sleep by SleepSign according to the previously established criteria (Lu et al., 2000, 2001). Briefly, NREM sleep was identified by a preponderance of high-amplitude, low-frequency (< 4 Hz) EEG activity and relatively low and unchanging EMG activity, whereas wakefulness was characterized by a preponderance of low-amplitude, fast EEG activity and highly variable muscle tone on EMG. REM sleep was identified by very low EMG activity and a low-amplitude monotonous EEG containing a predominance of theta range (6–9 Hz) EEG activity. After automatic scoring, defined sleep–wake stages were examined manually. Scoring was done before histological examination and so the scorers were unaware of the extent of the lesions. The amount of time, bout duration and total bouts spent in wake, NREM sleep and REM sleep of the second day of each recording were determined from the scored data. In addition, the circadian amplitude of sleep and wake was calculated using the following formula: (Lu et al., 2001). EEG power spectra for NREM and REM epochs were analysed offline using Fast Fourier Transformation (512 point, Hanning window, 0.5–24.5 Hz with 0.5 Hz resolution using SleepSign).
Histology
On completion of the EEG/EMG recordings, the animals were deeply anesthetized with 500 mg/kg of chloral hydrate and transcardially perfused with 50 mL saline, followed by 500 mL neutral phosphate-buffered formalin (Fischer Scientific, NJ, USA). The brains were removed, cryoprotected in 20% sucrose at 4°C and then sliced into four series of 40-μm sections on a freezing microtome. The sections were processed for Nissl staining and GAD67 immunohistochemistry to evaluate the extent of the lesions, as explained earlier (Qiu et al., 2003; Lu et al., 2006a). For Nissl staining, one series of sections was mounted on gelatin-coated slides, washed and incubated in 0.25% thionin solution for 2 min, then washed and dehydrated in gradient ethanols, and cleared in xylene before being coverslipped. For GAD67 immunostaining, sections were incubated with 0.3% H2O2 for 15 min to quench the endogenous peroxidase activity. After washing in 0.1 M phosphate-buffered saline (PBS; pH 7.4), the sections were incubated with primary antibody (anti-GAD7 mouse monoclonal antibody; Chemicon, CA, USA; 1: 5000 dilution) for 24 h at room temperature. On the second day, the sections were washed in PBS and incubated with secondary antibody (biotinylated donkey anti-mouse IgG; Jackson ImmunoResearch Laboratories, PA, USA; 1: 1000 dilution) for 1 h followed by a 1: 1000 dilution of avidin-biotin-peroxidase (Vector Laboratories, CA, USA) for 1 h at room temperature. The peroxidase reaction was visualized with 0.05% 3,3-diaminobenzidine tetrahydrochloride (DAB; Sigma, MO, USA) in 0.1 M phosphate buffer and 0.01% H2O2. After terminating the reaction by PBS-azide, sections were mounted, dehydrated and coverslipped. As controls, adjacent sections were incubated without the primary antibody to confirm that no non-specific staining had occurred.
Statistical analyses
All data were expressed as the mean ± SEM (n = 4–6). The statistical significance of time course data for sleep–wake profiles, sleep amount, stage transition, the number of each stage bouts and mean duration, and CI were assessed by two-tailed unpaired t-test or one-way ANOVA followed by Dunnett’s post hoc test. In all cases, P < 0.05 was taken as the level of significance.
Results
In our experience, sleep–wake behaviors recover within 3–5 days following the surgery, we recorded EEG/EMG for 48 h on two occasions, on post-lesion day 6 (week 1) and on post-lesion day 13 (week 2). As the sleep–wake data recorded at these two time points did not vary within individual animals, it suggests that sleep–wake data collected on day 7 (second 24 h of the recording started on day 6) reflect the new steady state obtained after the cell-specific lesions of the BG. Hence, we report only the sleep–wake data collected at day 7 after the lesions.
Cell body-specific lesions of the striatum decrease and destabilize wakefulness
The role of the striatum in sleep–wake regulation was investigated by placing ibotenate lesions in the striatum of the rats (n = 10). An example of a lesion site in the striatum is shown in Fig. 1. In six animals, the lesions were restricted to caudoputamen. The lesions in the other four animals extended to the NAc with varying degrees and were not included in the analysis.
Fig. 1.
Histology of striatal lesions. Thionin-stained (A–D) and GAD67-immunostained (E–H) coronal sections (rostrocaudal = top-down) from a typical case show the extents of the lesions. The black lines mark the lesion regions. Note that lesions in this case spare the right side of the caudal striatum. High-power Nissl-staining images show intact (I) and lesion (J, from the region marked by * in B) neuronal fields in the striatum. Corresponding high-power images of GAD67 immunostaining show intact (K) and lesion (L, from the region marked by * in F) neuronal fields in the striatum. aca, anterior commissure, anterior part; GP, globus pallidus; SCN, suprachiasmatic nucleus; SON, supraoptic nucleus.
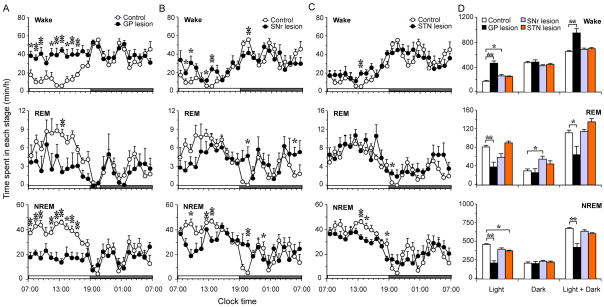
Striatial lesions that did not include the NAc produced a 14.95% reduction in wakefulness (Fig. 2A and B). The reduction in wakefulness was accompanied by a destabilization of the waking state, as indicated by an increase in the number of sleep–wake transitions and sleep–wake bouts and a decrease in NREM sleep bout duration during the dark period (Fig. 2C). The CI (i.e. diurnal amplitude) of wakefulness was significantly reduced (CI: control = 45.43 ± 3.91; lesion = 19.16 ± 7.24, P = 0.011) and was coupled to the appearance of prominent ultradian oscillations (Fig. 3). The most striking feature of the wake state instability was the absence of ultra-long (> 120 min, 2–3 per night observed in control animals) consolidated waking bouts in the lesioned animals (Fig. 3). Rats with striatal lesions appeared to move slowly during the night. Rats with striatal lesions that extended to the NAc showed a fragmented pattern of wakefulness, although their total sleep–wake time was not altered, In addition, these rats demonstrated ‘obsessive-compulsive disorder’ (OCD)-like behavior, that included profound hyperactivity in the form of repetitive chewing, excessive digging and grooming.
Fig. 2.
The effects of striatal lesions on sleep and wakefulness. (A) Light: dark = 12: 12 h time course of the hourly amounts of wake, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep of control (n = 6) and striatum-lesioned (n = 6) rats. Each circle represents the hourly mean ± SEM of wakefulness, REM and NREM sleep. (B) Total time spent in wake, REM and NREM sleep during the light and dark periods and over the 24-h day. (C) Results of sleep–wake transitions (N, W and R represent the stage for NREM, wakefulness and REM sleep, respectively). (D) Results of bout numbers (upper panel) and mean durations (lower panel) during light and dark periods. *P < 0.05, **P < 0.01, two-tailed unpaired t-test.
Fig. 3.
Examples of compressed 24-h electroencephalogram (EEG)/electromyogram (EMG) recordings and corresponding hypnograms of control, striatal, nucleus accumbens core (NAc) and globus pallidus (GP) lesions. As compared with controls, the striatal and GP lesions resulted in frequent sleep–wake transitions and the appearance of pronounced ultradian oscillation of wakefulness and sleep. In addition, striatal lesions eliminated ultra-long wake bouts (*), which were commonly observed in the control animals. Although the NAc lesions induced more frequent sleep–wake transitions, they did not affect the diurnal pattern and ultra-long wake bouts were preserved. Similar to NAc lesions, GP lesions did not eliminate ultra-long wake bouts. NREM, non-rapid eye movement; REM, rapid eye movement.
In order to clarify whether this OCD-like behavior observed in rats with NAc lesions masked the wake-reducing effects of the striatal lesion, we injected 45 μL of 10% ibotenic acid into the NAc bilaterally of four rats. We found that these animals with bilateral lesions confined to the NAc also displayed similar OCD-like behaviors. Whether these behaviors are truly OCD remains to be determined; however, the repetitive and compulsive nature of the behaviors was highly reminiscent of the OCD phenotype. Interestingly, in contrast to the striatal lesions, rats with lesions restricted to the NAc showed significant increases in the amount of wakefulness (26.72%) and a reduction in NREM bout duration (Figs 4 and 5); however, ultra-long wake bouts during the night were still observed and the diurnal pattern of sleep–wake was not affected in these animals (Fig. 3).
Fig. 4.
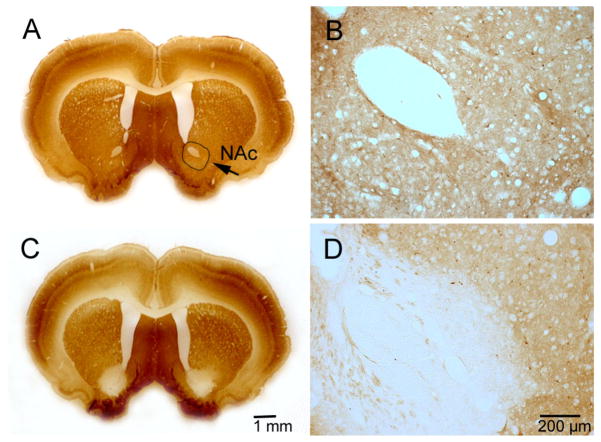
Histology of nucleus accumbens core (NAc) lesions. GAD67 immunostaining in a control (A, B) and a lesion case (C, D).
Fig. 5.
The effects of nucleus accumbens core (NAc) lesions on sleep and wakefulness. (A) Light: dark = 12: 12 h time course of the hourly amounts of wake, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep of control (n = 6) and NAc (n = 4) lesioned rats. Each circle represents the hourly mean ± SEM of wakefulness, REM and NREM sleep. (B) Total time spent in wake, REM and NREM sleep during the light and dark periods and over the 24-h day. (C) Results of sleep–wake transitions (N, W and R represent the stage for NREM, wakefulness and REM sleep, respectively). (D) Results of bout numbers (upper panel) and mean durations (lower panel) during light and dark periods. *P < 0.05, **P < 0.01, two-tailed unpaired t-test.
Cell body-specific lesions of the GP increase and destabilize wakefulness
We next injected ibotenic acid (30–45 nL, n = 6) into the GP bilaterally to clarify the role of the GP in sleep–wake regulation. During the first 24 h following the ibotenic acid injection, we observed repetitive chewing and scratching behaviors in the rats. These behaviors were likely due to the acute excitatory effects of ibotenic acid in the GP, as the chewing and scratching resolved within 24 h. Sleep–wake analysis showed that the rats with GP lesions (Fig. 6) had profound insomnia with a striking 45.52% increase in total wakefulness and pronounced fragmentation of sleep–wake behaviors, including more sleep–wake transitions and shortened sleep bouts (Figs 7 and 8). The CI of wakefulness in these animals was also reduced when compared with that of control animals (2.13 ± 2.47 vs 45.43 ± 3.91, P < 0.01; Fig. 7). However, the ultra-long nocturnal wake bouts were still observed in these animals (Fig. 3).
Fig. 6.
Histology of ibotenic acid lesions in the globus pallidus (GP), substantia nigra pars reticulata (SNr) and subthalamic nucleus (STN). The coronal sections (A–D) from rostral to caudal levels show lesion areas in the GP in a rat. (E, G and I) Intact normal morphology of the GP, SNr and STN from control rats. (F, H and J) The morphology of lesions in the GP, SNr and STN, respectively. The insert pictures of (e, g, i) and (f, h, j) are high-magnified images from the rectangular areas marked by ‘e’, ‘g’, ‘i’ and ‘f’, ‘h’, ‘j’, respectively).
Fig. 7.
Quantitative changes of sleep–wake times of ibotenic acid lesions in globus pallidus (GP), substantia nigra pars reticulate (SNr) and subthalamic nucleus (STN). (A–C) Diurnal patterns of hourly wake, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep amounts of control (n = 6) and GP (n = 6), SNr (n = 5) and STN (n = 6) lesion groups, *P < 0.05, **P < 0.01, two-tailed unpaired t-test. (D) Total amounts of wake, REM and NREM sleep in light period, dark period and light + dark period in each group. *P < 0.05, **P < 0.01, one-way ANOVA followed by Dunnett’s post hoc test.
Fig. 8.
Sleep–wake transition (A), bout number and mean duration (B) of wake, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep in dark and light periods of control and globus pallidus (GP) lesions. *P < 0.05, **P < 0.01, two-tailed unpaired t-test.
Consistent with that of previous studies (Levine et al., 1971; Palfai et al., 1984; Sandor et al., 1992), GP lesions also resulted in adipsia and aphagia, and a significant and rapid loss of body weight in rats. The rats with GP lesions lost 120 ± 16.31 g at week 2 after surgery, while the control rats gained about 14.67 ± 4.92 g during the same period. The weight loss in GP-lesioned animals was most pronounced during the first week after lesion.
Cell body-specific lesions of the STN and SNr have no significant effects on sleep–wake behavior
As the glutamatergic STN and GABAergic GP are reciprocally connected, we tested if STN lesions (n = 6 rats) would affect sleep–wake behavior. In the acute phase (i.e. 24 h following the ibotenic acid injection), we observed chewing and scratching behaviors similar to that observed after ibotenic acid injections into the GP. However, we did not observe any weight loss or abnormal motor behaviors in these animals, which we did observe in GP-lesioned animals. As shown in Fig. 7C and D, STN lesions resulted in only minor changes in the amount of sleep–wakefulness and did not alter sleep architecture.
As the SNr is a major output of the BG (projecting to the thalamus and brainstem), we also examined the effects of lesions (n = 5) of SNr neurons on sleep–wakefulness. Similar to the STN lesions, bilateral SNr lesions also produced only small changes in sleep–wake times and did not affect sleep architecture (Fig. 7B and D). We also did not observe any weight loss or abnormal motor behaviors in SNr-lesioned rats.
Power spectral analysis
The normalized power spectrum from lesion cases including the striatum, NAc and GP all showed a common pattern. Specifically, all lesions produced a shift in power from the theta to delta range in the EEG during wake, NREM sleep and REM sleep (Fig. 9), indicating that lesions of the BG produce a slowing of the cortical EEG in sleep–wake cycle.
Fig. 9.
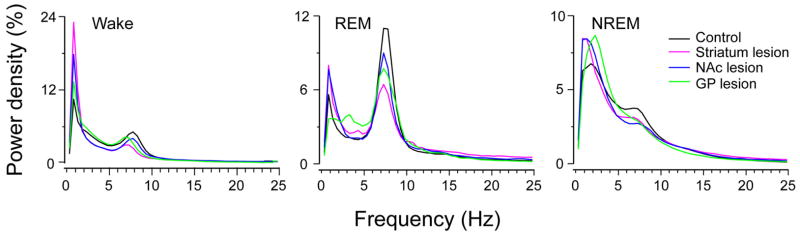
EEG power spectra during wake, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep over 24 h. The power spectrum was normalized to total power (0.5–24.5 Hz). Lesions in the striatum, nucleus accumbens core (NAc) and globus pallidus (GP) all produced a generalized slowing of the EEG, with less theta and more delta density during wake, REM and NREM sleep. In other words, all of the lesions produced a slowing of the cortical EEG.
Discussion
In the present study, we found that cell body-specific lesions in the BG produced significant changes in sleep–wake in rats. The striatal (caudoputamen) lesions produced a significant reduction in wakefulness, while the lesions of GP resulted in large increases in wakefulness. Both lesions, however, resulted in a destabilized sleep–wake architecture, with an increase in sleep–wake transitions and a reduction in the diurnal amplitude of wake–sleep cycle. Striatal lesions also eliminated ultra-long wake bouts (> 120 min), which were commonly observed in the control animals. On the other hand, lesions of the NAc (ventral striatum) produced an increase in wakefulness, but did not eliminate these ultra-long wake bouts. Finally, lesions in the STN and SNr did not produce any major changes in sleep–wake.
Striatal control of arousal
Rats with bilateral striatal lesions demonstrated a significant reduction in wakefulness. Most of these lesions were restricted to the rostral striatum, with the exception of two cases in which the caudal striatum was also affected. The reduction in wakefulness was observed whether or not the lesions included the caudal striatum, however. These observations suggest that the rostral striatum, but not caudal striatum, is critically involved in promoting wakefulness. These findings are not surprising as the caudal striatum receives inputs primarily from the auditory cortex (McGeorge & Faull, 1989; Sgambato et al., 1997). Interestingly, the reduction in wakefulness after striatal lesions was attenuated when the lesions included NAc. This finding is consistent with our observation that the lesions restricted to NAc produced an increase in wakefulness. Thus, it appears that these two cell groups play opposing roles in sleep–wake regulation and that the sleep–wake effects were largely balanced out when the lesions involved both cell groups. This also might explain why a previous study found transient increases in motor activity and wakefulness in rats after striatal lesions (Mena-Segovia et al., 2002). It is also possible that the increased wakefulness observed after NAc lesions could be secondary to OCD-like behaviors observed in those animals.
The ~15% reduction in wakefulness seen in the animals with striatal lesions is very significant considering the observations that wakefulness is not decreased following lesions or genetic ablation of several major and previously established ‘arousal’ groups, including the cholinergic basal forebrain neurons, serotonergic dorsal raphe neurons, the histaminergic tuberomammillary nucleus (Parmentier et al., 2002; Gerashchenko et al., 2004; Lu et al., 2006a; Blanco-Centurion et al., 2007), noradrenergic locus coeruleus(Blanco-Centurion et al., 2007), cholinergic pedunculopontine, and laterodorsal tegmental nuclei (Lu et al., 2006b) and orexin neurons in the lateral hypothalamus (Hara et al., 2001). Importantly, however, fragmentation of sleep–wake behavior was observed after some of these lesions (orexin knockout mice, pedunculopontine and laterodorsal tegmental nuclei and medial parabrachial nucleus lesions; Lu et al., 2006b). Thus far, lesions of only three other arousal centers, viz. medial parabrachial nucleus (40%; Lu et al., 2006b), the lateral hypothalamus (30%; Gerashchenko et al., 2003), and the dopaminergic ventral periaquaductal gray matter (vPAG, 20%; Lu et al., 2006a) have been shown to produce a greater than 15% reduction in baseline waking. As the rostral striatal lesions produced both a reduction in the amount of wakefulness and alterations in sleep–wake architecture, these findings suggest that this cell group may play an important role in promoting and maintaining wakefulness.
Lesions of the rostral striatum also decreased the CI of wakefulness and resulted in prominent ultradian oscillations. Disruption of circadian rhythm following lesions of the suprachiasmatic nucleus or its output relays, such as the ventral subparaventricular zone and the dorsomedial hypothalamic nucleus, results in a similar unmasking of prominent ultradian oscillations and loss of long wake bouts during the subjective night (Ibuka et al., 1977; Lu et al., 2001; Chou et al., 2003). On the other hand, lesions of the sleep-promoting ventrolateral preoptic nucleus do not alter the circadian pattern of sleep–wake behavior (Lu et al., 2000). Thus, we hypothesize that the circadian system may target arousal systems but not sleep systems to exert control over sleep–wake behavior. Fragmentation of sleep–wake behavior is perhaps the most common occurrence in human sleep disorders such as narcolepsy-cataplexy and insomnia, and many neurological disorders including Huntington’s disease and parkinsonism. Humans are arguably more vulnerable to fragmentation of wakefulness than non-human species as humans are required to perform maximally during the day. Wake and sleep fragmentation naturally result in daytime sleepiness and nighttime insomnia. Although the causes of insomnia are multiple, our results indicate that certain types of insomnia may be linked to dysfunction of arousal networks or circadian control, and that one effective way to treat this kind of insomnia may perhaps be through stabilization of daytime wakefulness by prescribing stimulants.
Pallidal control of arousal
On the basis of the anatomical interrelationship of the striatum and GP, we hypothesized that GP lesions would produce sleep–wake changes opposite to that of striatal lesions. Our data are in fact consistent with this hypothesis as GP lesions produced a large increase in wakefulness. Although GP-lesioned animals also suffered from weight loss, this may not be related to the increased wakefulness observed in these animals. The basis for this interpretation is: (i) increased wakefulness was observed even during the second week post-lesions when the weight loss was prevented by feeding the rats with a highly palatable diet; and (ii) lesions of either the ascending dopaminergic projections or SNc dopaminergic neurons by 6-hydroxydopamine also produced similar reductions in body weight but did not cause sleep–wake alterations (Qiu & Lu, unpublished observations). We therefore speculate that GP lesions and loss of dopamine signaling cause oral motor dysfunctions and this produces the observed weight loss. Nevertheless, the magnitude of increase (~45%) in wakefulness following GP lesions was huge and comparable to the effects of the lesions of the only established sleep-promoting cell group, i.e. ventrolateral preoptic nucleus (Lu et al., 2000). Hence, the GP may be another key player in the sleep–wake control mechanisms. The changes in wakefulness following striatal and GP lesions are consistent with the proposed neural circuitry for arousal control (see below). Besides the striatal and thalamic inputs, the GP receives glutamatergic inputs from the STN. The lack of sleep–wake changes following STN lesions suggests, however, that these inputs are not critically involved in pallidal control of arousal. We also observed weight loss, aphasia and adipsi in the GP-lesioned animals, which has been hypothesized to attribute to motoric impairment (Palfai et al., 1984).
Cortico-striato-pallidal loop controls sleep–wake cycle and cortical activation
The BG influence the cerebral cortex via three possible pathways: (i) corticopetal neurons in the GP (Zaborszky et al., 1999; Hur & Zaborszky, 2005); (ii) via ‘direct’ striatonigral pathway, projecting first to the SNr and then to the motor thalamus that project to the cortex; and (iii) via ‘indirect’ pathway that consists of a series of connections to the GP, STN and SNr, and finally to the motor thalamus (Nambu, 2008). However, the latter two pathways via the thalamus and SNr may not be important in relaying GP signals for arousal to the cerebral cortex because the ibotenic acid lesions of SNr or kainic acid lesions of the thalamus do not affect sleep–wake behavior significantly (Vanderwolf & Stewart, 1988). We therefore propose that the direct pallidal projections to the cerebral cortex are the most critical for arousal control (Fig. 10). In this framework, the cortico-striato-pallidal loop might relay and enhance cortical arousal signals and thereby regulate wake behavior. This loop may enhance and stabilize the excitatory state of the pyramidal neurons, i.e. cortical activation, which is consistent with that the power spectrum following lesions of the striatum, NAc and GP all showed a shifting of the EEG toward slow-oscillations during the sleep–wake states. Although the lesions of the striatum, NAc and GP differentially altered the sleep–wake amounts, fragmentation of sleep–wake states, however, was common after the lesions of these structures. Such fragmentation of sleep (or deconsolidation of sleep) would have led to greater homeostatic sleep drive and thus resulted in an increase in delta activity seen in the NREM EEG power analysis in the lesioned rats. Thus, we can conclude that the BG are not only involved in the regulation of neurobehavioral arousal but also involved in regulating the level of electrocortical arousal. It is, however, not clear whether the BG regulate cortical activation (EEG) via their projections to the thalamus or via direct pallidocortical projections.
Fig. 10.
Neural circuitry underlying the BG control of sleep–wake behavior. The striatum receiving cortical inputs projects to the GP, which then projects to the cerebral cortex directly or by the thalamus (mainly the mediodorsal thalamic nucleus). We hypothesize that the cortico-striato-pallidal loop regulates sleep–wake behavior and cortical activation. GP, globus pallidus; OC, optic chiasm.
As both the striatum and GP receive midbrain dopaminergic inputs, we hypothesized that this projection mediates, at least in part, dopaminergic control of arousal. Loss of dopaminergic D2 receptor-mediated inputs to the striatum would result in hyperactivity of MSN, resulting in GP inhibition. Although there has been a long controversy over whether and how dopamine controls arousal, several converging lines of evidence do support a role for dopamine in the control of arousal. For example, psycho-stimulants (e.g. methamphetamine and cocaine) promote wakefulness by inhibiting the dopamine reuptake transporter (DAT) and thereby enhancing the extracellular dopamine levels (Schenk, 2002). DAT knockout mice show about a 20% increase in wakefulness as compared with littermate controls (Wisor et al., 2001). DAT knockout mice are also refractory to the arousal effect of methamphetamine (Wisor et al., 2001). Despite this compelling data, midbrain and SNc dopaminergic neurons do not exhibit state-dependent discharge rates (Miller et al., 1983). Moreover, lesions of the and SNc produce hyperactivity and an increase rather than a decrease in wakefulness (Lai et al., 1999; Gerashchenko et al., 2006). On the other hand, we have recently identified a population of wake-active dopamine neurons in the vPAG, lesions of which produce a reduction in wakefulness of 20% (Lu et al., 2006a). Thus, we propose that vPAG dopaminergic projections to the striatum and GP may be the key circuitry in dopaminergic arousal circuitry.
In summary, our results show that both the striatum and GP are involved in the consolidation and promotion of wake and sleep, respectively, and that the BG comprise neural circuitry controlling sleep–wakefulness. In addition, our results suggest that the cortico-striato-pallidal loop may be critically involved in BG control of cortical arousal.
Acknowledgments
We thank Quan Hue Ha for her technical support. This work is supported by NS 051609 and NS 062727, and China Scholarship Council (2007106568).
Abbreviations
- CI
circadian index
- CP
cerebral peduncle
- DAT
dopamine reuptake transporter
- EEG
electroencephalogram
- EMG
electromyogram
- GABA
γ-aminobutyric acid
- GP
globus pallidus
- MSN
medium spiny neurons
- NAc
nucleus accumbens core
- NREM
non-rapid eye movement
- OCD
obsessive-compulsive disorder
- PBS
phosphate-buffered saline
- REM
rapid eye movement
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- vPAG
ventral periaquaductal gray matter
References
- Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long-Evans rats. Neuroscience. 2003;116:223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion CA, Miller JD, Shiromani PJ. Insomnia following hypocretin2-saporin lesions of the substantia nigra. Neuroscience. 2006;137:29–36. doi: 10.1016/j.neuroscience.2005.08.088. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Chou TC, Blanco-Centurion CA, Saper CB, Shiromani PJ. Effects of lesions of the histaminergic tuberomammillary nucleus on spontaneous sleep in rats. Sleep. 2004;27:1275–1281. doi: 10.1093/sleep/27.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected] The Journal of comparative neurology. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Ibuka N, Inouye SI, Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain research. 1977;122:33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Riquelme LA, O’Donnell P, Murer MG. Turning off cortical ensembles stops striatal Up states and elicits phase perturbations in cortical and striatal slow oscillations in rat in vivo. The Journal of physiology. 2006;577:97–113. doi: 10.1113/jphysiol.2006.113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The connexions of the striatum and globus pallidus: synthesis and speculation. Philosophical transactions of the Royal Society of London. 1971;262:441–457. doi: 10.1098/rstb.1971.0106. [DOI] [PubMed] [Google Scholar]
- Lai YY, Shalita T, Hajnik T, Wu JP, Kuo JS, Chia LG, Siegel JM. Neurotoxic N-methyl-D-aspartate lesion of the ventral midbrain and mesopontine junction alters sleep-wake organization. Neuroscience. 1999;90:469–483. doi: 10.1016/s0306-4522(98)00429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Ferguson N, Kreinick CJ, Gustafson JW, Schwartzbaum JS. Sensorimotor dysfunctions and aphagia and adipsia following pallidal lesions in rats. Journal of comparative and physiological psychology. 1971;77:282–293. doi: 10.1037/h0031650. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006a;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006b;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Cintra L, Prospero-Garcia O, Giordano M. Changes in sleep-waking cycle after striatal excitotoxic lesions. Behavioural brain research. 2002;136:475–481. doi: 10.1016/s0166-4328(02)00201-2. [DOI] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain research. 1983;273:133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Miyachi S. Cortico-basal ganglia circuits--parallel closed loops and convergent/divergent connections. Brain and nerve = Shinkei kenkyu no shinpo. 2009;61:351–359. [PubMed] [Google Scholar]
- Nambu A. Seven problems on the basal ganglia. Current opinion in neurobiology. 2008 doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfai T, Armstrong D, Courtney CL. Effect of l-dopa or bromocriptine on feeding and motor behavior of rats with lesions in the globus pallidus. Physiology & behavior. 1984;33:283–289. doi: 10.1016/0031-9384(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Zhang R, Sun FY. Enhancement of ischemia-induced tyrosine phosphorylation of Kv1.2 by vascular endothelial growth factor via activation of phosphatidylinositol 3-kinase. Journal of neurochemistry. 2003;87:1509–1517. doi: 10.1046/j.1471-4159.2003.02110.x. [DOI] [PubMed] [Google Scholar]
- Sandor P, Hajnal A, Jando G, Karadi Z, Lenard L. Microelectrophoretic application of kainic acid into the globus pallidus: disturbances in feeding behavior. Brain research bulletin. 1992;28:751–756. doi: 10.1016/0361-9230(92)90255-v. [DOI] [PubMed] [Google Scholar]
- Sastre JP, Buda C, Lin JS, Jouvet M. Differential c-fos expression in the rhinencephalon and striatum after enhanced sleep-wake states in the cat. The European journal of neuroscience. 2000;12:1397–1410. doi: 10.1046/j.1460-9568.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- Schenk JO. The functioning neuronal transporter for dopamine: kinetic mechanisms and effects of amphetamines, cocaine and methylphenidate. Progress in drug research. Fortschritte der Arzneimittelforschung. 2002;59:111–131. doi: 10.1007/978-3-0348-8171-5_4. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Abo V, Rogard M, Besson MJ, Deniau JM. Effect of electrical stimulation of the cerebral cortex on the expression of the Fos protein in the basal ganglia. Neuroscience. 1997;81:93–112. doi: 10.1016/s0306-4522(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Urbain N, Gervasoni D, Souliere F, Lobo L, Rentero N, Windels F, Astier B, Savasta M, Fort P, Renaud B, Luppi PH, Chouvet G. Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat. The European journal of neuroscience. 2000;12:3361–3374. doi: 10.1046/j.1460-9568.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Stewart DJ. Thalamic control of neocortical activation: a critical reevaluation. Brain research bulletin. 1988;20:529–538. doi: 10.1016/0361-9230(88)90143-8. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. The Journal of comparative neurology. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Annals of the New York Academy of Sciences. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]