Abstract
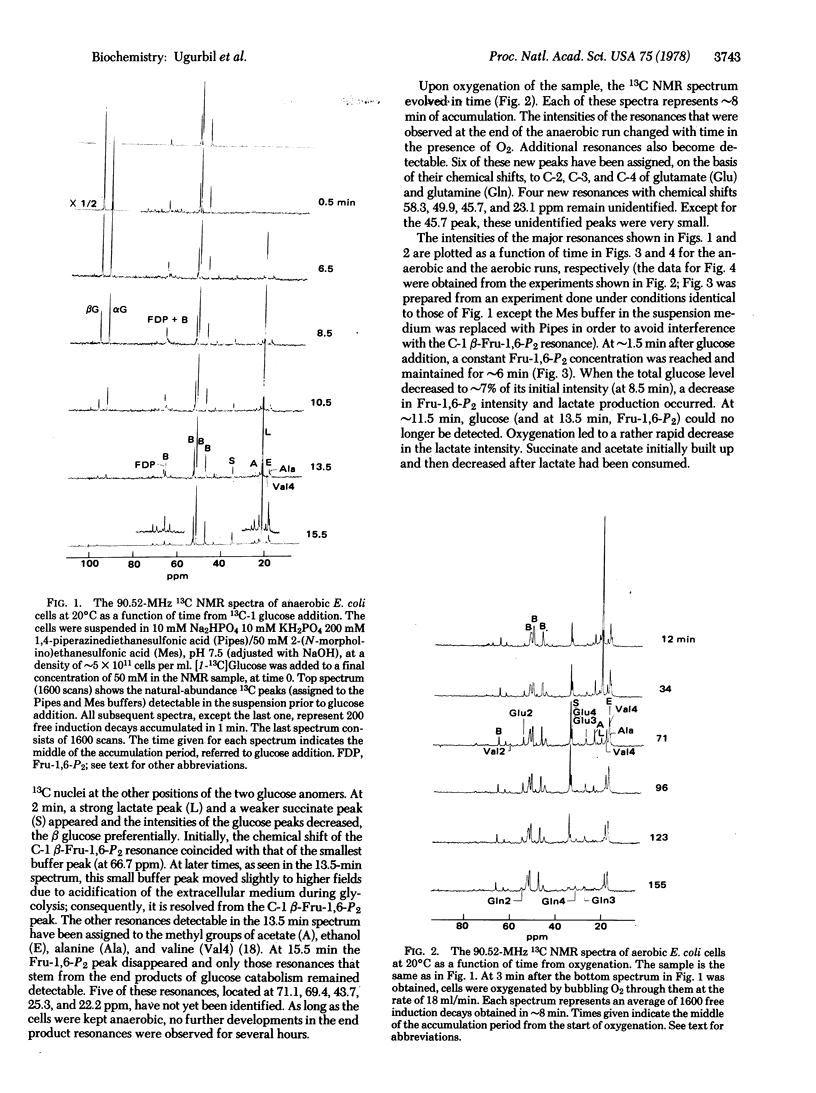
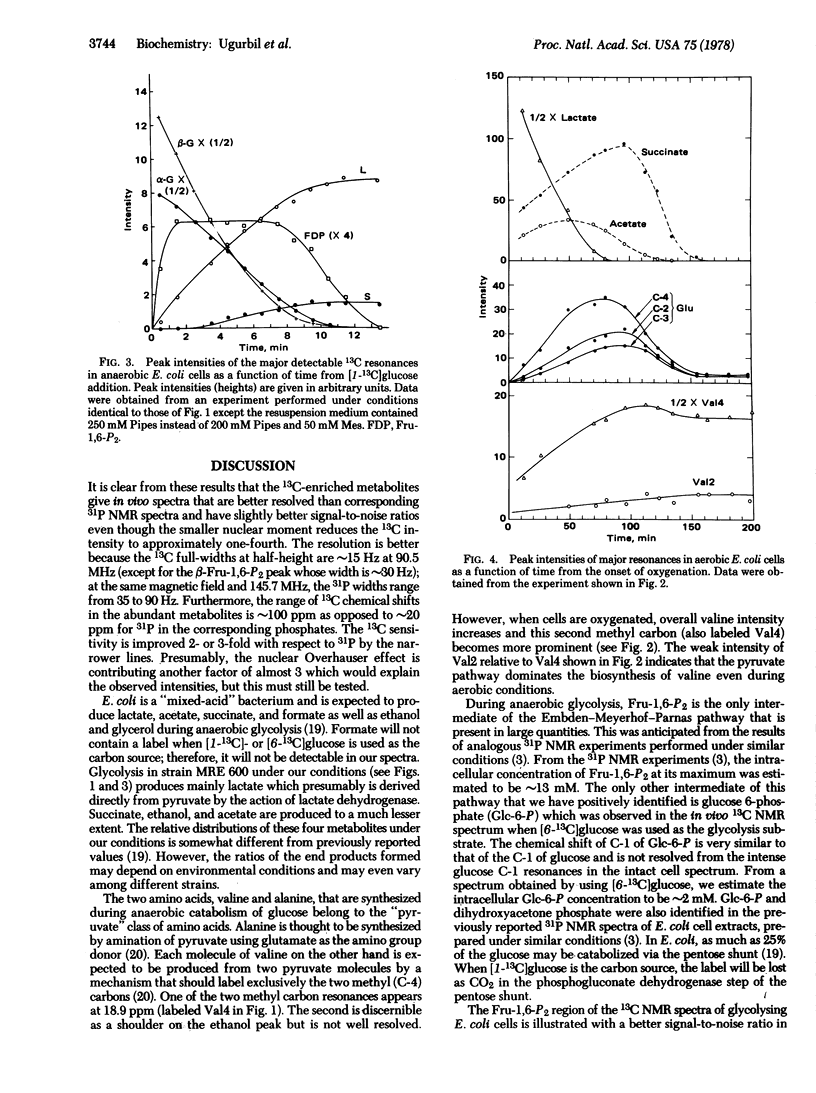
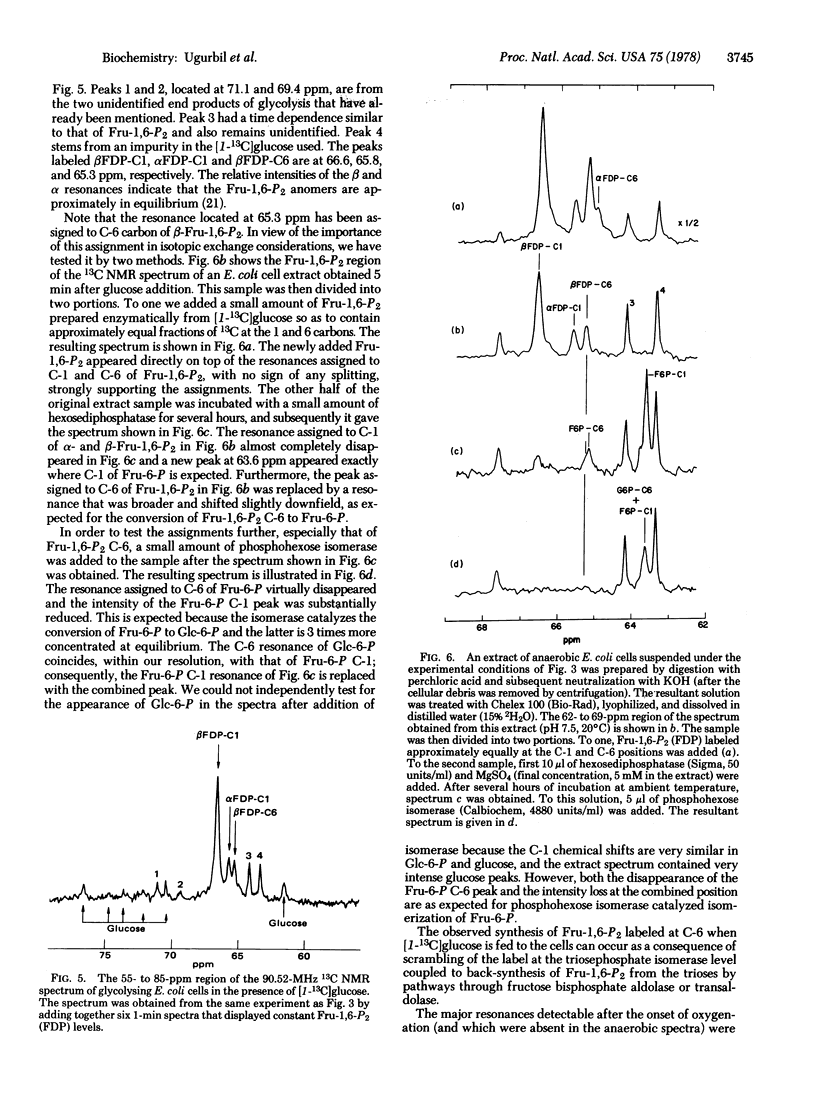
High-resolution 13C nuclear magnetic resonance spectra of suspensions of Escherichia coli cells have been obtained at 90.5 MHz by using the Fourier transform mode. Anaerobic cells incubated with [I-13C]glucose show a time course of glycolysis in which the alpha and beta glucose anomers disappear at different rates, lactate, succinate, acetate, alanine, and valine accumulate as end products of glycolysis, and fructose bisphosphate appears as an intermediate. It is shown that fructose bisphosphate is labeled at C-1 and C-6 during [I-13C]-glucose catabolism. Upon oxygenation, glutamate appears with the 13C ENRICHMENT AT THE C-4, C-3, and C-2 positions, with the C-4 most intense. From the position of the 13C label we conclude that valine is formed by condensation of pyruvate and that carbon enters the tricarboxylic acid cycle mainly through acetyl CoA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J., Engle J. L., Mildvan A. S. Magnetic resonance studies of the anomeric distribution and manganese binding properties of fructose phosphates. Biochem Biophys Res Commun. 1972 May 26;47(4):852–858. doi: 10.1016/0006-291x(72)90571-2. [DOI] [PubMed] [Google Scholar]
- Eakin R. T., Morgan L. O., Gregg C. T., Matwiyoff N. A. Carbon-13 nuclear magnetic resonance spectroscopy of living cells and their metabolism of a specifically labeled 13C substrate. FEBS Lett. 1972 Dec 15;28(3):259–264. doi: 10.1016/0014-5793(72)80726-9. [DOI] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainosho M., Ajisaka K., Nakazawa H. In situ analysis of the microbial fermentation process by natural abundance 13C and 31P NMR spectroscopy. Production of adenosine-5'-triphosphate from adenosine. FEBS Lett. 1977 Aug 15;80(2):385–389. doi: 10.1016/0014-5793(77)80482-1. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Cary L. W., Bhacca N. S., Younathan E. S. Tautomeric composition of D-fructose phosphates in solution by Fourier transform carbon-13 nuclear magnetic resonance. Biochem Biophys Res Commun. 1973 Apr 2;51(3):543–550. doi: 10.1016/0006-291x(73)91348-x. [DOI] [PubMed] [Google Scholar]
- Matwiyoff N. A., Needham T. E. Carbon-13 NMR spectroscopy of red blood cell suspensions. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1158–1164. doi: 10.1016/0006-291x(72)90590-6. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Ciba Found Symp. 1975;(31):225–241. doi: 10.1002/9780470720134.ch13. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Stejskal E. O., Beard C. F. Carbon-13 Nuclear Magnetic Resonance Analysis of Metabolism in Soybean Labeled by CO(2). Plant Physiol. 1975 Jun;55(6):1048–1053. doi: 10.1104/pp.55.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séquin U., Scott A. I. Carbon-13 as a label in biosynthetic studies. Science. 1974 Oct 11;186(4159):101–107. doi: 10.1126/science.186.4159.101. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]


