Abstract
Immunotherapeutic approaches reducing α-synuclein deposits may provide therapeutic benefit for Dementia with Lewy Bodies (DLB). Immunization with full-length human α-synuclein (hα-Syn) protein in a Parkinson's disease mouse model decreased the accumulation of the aggregated forms of this protein in neurons and reduced neurodegeneration. To enhance the immunogenicity of candidate vaccines and to avoid the risk of autoreactive anti-hα-Syn T-helper (Th) cell responses, we generated three peptide-based epitope vaccines composed of different B-cell epitopes of hα-Syn fused with a “non-self” Th epitope from tetanus toxin (P30). Immunization of mice with these epitope vaccines produced high titers of anti-hα-Syn antibodies that bound to Lewy bodies (LBs) and Lewy neurites (LNs) in brain tissue from DLB cases and induced robust Th cell responses to P30, but not to hα-Syn. Further development of these first generation epitope vaccines may facilitate induction of anti-hα-Syn immunotherapy without producing potentially harmful autoreactive Th cell responses.
Keywords: Immunotherapy, Parkinson's and Alzheimer's diseases, epitope vaccine, B cell epitope of α-synuclein, T cell epitope of tetanus toxin
1. Introduction
Although the physiological function of α-synuclein (α-Syn) is not well defined, the deposition of misfolded aggregates of α-Syn is a pathological feature of several neurodegenerative diseases such as Parkinson's disease (PD), PD Dementia (PDD) and Dementia with Lewy Bodies (DLB) collectively known as Lewy Body Disorders (LBD) [22]. In addition, up to 50% of AD cases have LB inclusions in the amygdala and limbic structures associated with an accelerated cognitive decline [15]. Neuropathological studies have shown that aggregates of α-Syn, Aβ and tau appear in the same neuronal structures, providing a pathological basis for the clinical observations of the overlap between LBD and AD [8, 17]. Thus, reducing pathological forms of α-Syn may provide clinical benefit to patients with LBD [6, 8, 15]. Previously, Masliah et al. reported that immunization of PD transgenic (PD/Tg) mice with full-length human α-Syn (hα-Syn) decreased accumulation of misfolded α-Syn in neuronal cell bodies and synapses, and reduced neurodegeneration. Importantly, mice that produce high affinity antibodies showed greater reductions in α-Syn relative to mice producing lower affinity and lower titers of anti-α-Syn antibodies [13, 14]. Previously, we developed an epitope-based active vaccination approach [1] to avoid the harmful autoreactive T helper (Th) cell responses that occurred in a subset of AD patients from AN1792 trial [11, 18]. In this report we have adopted the same epitope vaccine strategy with aim to induce a strong anti-hα-Syn antibody production without generation of anti-hα-Syn cellular responses.
2. Materials and methods
2.1. Mice, epitope vaccine, peptides
Female, 6-8 weeks old B6SJL mice were obtained from the Jackson Laboratory and housed under the guidelines of NIH and an approved IACUC protocol at UCI. Linear peptides α-Syn85-99 (AGSIAAATGFVKKDQ), α-Syn109-126 (QEGILEDMPVDPDNEAYE), α-Syn126-140 (EMPSEEGYQDYEPEA) and P30 (FNNFTVSFWLRVPKVSASHLE) peptide from Tetanus toxoid (TT) were synthesized by GenScript. Peptides α-Syn85-99-P30, α-Syn109-126-P30, and α-Syn126-140-P30 were synthesized as Multiple Antigenic Peptides (MAPs) (Life Technologies).
2.2 Immunizations
Mice (n=4/group) were immunized with Syn85-99-P30-MAP, α-Syn109-126-P30-MAP and α-Syn126-140-P30-MAP (50 μg/mouse) formulated in QuilA adjuvant (20 μg/mouse), subcutaneously (s.c), as previously described [1]. A control group of mice was injected with QuilA adjuvant only (n=4). Mice were boosted at two-week intervals (days 0, 14, 28 and 42); sera were collected before injection (pre-bleed), at day 12 after 2nd and 3rd immunizations, and at day 7 after the last immunization for detection of humoral immune responses. Mice were terminated 7 days after the 4th immunization, and cellular immune responses were analyzed in splenocytes.
2.3. Analyses of cellular immune responses
The cultures of splenocytes from experimental and control mice were re-stimulated in vitro with indicated peptides/protein for 18 hours, and cells producing IFNγ were detected by ELISPOT kit as recommended by manufacturer (BD Pharmingen). SFCs (spot forming colonies) were counted using a CTL-ImmunoSpot S5 Macro Analyzer (Cellular Technology Ltd).
2.4. Analyses of humoral immune responses
Total anti-hα-Syn antibodies were detected as described previously [1] except that the wells of the ELISA plates were coated with 1 μg of hα-Syn protein (rPeptide). The concentrations of antibodies were calculated using calibration curve generated with anti-Syn (SNCA) monoclonal antibody (GenScript). To analyze the isotypes of antibodies in individual sera (dilution 1:1000) of mice we used HRP-conjugated anti-IgG1, IgG2ab, IgG2b and IgM specific secondary antibodies (Bethyl Laboratories, Inc.) in ELISA. The titers of antibodies specific to α-Syn85-99, α-Syn109-126 and α-Syn126-140 were detected in ELISA plates coated with 1 μg/ml of the appropriate peptide. Endpoint titers were calculated as the reciprocal of the highest sera dilution that gave a reading twice above the background levels of pre-immune sera at the same dilution (cutoff).
2.5. Brain extraction, immunoprecipitation and western blot analysis
Brain homogenates were prepared from DLB cases using lysis buffer (Cell Signaling Technology) supplemented with 1mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). Lysates were centrifuged (14,000×g, 1 hr, 4°C), supernatants were collected, and protein concentrations were determined by BCA protein assay kit (Pierce). α-Syn in the lysates was detected by immunoprecipitation (IP)/western blot (WB), as previously described [19]. Briefly, IP was performed using different immune sera (1.5 μl) containing 0.15μg of appropriate anti-α-Syn antibodies and protein G-sepharose beads. Samples were subjected to electrophoresis in NuPAGE 10% Bis-Tris gel in MES buffer under reducing conditions (Life Technologies) and electrotransferred onto nitrocellulose membrane (GE Healthcare). The α-Syn was detected using rabbit anti-α-Syn polyclonal antibody (Millipore) and goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology). As a positive control, 0.5μg of full-length hα-Syn was loaded onto the gel. WBs were scanned and converted into digital files to calculate the optical density of α-Syn protein spots with NIH ImageJ software, version 1.45s. The relative optical density was presented as an intensity of bands normalized to immunoglobulin bands.
2.6. Immunohistochemistry and quantitative image analyses
The brain samples were obtained from the Brain Bank and Tissue Repository (MIND, UCI) as formalin fixed blocks of anterior cingulate of control normal brains (n=4) and brains of the human subjects with established DLB pathology (n=4). The brains were processed for immunohistochemistry (IHC) by previously published methods [19], and LBs and LNs were detected with immune sera specific to α-Syn85-99-P30-MAP, α-Syn109-126-P30-MAP and α-Syn126-140-P30-MAP (sera were normalized to the 1.5 μg/ml based on ELISA data). As a positive and negative controls, we used anti-α-Syn monoclonal antibody, LB509 (Abcam) and immune sera from mice immunized with irrelevant antigen, respectively. Epitope retrieval was performed by incubation of sections in Sodium Citrate Buffer (pH 6.0, 90°C, 30′). Binding of antibodies to LBs and LNs in DLB and control brain sections (40μm thick) were visualized as described earlier [4, 21] using ABC/DAB kit (Vector Labs). A Sony high-resolution CCD video camera (XC-77) was used to capture images of LBs at 10× magnification, and numbers of aggregates were counted in full immunostained sections of the same size in all brains. The averages of LB-positive profiles per section for each brain were calculated to provide a mean semi-quantitative score for LB hα-Syn load that was determined via visual microscopic inspection performed by three independent observers blindly with respect to treatment conditions.
LNs were analyzed using NIH ImageJ program, as described previously [5]. For every section, eight images (802×650μm each) of the anterior cingulate brain regions were captured with a 10× objective. LNs load was assessed by selecting an area without cell bodies, setting the threshold levels and expressing the data as pixel intensity (arbitrary units).
2.7. Statistical Analysis
Significance of differences between groups in antibody production (ELISA), number of INF-γ producing cells (ELISPOT), IHC staining was analyzed using one-way ANOVA: Tukey paired comparison post-test, Graph Pad Prism 6.
3. Results
3.1. Immunogenicity of novel epitope vaccines targeting three different B cell antigenic determinants of hα-Syn
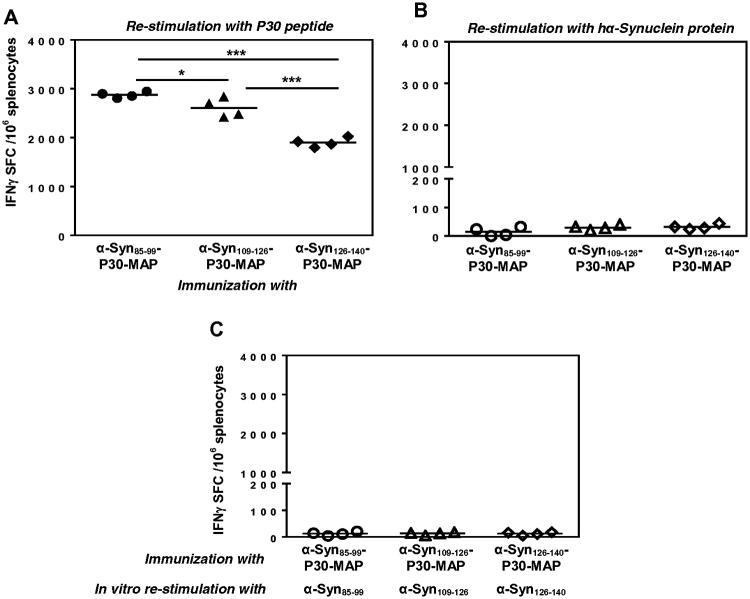
Masliah et al. previously reported that immune sera from PD/Tg mice vaccinated with full-length hα-Syn recognized four peptides (aa 85-99, 109-123, 112-126, and 126-138) within the C-terminus region of this protein. However, the cellular immune responses to vaccination with hα-Syn were not analyzed [13]. To reduce the risk of hypothetically harmful Th-cell-mediated immune responses to hα-Syn-based immunotherapy in humans and to generate a more immunogenic vaccine based on strong foreign Th antigenic epitope, we designed three epitope vaccines, α-Syn85-99-P30-MAP, αSyn109-126-P30-MAP, and α-Syn126-140-P30-MAP (Fig.1A,B) and tested their immunogenicity in mice. Accordingly, we first analyzed the specificity of cellular immune responses in splenocytes of mice immunized with epitope vaccines. Data demonstrated that all three vaccines induced effective cellular immune responses: in vitro re-stimulation of immune splenocytes with P30 peptide generated high numbers of cells producing IFN-γ (Fig. 1A). The response was stronger in mice vaccinated with α-Syn85-99-P30-MAP than that in mice vaccinated with αSyn109-126-P30-MAP and α-Syn126-140-P30-MAP. Importantly, re-stimulations of the same splenocytes with hα-Syn protein (Fig 1B) and peptides spanning aa 85-99, aa109-126 or aa126-140 of this molecule (Fig 1C) were not effective in activation of IFN-γ-positive cells. As expected, splenocytes from the control group of mice injected with adjuvant only also induced background levels of IFN-γ producing cells after re-stimulations with P30, hα-Syn, or peptides spanning aa 85-99, 109-126 or 126-140. Therefore, none of three B-cell epitopes previously selected from hα-Syn [13] possessed Th-cell epitopes in mice of the H-2bxs haplotype (Fig.1B, C).
Figure 1.
Cellular immune responses generated by α-Syn85-99-P30-MAP, α-Syn109-126-P30-MAP and α-Syn126-140-P30-MAP vaccines. Cellular responses are specific to P30 (A), but not to full-length hα-Syn protein (B), or peptides spanning aa 85-99, aa109-126, or aa126-140 of hα-Syn (C). Number of cells producing IFN-γ cytokine was detected by ELISPOT assay. Final concentration of each peptide and hα-Syn protein was 10 μg/ml. Horizontal lines indicate average data obtained with 4 mice per group.
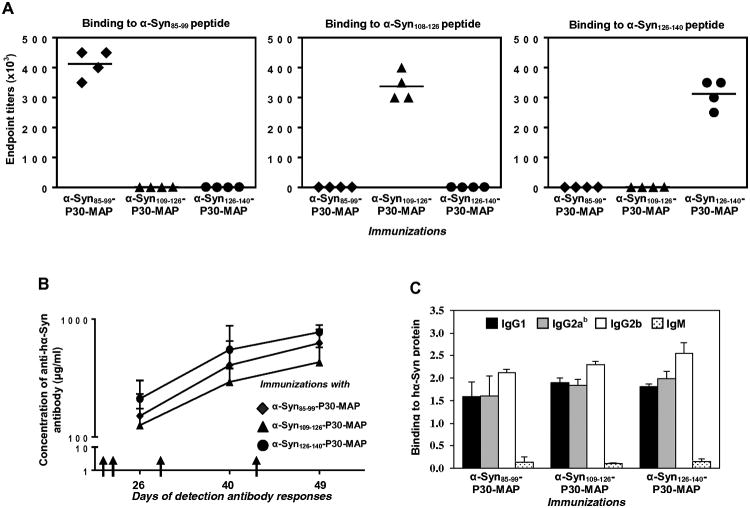
As mentioned above, immunization of mice with full-length hα-Syn induced antibodies to α-Syn85-99, α-Syn109-126, and α-Syn126-140 peptides [13]. However, the ability of these peptides to stimulate B-cells and generate anti-hα-Syn antibody responses was not demonstrated. Accordingly, we measured anti-α-Syn antibody production in mice vaccinated with α-Syn85-99-P30-MAP, αSyn109-126-P30-MAP, and α-Syn126-140-P30-MAP epitope vaccines. These vaccines induced antibodies specific to B-cell epitopes spanning aa 85-99, 109-126 and 126-140 of hα-Syn, respectively (of note, no cross-reactivity was detected). Notably, α-Syn85-99-P30-MAP induced the highest titers of antibody, which are significantly higher than the titers induced by α-Syn126-140-P30-MAP (P<0.05), but not to α-Syn109-126-P30-MAP (Fig 2A). Thus, all three epitope vaccines stimulated B-cells specific to different antigenic determinants of hα-Syn (Fig 2A) without activation of autoreactive Th cell responses (Fig 1D). Importantly, all three epitope vaccines induced a strong antibody response to full-length hα-Syn as well (Fig 2B). It is interesting that mice vaccinated with α-Syn126-140-P30-MAP induced the highest titers of anti-hα-Syn antibodies, significantly higher than titers induced by α-Syn109-126-P30-MAP (P<0.05). Analyses of the IgG isotype profiles of anti-hα-Syn antibodies demonstrated that all three epitope vaccines induced comparable levels of IgG1, IgG2ab and IgG2b and very low amounts of IgM antibodies (Fig 2D), suggesting Th1 polarized immune responses (IgG1/IgG2ab ratio was approximately 1).
Figure 2.
Humoral immune responses generated by α-Syn85-99-P30-MAP, α-Syn109-126-P30-MAP and α-Syn126-140-P30-MAP vaccines were analyzed by ELISA. (A) Endpoint titers of antibodies specific to α-Syn85-99, α-Syn109-126, α-Syn126-140 peptides were detected in sera collected after third immunization. Sera from individual mice were diluted from 1:1,000 to 1:600,000. (B) Concentrations of antibodies specific to full-length hα-Syn were calculated using a calibration curve generated with anti-Syn (SNCA) monoclonal antibody. (C) The isotypes of antibodies detected in immune sera (collected after 3rd immunization), sera were diluted 1:1,000. Data were obtained with individual sera of mice (n=4/group).
3.2. Potential therapeutic efficacy of anti-hα-Syn antibodies
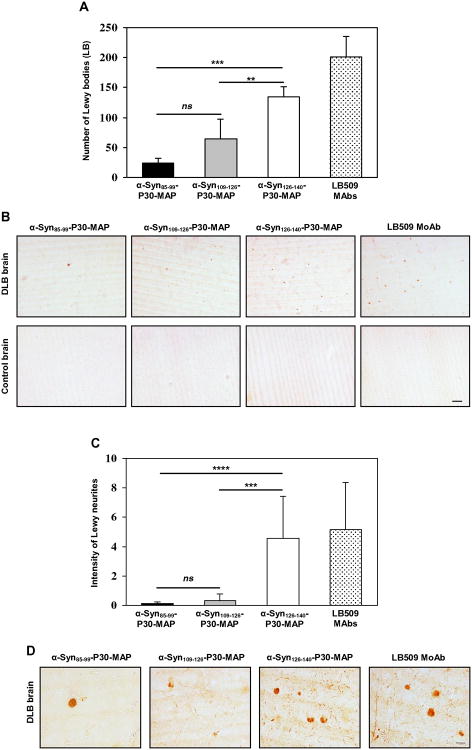
To demonstrate potential therapeutic efficacy of the anti-hα-Syn antibodies generated by epitope vaccines, we analyzed binding of antisera to LBs and LNs in brain tissue from four DLB cases and four non-DLB control cases. Sera from all vaccinated mice stained LBs and LNs in the brains of DLB cases, but not in control brains (Fig. 3). Remarkably, quantitative analyses of LBs and LNs revealed significant differences in numbers of LBs (Fig. 3A,B) and in LNs load (Fig. 3C,D) detected by anti-α-Syn126-140 and anti-α-Syn85-99, or anti-α-Syn109-126 antibodies in DLB brains. To confirm this observation we tested binding of antibodies specific to all three B-cell epitopes to the hα-Syn isolated from DLB brains by IP/WB. Despite the similar concentrations, antibodies induced by α-Syn126-140-P30-MAP immunoprecipitated higher amount of hα-Syn, from brain extracts than antibodies generated by α-Syn85-99-P30-MAP and α-Syn109-126-P30-MAP (Fig. 4A,B).
Figure 3.
Therapeutic potency of antibodies generated by epitope vaccines detected by IHC. (A, B, C, D) Antibodies recognized Lewy Bodies (LBs) (A, B) and Lewy Neurites (LNs) (C, D) in DLB, but not in control brain sections. Of note, anti-α-Syn126-140 recognized higher number of LBs and LNs than anti-α-Syn85-99 and anti α-Syn109-126 antibodies. (A) Number of LBs was calculated based on 10× magnification of DLB brain images. (B) Representative images of DLB and non-DLB brains are presented (original magnification 10×, scale bar=100μm). (C) Intensity of LNs was detected based on 10× magnification of DLB brain images. (D) Representative images of DLB brains (original magnification 40×, scale bar = 20μm).
Figure 4.
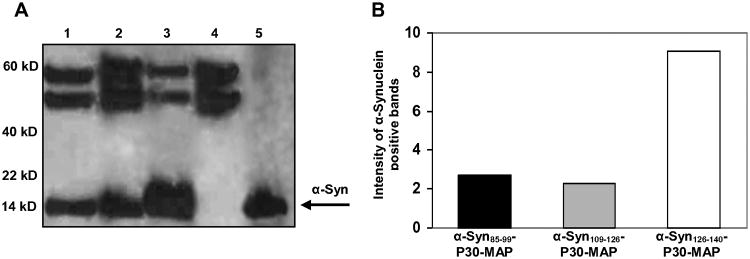
Immune sera generated by epitope vaccines recognized native hα-Syn in DLB brain homogenate. (A) Brain homogenate was IP with anti-α-Syn85-99 (Ln 1), anti-α-Syn109-126 (Ln 2), anti-α-Syn126-140 (Ln 3), or irrelevant anti-P30 (Ln 4) immune sera. Full-length hα-Syn was used as the positive control (Ln 5). (B) Antibodies specific to α-Syn126-140 immunoprecipitated higher amount of hα-Syn from the DLB brain homogenate. Experiment was repeated with two different brains.
4. Discussion
Passive and active immunotherapy strategies for neurodegenerative disorders have emerged following the seminal report of Schenk et al. [20], which established that antibodies against Aβ could reduce amyloid plaque load in a transgenic mouse model of AD [11]. More recently, several groups demonstrated that immunotherapeutic strategy is also effective in reducing the levels of intracellular tau [9] and α-Syn [13, 14] aggregates. However, it is well-known that the first active immunotherapy clinical trial was halted because of adverse events that have been attributed to the use of full-length fibrillar Aβ, which may generate autoreactive Th-cell [11, 18]. To avoid this, we have utilized an alternative active vaccination strategy in which we kept an immunogenic self B-cell epitope of Aβ and replaced the self Th-cell epitope(s) of this self-antigen with foreign universal Th-cell epitope(s) [1]. The safety and efficacy of vaccines targeting misfolded Aβ have been tested in wild-type and Tg mice [5, 16, 19], rabbits [7], guinea pigs, and monkeys [5]. Here we reported on generating and testing novel epitope vaccines targeting pathological hα-Syn peptides.
Immunogenicity of the epitope vaccines composed of three different hα-Syn peptides fused with a foreign universal Th cell epitope, P30 from TT, has been tested in mice. Data demonstrate that all epitope vaccines induced cellular immune responses in mice specific to only foreign Th epitope, P30. Analyses of immune sera showed that each epitope vaccine induced antibodies specific to appropriate hα-Syn peptide incorporated into the vaccine design (Fig 2A). It is interesting, that α-Syn126-140-P30-MAP vaccine induced lower titers of antibodies specific to the appropriate peptide compared with other two vaccines, but these antibodies better recognized full-length recombinant hα-Syn. Importantly, antibodies generated by all three epitope vaccines bound specifically to LBs and LNs in DLB brain sections (Fig. 3) and native hα-Syn in DLB brain homogenates (Fig. 4). Again, using two different techniques we found that antibodies specific to α-Syn126-140 bound higher numbers of LBs/LNs and immunprecipitate higher amount of hα-Syn from the brain homogenates than anti-α-Syn85-99 and anti-α-Syn109-126 antibodies (Fig. 3, 4). These data showed not only the feasibility of epitope vaccine strategy for α-Syn per-se, but also suggest the use of α-Syn126-140 B-cell epitope for generation of the immunogenic and effective vaccine for further testing. Previously, in the studies with passive vaccination of Tg mice, it was shown that monoclonal antibodies to different α-syn epitopes may clear α-syn aggregates via different mechanisms [2]. These data and data presented here underscore the importance of choosing “the right” epitope for generation of effective epitope vaccine. Based on these data, we are currently engineering a novel α-Syn-immunotherapeutic possessing α-Syn126-140 fused with the multiple universal Th cell epitopes described in [7] instead of P30 only. We believe that this second-generation epitope vaccine will be more immunogenic in human population with highly polymorphic MHC genes controlling the immune responses.
As to the mechanism of action of antibodies, recent reports indicate that although α-Syn is a cytoplasmic protein, it can also be secreted by neurons and may contribute to cell-to-cell propagation of synucleinopathies similar to the mechanism of prion pathogenesis [3, 12]. Moreover, postmortem analysis of brain tissue of PD patients that received fetal tissue transplants has established that neurons in the graft develop α-Syn inclusions [10]. Thus, anti-α-Syn antibodies may form extracellular immune complexes with secreted α-Syn, thereby contributing to the clearance of α-Syn from the CNS. In fact, it is shown that direct injection of anti-α-syn antibodies into the brain of Tg mice facilitates uptake of extracellular α-syn aggregates by microglia and prevents cell-to-cell transmission of α-syn aggregates. α-syn/antibody complexes are internalized via Fcγ receptor on the surface of microglia and are more efficiently transported to lysosomes through different pathway distinct from pathway of α-syn alone[2]. In addition, it was suggested that anti-α-Syn antibody could enter into the cells and induce lysosomal degradation of intracellular α-Syn aggregates [13]. It is likely that multiple mechanisms may contribute to the clearance of both extracellular and intracellular pathological aggregates of proteins, such as Aβ, α-Syn and tau, and hopefully soon we will learn more about these mechanisms. However, regardless of these mechanisms, we would like to mention that our groups hypothesize that any benefit from immunotherapy for CNS proteinopathies may be dependent on early intervention in the clinical course of the disease before extensive neurodegeneration has occurred.
5. Conclusion
To our knowledge, this is the first report on the generation of an epitope vaccine targeting hα-synuclein and demonstrating the feasibility of this approach. Here we demonstrated the “proof of principle” for generation of epitope vaccine. The further improvement of these epitope vaccines may lead to a development of a safe and effective immunotherapeutic inducing high titers of anti-hα-Syn antibody without producing potentially harmful autoreactive Th-cell responses in a human population with high levels of genetic diversity.
Highlights.
Three novel PD peptide-based epitope vaccines were designed and their immunogenicity was tested in mice
Each epitope vaccine was composed of one B-cell epitope of hα-Synuclein (hα-Syn) fused with the same “non-self” Th epitope from tetanus toxin (P30)
All tested vaccines generated strong cellular responses specific to P30 and high titers of anti-hα-Syn antibodies in mice
Generated antibodies bound to Lewy bodies and Lewy neurites in brain tissue from DLB cases and recognize hα-Syn in brain extracts.
Acknowledgments
We would like to thank Dr. Annette Marleau for the help with editing this manuscript and her valuable comments. This work was supported by funding from NIH (AG-20241, NS-50895 and NS-057395) and Alzheimer's Association (IIRG-12-239626 and NIRG-13-281227). HD was supported by NIA T32 training grant (AG000096). Additional support for DLB case tissues was provided by University of California, Irvine Alzheimer's Disease Research Center Grant P50 AG16573.
Footnotes
Conflict of interest: Authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 2.Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E, Lee SJ. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 4.Chai SK, Clavijo P, Tam JP, Zavala F. Immunogenic properties of multiple antigen peptide systems containing defined T and B epitopes. J Immunol. 1992;149:2385–2390. [PubMed] [Google Scholar]
- 5.Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Poghosyan A, Marleau AM, Movsesyan N, Kiyatkin A, Rasool S, Larsen AK, Madsen PJ, Wegener KM, Ditlevsen DK, Cribbs DH, Pedersen LO, Agadjanyan MG. Immunogenicity, Efficacy, Safety, and Mechanism of Action of Epitope Vaccine (Lu AF20513) for Alzheimer's Disease: Prelude to a Clinical Trial. J Neurosci. 2013;33:4923–4934. doi: 10.1523/JNEUROSCI.4672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallardo G, Schluter OM, Sudhof TC. A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci. 2008;11:301–308. doi: 10.1038/nn2058. [DOI] [PubMed] [Google Scholar]
- 7.Ghochikyan A, Davtyan H, Petrushina I, Hovakimyan A, Movsesyan N, Davtyan A, Kiyatkin A, Cribbs DH, Agadjanyan MG. Refinement of a DNA based Alzheimer's disease epitope vaccine in rabbits. Hum Vaccin Immunother. 2013;9:1002–1010. doi: 10.4161/hv.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giasson BI, Lee VM, Trojanowski JQ. Interactions of amyloidogenic proteins. Neuromolecular Med. 2003;4:49–58. doi: 10.1385/NMM:4:1-2:49. [DOI] [PubMed] [Google Scholar]
- 9.Gu J, Sigurdsson EM. Immunotherapy for tauopathies. J Mol Neurosci. 2011;45:690–695. doi: 10.1007/s12031-011-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobello K, Ryan JM, Liu E, Rippon G, Black R. Targeting Beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:628070. doi: 10.1155/2012/628070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.McGeer PL, McGeer EG. The alpha-synuclein burden hypothesis of Parkinson disease and its relationship to Alzheimer disease. Exp Neurol. 2008;212:235–238. doi: 10.1016/j.expneurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine- a novel immunotherapeutic strategy. PLos ONE. 2008;3:e21–24. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Orgogozo JM, Gilman S, Dartigues JM, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 19.Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH, Agadjanyan MG. Alzheimer's Disease Peptide Epitope Vaccine Reduces Insoluble But Not Soluble/Oligomeric A{beta} Species in Amyloid Precursor Protein Transgenic Mice. J Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse [see comments] Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 21.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015–1025. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]