Abstract
Background
Neurocognitive sequelae following treatment for pediatric acute lymphoblastic leukemia (ALL) has been reported in a significant proportion of survivors, including those treated only with chemotherapy. Early identification of children “at risk” for neurocognitive problems is not yet reliable. Biomarkers of oxidative stress (e.g., oxidated phosphatidylcholine) in cerebral spinal fluid (CSF) have been correlated with intensity of methotrexate (MTX) treatment, suggesting an association with acute central nervous system toxicity.
Procedure
This study examined the association between oxidized CSF phospholipids and executive functions throughout chemotherapy. Measures of oxidative stress and executive functions were examined in 88 children newly diagnosed with ALL. The children were followed over three years with neurocognitive testing and parent ratings of executive functions.
Results
Results demonstrated an association between increased oxidative stress following induction and consolidation and decreased executive function two years later. Younger age at diagnosis was associated with both an increase in oxidative stress and in executive dysfunction; younger age was associated with poorer ability to organize materials in one's environment (r(48) = 0.28, p < 0.05) and with greater oxidated phosphatidylcholine in CSF at the end of chemotherapy ( r(48) = −0.27, p < 0.05). As such, younger age appears to be the most prominent moderator of neurocognitive decline.
Conclusions
These results link functional changes to CSF biomarkers and underscore the importance of monitoring cognitive development in young children treated for ALL. Children with less advanced central nervous system development may be particularly vulnerable to the effects of chemotherapy.
Keywords: oxidative stress, neurocognitive, leukemia
Introduction
Advances in treatment for pediatric acute lymphoblastic leukemia (ALL) have led to increased survival rates that now exceed 80%.[1,2] Therapies responsible for the dramatic improvement initially consisted of prophylactic cranial-spinal (C-S) radiation to prevent CSN relapse, though this approach often lead to neurocognitive difficulties and other undesirable late-effects.[3,4] Successful efforts to reduce neurotoxicities resulted in replacing C-S radiation with intensified intrathecal and intravenous methotrexate (MTX).[5,6] Although removing radiation from frontline therapy has improved neurocognitive outcomes, high-dose MTX still poses substantial risk to neurocognitive functions. Risk factors for neurotoxicity associated with MTX include younger age at treatment and being female.[7,8]
MTX has been routinely associated with leukoencephalopathy.[9,10] With disproportionately greater amounts of white matter in the frontal lobes, connections involving these areas may be at particularly high risk for dysfunction and may account for much of the cognitive, intellectual, academic, and psychiatric sequelae associated CNS treatment. Reduced frontal lobe white matter volume has been reported in long-term survivors of pediatric leukemia.[11]
The frontal lobes are also widely associated with executive functions, which have been defined as the ability to coordinate other functional systems through attending, planning, and monitoring behavior.[12] Executive functions are one's ability to self-regulate behavior through cognitive flexibility and initiate or switch purposive behavior to appropriately function within a given situation.[13] Although there is clearly some overlap between intellectual/cognitive functions and executive functions, it is somewhat surprising that there are not more studies specifically examining executive functions associated with chemotherapy given the connection between executive functions and frontal lobe white matter.
It is unclear whether leukoencephalopathy associated with MTX is due to oxidative stress or other factors, but an association between MTX and markers of oxidative stress is emerging and demonstrates treatment-related cellular breakdown within the CNS. Elevated concentration of phosphatidylcholine (PC), the most abundant CSF phospholipid and a critical component of CNS cell membranes, has been used to identify acute CNS injury associated with ALL and its chemotherapy.[14] More recently, the oxidized fraction of PC has been used as a sensitive biological marker of oxidative stress.[15] Studies with rats and humans provide converging evidence that MTX treatment triggers a decrease in protective factors against free radical damage[16,17] making polyunsaturated fatty acid chains within cell membrane more susceptible to attack by reactive oxygen species.[18] Oxidant attacks cause further lipid peroxyl radicals to form and propagates a cascade of cell membrane damage.[15,19] As increased levels of oxidized PC following MTX therapy for ALL have been associated with unfavorable intellectual, academic, spatial-perceptual, and working memory abilities,[8,20] oxidative stress may be a mechanism through which declines in functioning occur.
Despite evidence of oxidative stress, leukoencephalopathy, and neuropsychological dysfunction, the relation between executive functions and oxidative injury in patients treated with MTX has not yet been established. The main aim of this study was to investigate the association between markers of oxidative stress (following induction and consolidation phases of chemotherapy) and difficulties with executive functions at post-consolidation and at the end of therapy. The effects of known covariates (e.g. higher dose of MTX, younger age at treatment, female sex) in the association between markers of oxidative stress and executive functions were also investigated.
Methods
Subjects
The sample included 88 children newly-diagnosed with pediatric ALL, who were consecutively recruited from two major southwestern cancer centers. Parents or legal guardians completed the informed consent process, and assent was obtained from patients when appropriate. The study was approved by the necessary institutional review boards.
Participants were treated on Pediatric Oncology Group (POG) protocols 9201, 9406, 9605, 9806, 9904, 9905, or 9906. Most children were treated with 1 gram (gm) of MTX/m2 over 24 hour infusion or 2 gm of MTX/m2 over 4 hour infusion by IV. Additionally, 6 children were treated with 100 mg of MTX/m2 over 24 hour infusion and 1 child was treated with 2.5 mg MTX/m2 over 4 hour infusion. These seven participants were not included in evaluation related to dose of methotrexate, but were included in analyses of the overall treatment group. Intrathecal MTX was administered in age-titrated doses according to protocol, and the number of IT injections was equivalent across the various protocols. Patients were excluded if diagnosed with CNS involvement any time during the course of therapy, if relapsed during therapy, or if treated with cranial irradiation. All children were between the ages of 3 and 16 years, the appropriate age range for reliable administration of the standardized neurocognitive tests used in this study. The mean age at diagnosis for the clinical sample was 7.1 years (SD = 3.1), and mean parent education level was 13.3 years (SD = 2.7). Males comprised 41% the sample.
CSF Sample Phospholipids
CSF samples were harvested from patients at the diagnosis lumbar puncture (LP) and during subsequent induction and consolidation LPs required for IT MTX therapy. Induction and consolidation PC measurements represent mean levels over those treatment phases. Protocols used in the process leading to CSF PC component quantification have been described previously.[15] The concentration of unoxidized PC in CSF samples was converted to μg/mL. Oxidized PC was recorded as a fraction for each sample (i.e. peak area of oxidized PC: peak area of unoxidized PC) instead of a concentration. Results are expressed as changes in unoxidized PC concentration and oxidized PC fraction from diagnosis to induction and diagnosis to consolidation.
Executive Function Measures
The Behavior Rating Inventory of Executive Function (BRIEF)[21] is a parent-completed questionnaire used to evaluate executive functions within the domains of behavioral regulation and metacognitive abilities. Specifically, it assesses nine domains of executive functions, including: 1) Inhibition- the ability to resist impulses and stop behavior appropriately; 2) Shifting- the ability to transition, tolerate change, and shift attention; 3) Emotional control- the expression and regulation of emotion; 4) Self-monitoring- the awareness of self-behavior on others; 5) Initiation- the ability to get started on physical or mental activities; 6) Working memory- the capacity to hold information in one's mind in order to complete tasks; 7) Planning and organization- the ability to determine the best way to achieve a goal and bring organized structure to information; 8) Organization of materials- the ability to organize things in one's environment; and 9) Task monitoring- the ability to attend to one's success or failure and modify strategy appropriately. The BRIEF transforms raw scores for each domain into sex and age-adjusted T-scores (M=50, SD=10) based on a nationally representative normative sample. BRIEF assessments were conducted at approximately one year post-consolidation and at the end of therapy.
The Behavior Assessment System for Children Parent Rating Scale (BASC-PRS) is a widely-used, clinical assessment tool that evaluates behaviors and adaptive skills.[22] One of the scales from the BASC is Attention Problems; attention is a core component of proper executive functions. The raw scores for Attention Problems were also converted to sex and age-adjusted T-scores (M=50, SD=10) based on a nationally representative normative sample. BASC-PRS assessments were conducted at the same time points as the BRIEF.
Verbal fluency was assessed as part of the neurocognitive evaluation associated with this study.[23] Verbal fluency is a performance-based measure of executive functions that combines aspects of initiation and working memory. Using standard rules for administration, children were asked to name as many animals as they could within the span of one minute. Raw scores were converted to age adjusted scaled scores (M=10, SD=3). Verbal fluency evaluations were conducted at the same time as the BRIEF and BASC questionnaires.
Data Analysis
All data was analyzed using SPSS 16. T-tests were conducted to examine the change in oxidized PC levels across treatment phases and the change associated with the 1 gm MTX/m2 IV treatment regimen vs. the 2 gm MTX/m2 IV treatment. Bivariate Pearson Product correlations were used to evaluate the association between oxidative stress (oxidative PC fraction reactivity) and executive functions (BRIEF, BASC-PRS Attention Problems, and Semantic Fluency). Bivariate Pearson Product correlations were also used to evaluate whether certain covariates (age at diagnosis or parent education) were associated with amount of oxidative reactivity post-consolidation and at the end of therapy. Chi Square (χ2) was used to examine oxidative PC fraction differences related to dichotomous risk factors such as sex and treatment dose (1 g MTX/m2 or 2 g MTX/m2). To accomplish this, a median split was used to create low and high groups of oxidated PC fraction reactivity. A repeated measures analysis of variance was used to examine changes in executive function from post-consolidation to end of therapy time point points.
Measures from the BRIEF, BASC-PRS, and Verbal Fluency were also compared to normative data. Using participants’ standard errors from each test/subtest, 95% confidence intervals were created for each measure to identify differences between patients and national norms. Normative means falling outside of a confidence interval were considered reflective of a group difference for that particular measure.
Results
Table I presents basic demographic and treatment data related to the risk factors of interest in this study. There were no significant differences between the 1 gm/m2 and 2 gm/m2 group for Age at Diagnosis, F (1, 71) = 1.7, p > 0.05 or Parent Education, F(1,67) = 0.028, p > 0.05. Although there were more females in the sample overall, the proportion of males to females did not differ by treatment group (χ2 (1, 77) = 0.24, p > 0.05). In addition, the 1 gm/m2 and 2 gm/m2 did not differ in regard to risk stratification (χ2 (1, 81) = 2.42, p > 0.30).
Table I.
Demographics by Treatment Group
| MTX | # Intrathecal MTX | Sex | Age at Diagnosis | Parent Education | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | N | Male | Female | M | SD | N | M | SD | N | |
| 1gm/m2 | 16.68 | 1.28 | 28 | 21 | 34 | 6.5 | 2.8 | 52 | 13.3 | 2.9 | 47 |
| 2gm/m2 | 16.00 | 1.07 | 8 | 11 | 11 | 7.5 | 3.2 | 21 | 13.4 | 1.7 | 22 |
| Total | 16.53 | 1.25 | 36 | 32 | 45 | 6.8 | 2.9 | 73 | 13.3 | 2.7 | 69 |
| F = 1.87, p > 0.05 | X2 = 0.24, p > 0.05. | F = 1.7, p > 0.05 | F = 0.028, p > 0.05. | ||||||||
Note: Age and Parent Education expressed in years.
Oxidized PC levels significantly increased from diagnosis (M = 0.015, SD = 0.006) to post-induction (M = 0.026, SD = 0.010), t(46) = 6.49, p < 0.001), and from diagnosis to post-consolidation (M = 0.026, SD = 0.0090) for both groups combined, t(52) = 8.35, p < 0.001). Although the 1 gm/m2 group demonstrated a larger change in oxidized PC from diagnosis to post-induction (M = 0.012, SD = 0.013) compared to the 2 gm/m2 group [(M = −0.006, SD = 0.005), t (41) = 2.41, p < 0.02], this change occurred prior to administration of the various IV MTX doses during consolidation therapy. The change in oxidized PC from post-induction to post-consolidation, reflecting the change associated with consolidation therapy, was larger for the 2 gm/m2 group (M = 0.006, SD = 0.010) compared to the 1 gm/m2 group [M = −0.002, SD = .010), t(43) = 2.10, p < 0.05].
The overall group performance on measures of executive functions were elevated, though the patients did not significantly differ from the normative sample. However, patients displayed distributions that were positively skewed with greater variability than standardized norms, suggesting the presence of a subset of patients that may have been more adversely affected than others. Table II shows the means and standard deviations for each measure, stratified by IV MTX dose group. The vast majority of scores fall in the average range, though mild elevations and significant variability exists within certain scales.
Table II.
Executive Function Mean and SD by Oxidative Reactivity
| Change in Oxidative Stress Marker |
||||
|---|---|---|---|---|
| Diagnosis to Induction |
Diagnosis to Consolidation |
|||
| Post-Consolidation | High Reactors Mean (SD) |
Low Reactors Mean (SD) |
High Reactors Mean (SD) |
Low Reactors Mean (SD) |
| Inhibition | 49.9 (9.96) | 50.5 (10.89) | 50.7 (11.11) | 48.5 (7.95) |
| Shift | 52.1 (11.33) | 49.2 (8.09) | 48.6 (9.14) | 52.7 (11.08) |
| Emotional Control | 51.9 (12.51) | 54.7 (14.17) | 49.8 (11.15) | 54.3 (14.60) |
| Initiation | 49.5 (9.69) | 50.3 (10.49) | 49.6 (10.78) | 50.5 (9.36) |
| Working Memory | 52.1 (10.27) | 51.1 (9.37) | 51.6 (10.16) | 51.1 (8.96) |
| Plan / Organize | 48.9 (8.33) | 52.2 (10.04) | 50.7 (10.20) | 50.1 (7.92) |
| Organize Materials | 52.2 (10.90) | 50.5 (11.44) | 49.7 (9.37) | 52.8 (13.50) |
| Task Monitoring | 48.4 (8.72) | 49.5 (12.08) | 49.0 (9.45) | 48.5 (11.27) |
| Attention | 50.8 (14.02) | 49.4 (11.57) | 50.7 (13.92) | 49.3 (10.64) |
| Verbal Fluency | 51.3 (25.70) | 50.0 (17.3) | 50.1 (22.07) | 48.3 (19.47) |
| End of Treatment | ||||
| Inhibition | 52.9 (8.43) | 46.1 (6.49) | 48.6 (6.70) | 49.7 (8.48) |
| Shift | 51.9 (8.97) | 44.1 (5.19) | 47.8 (8.27) | 48.8 (8.32) |
| Emotional Control | 52.6 (11.09) | 49.2 (10.65) | 48.0 (9.39) | 51.2 (11.38) |
| Initiation | 50.8 (10.52) | 54.6 (7.35) | 45.2 (7.55) | 50.7 (9.48) |
| Working Memory | 54.2 (9.54) | 48.3 (7.10) | 51.9 (9.21) | 51.4 (8.51) |
| Plan / Organize | 54.5 (9.06) | 49.7 (9.47) | 51.8 (9.04) | 52.9 (8.91) |
| Organize Materials | 55.6 (10.44) | 48.1 (11.67) | 52.3 (8.67) | 51.9 (12.22) |
| Task Monitoring | 50.7 (8.22) | 46.6 (9.18) | 47.7 (6.23) | 48.8 (9.23) |
| Attention | 54.6 (14.08) | 49.3 (8.97) | 58.5 (10.64) | 50.5 (9.28) |
| Verbal Fluency | 53.0 (20.58) | 46.3 (11.55) | 52.3 (16.06) | 43.3 (15.09) |
Note: All scores reported as T-Scores (Mean = 50 and Standard Deviation = 10), with higher scores reflecting more pathology.
Increased oxidative stress was associated with measures of executive functions (poorer working memory, poorer ability to organize materials, and attention problems) collected at the end of therapy. Table II presents the significant correlations between these executive functions and oxidized PC reactivity at induction and consolidation. All correlations support the hypothesis that increased measures of oxidative stress were associated with greater executive dysfunction. No significant correlations were found between oxidized PC reactivity and executive functions at the post-consolidation assessment time.
The only patient factor associated with oxidative stress was age at diagnosis r (48) = 0.268, p < 0.05, suggesting younger age was associated with higher oxidized PC reactivity at consolidation. Although most risk factors were noted to affect executive functions, only age at diagnosis was associated with oxidative stress and executive functions. Executive abilities varying by age at diagnosis include Verbal Fluency at the post-consolidation time point, r (47) = −0.58, p < 0.001, Verbal Fluency at the end of therapy time point, r (51) = −0.63, p < 0.001, and Organization of Materials at the end of therapy time point, r (44) = 0.28, p < 0.05. These results suggest older age at diagnosis was associated with poorer verbal fluency performance and better organization of materials.
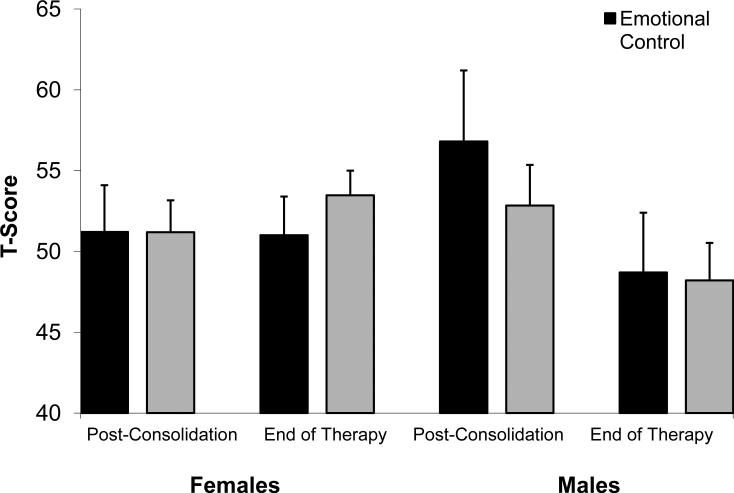
Although other factors were not associated with oxidative stress, many did affect executive functions. Females had poorer working memory than males at the end of therapy, (χ2 (1, N = 46) = 5.4, p > 0.05). On measures of emotional regulation, males made significant improvements from post-consolidation to end of therapy, while females did not F (1,28) = 8.0, p > 0.01). This sex by time interaction can be seen in Figure 1. Parent education level was not associated with executive functions after controlling for age of diagnosis (age at diagnosis and parent education were positively correlated r (71) = −0.277, p = 0.01). Controlling for parent education did not change the association between age of diagnosis and executive functions (Table III).
Figure 1.
Parent ratings on measures of Emotional Control and Working Memory functions from post-consolidation to end of therapy for females and males. Higher scores represent more problematic symptoms.
Table III.
End of Therapy Executive Functions and Oxidized Phosphatidylcholine Fraction (OP).
| Executive Function | OP at Induction | OP at Consolidation |
|---|---|---|
| Working Memory | 0.398* | 0.346* |
| Organization of Materials | 0.327* | 0.335* |
| Attention Problems | 0.437* | ns |
All correlations significant at the p< 0.05 level and suggest a significant increase in executive function problems with increasing oxidized PC fraction.
Discussion
Methotrexate (MTX) has been associated with cognitive and intellectual dysfunction,[24,25] leukoencephalopathy,[9,10] and oxidative stress.[15, 20] The present results further support the hypothesis that oxidative stress, as reflected through oxidized PC, increases with treatment intensity and, to some extent MTX dosage. Our study specifically examined the association between oxidative stress induced by MTX treatment and specific executive dysfunction. The hypothesis that poorer executive functioning would be associated with increased oxidative stress was supported. Significant correlations were found between greater oxidized PC reactivity (at induction and consolidation) and lower executive functioning at the end of chemotherapy treatment. Admittedly, the decline in executive functioning was small, with performances still falling in the average range, though the decline was consistent and may reflect a beginning trend that results in clinically significant late-effects.
The specific functional areas associated with oxidative stress include working memory, organizational abilities, and verbal fluency. The delayed onset in problems with executive function (i.e. end of therapy but not one year post diagnosis) suggests that oxidative stress results in reduced development that may take time to manifest in observable behaviors. Oxidative stress may be an initial pathophysiological event that leads to apoptosis. Progressive degeneration of white matter and/or neurons may account for later manifestation of effects on executive functions. MTX has been associated with delayed onset and long-term cognitive effects in other studies, so this finding is not unique.[26,27] Furthermore, the pattern of problems in fluency and working memory is commonly reported in long-term survivor studies.[27,28]
The expectation that common factors associated with neurotoxicity (i.e., age at diagnosis, sex, treatment intensity, parent education) would be associated with more oxidative stress and poorer executive function outcome was partially supported. Younger age at diagnosis was associated with more oxidative stress after consolidation, but the other risk factors were not. As age and oxidative stress were related to similar executive functions, there does appear to be a connection between the vulnerability of younger children to oxidative stress and greater problems in organization and working memory. White matter also appears particularly vulnerable to oxidative stress because of its high lipid content.[29-31] As such, younger children may be more vulnerable to oxidative stress because of relatively more myelin development during this phase.[32]
Although literature suggests that younger age at diagnosis is largely associated with greater cognitive sequelae,[1,2,7] the present study's results appear contradictory. Children treated at a younger age were reported to have more problems with organization, though demonstrated fewer problems with verbal fluency. This pattern may not be completely inconsistent with the age sensitivity theory, as emerging research suggests that verbal fluency develops later than other executive functions, with full development not occurring until late adolescence or early adulthood.[33,34] Thus, verbal fluency problems may be more difficult to identify in younger children due to increased normal variability among the younger cohort. As children's verbal fluency skills develop, the variability in normal performance decreases making identification of problems in an older subset much easier. In addition, children treated at a younger age may complete therapy prior to the developmental window when verbal fluency matures, while children treated at an older age may have the development within this window disrupted by treatment agents.
No appreciable differences were found between the overall sample of participants diagnosed with ALL and the age-standardized norms on executive function measures (the BRIEF, BASC-PRS, and verbal fluency). This may be due to the fact that the children are still in the early stages of recovery and have not yet entered the long-term survival stage. The fact that the performance is correlated to biological markers of oxidative stress indicates that the outcomes are associated with physiological changes. These changes may reflect the onset of a neurological impact which may become more evident as the children age and reach long-term survivorship status. If this is the case, the biological markers of oxidative stress may be useful in the early identification of children at future risk for neurocognitive decline. With such an identifiable risk factor, preventative treatment strategies could be implemented.
There were limitations associated with this study. First, due to some missing data points within our data set, certain risk group comparisons were limited and reduced in power. With increased power, stronger associations between executive dysfunction and oxidative stress could have emerged, though it is plausible that the missing data could have gone in a direct opposite of the currently apparent trend. Similarly, more associations between oxidative stress and known risk factors for toxicity and neurocognitive dysfunction might have been identified. Another limitation of this study was its prospective cohort design. While this design is ideal for establishing the relation between oxidative stress and executive functions for a specified period of time, longitudinal studies are still needed to better understand the course of executive dysfunction and the clinical impairment that may result if the current trend continues into the late-effect stage of cancer survivorship (i.e. ≥ 5 year post-diagnosis).
In summary, oxidative stress from MTX treatment appears to be associated with poorer executive functions in children at the end of chemotherapy. Longitudinal studies of ALL survivors are needed to determine whether these deficits develop into long-term effects or remit over time. The existing literature would suggest deficits are a byproduct of CNS integrity and cognitive problems may persist. This study adds converging evidence that age at diagnosis is a risk factor for poorer neuropsychological outcome. The emergence of certain deficits, however, may be associated with the developmental stage of the CNS at the time of evaluation. The results from this study suggest oxidative stress is one mechanism responsible for difficulties with executive functions and underscores the importance of taking special precautions when treating younger children with ALL.
Table IV.
Correlations between Executive Functions and Age Diagnosed and Parent Education
| Executive Function | Age at Diagnosis (AD) | Parental Education (PE) | ||
|---|---|---|---|---|
| Original | PE Adjusted | Original | AD Adjusted | |
| Verbal Fluency1 | 0.58** | 0.52* | −0.30* | ns |
| Verbal Fluency2 | 0.63** | 0.64** | −0.34** | ns |
| Organization of Materials2 | 0.28* | 0.58** | −0.35** | ns |
Correlation is significant at the 0.05 level.
Correlation is significant at the 0.01 level.
Note: Positive correlations suggest an increase in executive function problems associated with increasing age/parent education, while negative correlations suggest decreased executive dysfunction associated with these factors. PE Adjusted represents correlation between Age at Diagnosis and Executive Functions adjusting for Parent Education. AD Adjusted represents correlation between Parent Education and Executive Functions adjusting for Age at Diagnosis.
Acknowledgments
Support: Supported in part by NIH grants HD 37816 (K. Kaemingk) and NR 04905 (I.M. Moore).
References
- 1.Gurney JG, Bondy ML. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th ed. Lippincott, Williams, & Wilkins; Philadelphia, PA: 2006. pp. 1–13. [Google Scholar]
- 2.Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute; Bethesda, MD: 1999. [Google Scholar]
- 3.Dickerman JD. The late effects of childhood cancer therapy. Pediatrics. 2007;119(3):554–568. doi: 10.1542/peds.2006-2826. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic M, Brouwers P, Valsecchi MG, et al. Association of 1800 cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia. ISPACC. International Study Group on Psychosocial Aspects of Childhood Cancer. Lancet. 1994344(8917):224–227. doi: 10.1016/s0140-6736(94)92997-1. [DOI] [PubMed] [Google Scholar]
- 5.Spiegler BJ, Kennedy K, Maze R, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol. 2006;24(24):3858–3864. doi: 10.1200/JCO.2006.05.9055. [DOI] [PubMed] [Google Scholar]
- 6.Waber DP, Turek J, Catania L, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol. 2007;25(31):4914–4921. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 7.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer. 2003;39(3):359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 8.Carey ME, Hockenberry MJ, Moore IM, et al. Brief report: effect of intravenous methotrexate dose and infusion rate on neuropsychological function one year after diagnosis of acute lymphoblastic leukemia. J Pediatr Psychol. 2007;32(2):189–193. doi: 10.1093/jpepsy/jsj114. [DOI] [PubMed] [Google Scholar]
- 9.Montour-Proulx I, Kuehn SM, Keene DL, et al. Cognitive changes in children treated for acute lymphoblastic leukemia with chemotherapy only according to the Pediatric Oncology Group 9605 protocol. J Child Neurol. 2005;20(2):129–133. doi: 10.1177/08830738050200020901. [DOI] [PubMed] [Google Scholar]
- 10.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26(5):1263–1269. [PMC free article] [PubMed] [Google Scholar]
- 11.Carey ME, Haut MW, Reminger SL, et al. Reduced Frontal White Matter Volume in Long-Term Childhood Leukemia Survivors: A Voxel-Based Morphometry Study. AJNR Am J Neuroradiol. 2008 doi: 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuss DT, Alexander MP, Benson DF. Frontal lobe functions. In: Trimle MR, Cummings JL, editors. Contemporary behavioral neurology. Butterworth-Heinemann; Boston: 1997. pp. 169–187. [Google Scholar]
- 13.Lamar M, Zonderman AB, Resnick S. Contribution of specific cognitive processes to executive functioning in an aging population. Neuropsychology. 2002;16(2):156–162. doi: 10.1037//0894-4105.16.2.156. [DOI] [PubMed] [Google Scholar]
- 14.Moore IM, Espy KA, Kaufmann P, et al. Cognitive consequences and central nervous system injury following treatment for childhood leukemia. Semin Oncol Nurs. 2000;16(4):279–290. doi: 10.1053/sonu.2000.16582. discussion 291-279. [DOI] [PubMed] [Google Scholar]
- 15.Miketova P, Kaemingk K, Hockenberry M, et al. Oxidative changes in cerebral spinal fluid phosphatidylcholine during treatment for acute lymphoblastic leukemia. Biol Res Nurs. 2005;6(3):187–195. doi: 10.1177/1099800404271916. [DOI] [PubMed] [Google Scholar]
- 16.Babiak RM, Campello AP, Carnieri EG, et al. Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct. 1998;16(4):283–293. doi: 10.1002/(SICI)1099-0844(1998120)16:4<283::AID-CBF801>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Jahovic N, Cevik H, Sehirli AO, et al. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pineal Res. 2003;34(4):282–287. [PubMed] [Google Scholar]
- 18.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39(8):1529–1542. [PubMed] [Google Scholar]
- 19.Farooqui AA, Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18(6):599–608. doi: 10.1023/A:1020625717298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore IM, Miketova P, Hockenberry M, et al. Methotrexate-induced alterations in beta-oxidation correlate with cognitive abilities in children with acute lymphoblastic leukemia. Biol Res Nurs. 2008;9(4):311–319. doi: 10.1177/1099800407313268. [DOI] [PubMed] [Google Scholar]
- 21.Gioia GA, Isquith PK, Guy SC, et al. Behavior Rating Inventory of Executive Function. Psychological Assessment Resources, Inc.; Odessa, FL: 2000. [Google Scholar]
- 22.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children - Manual. American Guidance Service, Inc.; Circle Pine, MN: 1992. [Google Scholar]
- 23.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. Harcourt Assessment, Inc.; San Antonio: 2001. [Google Scholar]
- 24.Hockenberry M, Krull K, Moore K, et al. Longitudinal evaluation of fine motor skills in children with leukemia. J Pediatr Hematol Oncol. 2007;29(8):535–539. doi: 10.1097/MPH.0b013e3180f61b92. [DOI] [PubMed] [Google Scholar]
- 25.Espy KA, Moore IM, Kaufmann PM, et al. Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: A prospective study. Journal of Pediatric Psychology. 2001;26(1):1–9. doi: 10.1093/jpepsy/26.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Cole PD, Kamen BA. Delayed neurotoxicity associated with therapy for children with acute lymphoblastic leukemia. Ment Retard Dev Disabil Res Rev. 2006;12(3):174–183. doi: 10.1002/mrdd.20113. [DOI] [PubMed] [Google Scholar]
- 27.Moore BD., 3rd Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 2005;30(1):51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 28.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children's Oncology Group. Arch Pediatr Adolesc Med. 2007;161(8):798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 29.Bongarzone ER, Pasquini JM, Soto EF. Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. J Neurosci Res. 1995;41(2):213–221. doi: 10.1002/jnr.490410209. [DOI] [PubMed] [Google Scholar]
- 30.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23(3):263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 31.Gerstner B, DeSilva TM, Genz K, et al. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008;28(5):1236–1245. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178(3):296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: a dimensional and developmental study. Dev Neuropsychol. 2004;26(2):571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- 34.Matute E, Rosselli M, Ardila A, et al. Verbal and nonverbal fluency in Spanish-speaking children. Dev Neuropsychol. 2004;26(2):647–660. doi: 10.1207/s15326942dn2602_7. [DOI] [PubMed] [Google Scholar]