Abstract
Background
Dexamethasone is used in acute lymphoblastic leukemia (ALL) treatment, though long-term impact on central nervous system (CNS) function is unclear. As glucocorticoids influence hippocampal function, we investigated memory networks in survivors of childhood ALL treated with dexamethasone or prednisone.
Procedure
Neurocognitive assessment and functional magnetic resonance imaging (fMRI) were conducted in 38 adult survivors randomly recruited from cohorts treated on one of two standard treatment protocols, which differed primarily in the glucocorticoid administered during continuation therapy (dexamethasone [n=18] vs. prednisone [n=20]). Groups did not differ in age at diagnosis, age at evaluation, or cumulative intravenous or intrathecal methotrexate exposure.
Results
Survivors treated with dexamethasone demonstrated lower performance on multiple memory-dependent measures, including story memory (p=0.01) and word recognition (p=0.04), compared to survivors treated with only prednisone. Dexamethasone treatment was associated with decreased fMRI activity in the left retrosplenial brain region (effect size =1.3), though the small sample size limited statistical significance (p=0.08). Story memory was associated with altered activation in left inferior frontal-temporal brain regions (p=0.007).
Conclusions
Results from this pilot study suggest that adult survivors of ALL treated with dexamethasone are at increased risk for memory deficits and altered neural activity in specific brain regions and networks associated with memory function.
Keywords: Leukemia, fMRI, memory, survivors, glucocorticoid, retrosplenium
INTRODUCTION
The 5-year survival rate for acute lymphoblastic leukemia (ALL) has increased from 5% in the early 1960s to over 80% today [1, 2]. Contemporary ALL treatment protocols include high doses of glucocorticoid steroids. Prednisone is a glucocorticoid used in early therapeutic protocols, though dexamethasone has been used on recent protocols with improved event-free survival [2].
CNS late effects, including neurocognitive impairment and leukoencephalopathy, have been observed in ALL survivors treated with only chemotherapy [3, 4]. The long-term impact of dexamethasone on CNS integrity is unclear, though increased academic and memory problems have been reported in survivors treated with dexamethasone compared to prednisone [5]. A recent report comparing neurocognitive performance in patients randomized to dexamethasone or prednisone treatment found a difference in word reading though not memory [6]. These conflicting results might be due to differences in time of follow-up, cumulative dose of glucocorticoid administered or neurocognitive assessment procedures.
Glucocorticoid receptors play an important role in memory storage and consolidation [7]. Prolonged exposure to glucocorticoids can inhibit glucose utilization, which increases concentration of glutamate and leads to excitotoxic neuronal death [8]. Reduced synaptic plasticity in hippocampal neurons and reduced CNS development following prolonged use of glucocorticoids has been reported [9]. In a double-blind placebo-controlled study, healthy adults who received dexamethasone demonstrated impaired immediate and delayed memory recall [10]. Glucocorticoids have also been shown to alter hippocampal and prefrontal activation during memory retrieval tasks through functional magnetic resonance imaging (fMRI) in healthy controls [11].
To date, no studies have reported brain activation patterns associated with memory function in long-term survivors treated with different glucocorticoids for childhood ALL. This study reports neurocognitive testing and brain fMRI in adult survivors of childhood ALL treated with prednisone or dexamethasone during continuation therapy. We hypothesized that survivors who received dexamethasone would be at higher risk for memory deficits compared to survivors treated with prednisone, and that such deficits would be associated with altered neural activity within memory networks.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at St. Jude Children’s Research Hospital (SJCRH). Written informed consent was obtained from all participants.
Participants
Survivors included in these analyses were participants in the St. Jude Lifetime Cohort (SJLIFE) study, which evaluates medical and psychosocial late effects in adult survivors of childhood cancer [12]. To be eligible for SJLIFE, survivors must have been treated at SJCRH for childhood cancer, currently ≥18 years of age and ≥10 years from time of diagnosis. From this cohort, we identified survivors treated for ALL on either: Total Therapy XIII (TOTXIII)A (1992 to 1994) or TOTXIIIB (1994 to 1998) [13]. Survivors were excluded if they received cranial radiation or nonstandard glucocorticoid therapy, relapsed, had secondary cancer, were not proficient in English, or had a pre-existing non-cancer related neurological disorder. Of the 415 patients treated on TOTXIII, 84 died prior to study recruitment, 5 were permanently discharged, 66 were excluded due to being <18 years of age, and 2 were in jail or house arrest. Of the remaining 258 survivors eligible for SJLIFE, 123 were treated on TOTXIIIA and 135 were treated on TOTXIIIB. From this combined group, 50 survivors were randomly identified and targeted for recruitment into the current study. 24 survivors treated on TOTXIIIA were contacted. 22 agreed to participate and were scheduled for a campus visit. 21 survivors treated on TOTXIIIB were contacted. 18 agreed to participate and were scheduled for a campus visit. Two of the 40 recruited survivors withdrew during data collection, leaving 38 participants with evaluable data.
Supplementary Table I compares treatment schemas for TOTXIIIA and TOTXIIIB. Prednisone was used for remission induction and reinduction in both protocols. During continuation, prednisone (40 mg/m2 per day for 7 days every 4 weeks) was used in TOTXIIIA and dexamethasone (8mg/m2 per day for 7 days every 4 weeks) in TOTXIIIB. Survivors treated on TOTXIIIA will be referred to as those treated with no dexamethasone.
Neurocognitive Testing
All survivors completed a neurocognitive evaluation with certified examiners under the supervision of a clinical neuropsychologist. Examiners were blind to steroid exposure. Assessed neurocognitive domains (and instruments) included: intelligence (Wechsler Abbreviated Scale of Intelligence [14]), academics (Woodcock-Johnson-III Tests of Achievement [15] [letter-word identification and calculation subtests]), and memory (Test of Memory and Learning: 2nd edition [16] [word selective reminding, visual selective reminding, memory for stories, paired recall, and facial memory subtests]).
fMRI Data Collection
Brain imaging was conducted within four days of neurocognitive testing using a 3-T Siemens Trio scanner (Malvern, PA, USA). T2-weighted echo planar imaging (EPI) pulse sequences were obtained using the standard quadrature headcoil and the following parameters: field of view, 192mm; matrix, 64 × 64; slice thickness, 5mm; pulse sequence bandwidth per pixel, 1953Hz; TE, 30ms; 32 slices per volume; and TR, 2060ms. High-resolution three dimensional T1-weighted images were acquired for anatomic visualization (TR=1.8s, TE=2.74ms, flip angle=15°, voxel size= 1×1×1mm3).
Two memory recognition tasks were conducted during fMRI data collection: word recognition and facial recognition. Tasks were modeled after the experimental design published by Golby and colleagues [17]. We selected this paradigm given its sensitivity to medial temporal lobe function, the apparent site of altered hippocampal development following prolonged use of glucocorticoids [9]. Word and face stimuli were presented during separate scans. Before starting each scan, survivors were instructed to remember stimuli for a later test. Survivors were presented with 96 stimuli in 16 blocks of six stimuli per block. Stimuli were visible for 3,500ms, with an interstimulus interval of 500ms. Alternating blocks contained either all new stimuli or repeated stimuli. The word recognition task consisted of visually presented pairs of common words. Survivors were instructed to silently generate a sentence containing both words and to use the same sentence for word pairs that repeated. Survivors responded by button press after generating a sentence. For facial recognition, photographs of male and female faces were presented using the same parameters. After scanning, a recognition test was administered. For each type of stimulus, survivors viewed 10 previously presented items and 10 foils.
Statistical Analysis
Descriptive statistics were calculated for demographic and treatment characteristics as well as task performance during neurocognitive and fMRI testing. Scores on neurocognitive measures were transformed into age-adjusted standard scores using national normative data. Group performance was compared using Mann-Whitney U or Fisher’s exact tests. Education level is reported as years of educational attainment and analyzed via median regression to adjust for age at evaluation. Performance on neurocognitive measures were compared to population norms (mean=0, standard deviation=1) using one-sample median sign tests. We did not correct for multiple comparisons, as our a priori hypothesis was those treated with dexamethasone would be at a higher risk for neurocognitive problems and we used a conservative two-sided alpha for all analyses. Neurocognitive measures that differed between groups were examined in reference to fMRI.
Performance on the post-fMRI recognition tasks was compared against chance (mu=10) for the total sample via one-sample median sign tests. Reaction times of new versus old images were compared using Wilcoxon signed ranks tests. Spearman correlations were conducted between task performance (word or face) and the neurocognitive measures that demonstrated significant group differences.
Image analysis was conducted with Statistical Parametric Mapping software (SPM5, Wellcome Institute of Neurology, London, UK). Images from each subject were slice-time corrected, realigned to correct for interscan head motion, normalized to the Montreal Neurological Institute brain template and smoothed with a 6mm full width at half-maximum Gaussian kernel. The smoothed and normalized images were resliced to 2mm isotropic resolution. Data from individual subjects were analyzed according to a fixed-effect general linear model, with stimulus-related activation as a delayed boxcar function and treating low-frequency signal components as nuisance covariates. Differences in global signal intensity were corrected by using proportional scaling to a common mean. This analysis identified for each subject those regions that were significantly more active for novel than for repeated stimuli (i.e. activational change). Contrast images were then used as variables in second-level, random-effect analyses to identify patterns of brain activation. Neuropsychological score were entered as covariates to identify areas correlated with activation. Group differences were tested with a two-sample t test or non-parametric permutation test. Voxels were considered active for p<0.05, corrected for multiple comparisons and with a minimum cluster size of 5 voxels. Additional cluster-level analysis was conducted with threshold p<0.001 (uncorrected for multiple comparisons) and minimum cluster size of 5 voxels. Results for all survivors are reported for which the cluster size statistic was p<0.05 corrected for multiple comparisons. Coordinates for the location of clusters of activation were converted to Talairach space by using the transformation method developed by the MRC Cognition and Brain Sciences Unit (Cambridge, UK, available online at http://imaging.mrc-cbu.cam.ac.uk/imaging/CbuImaging). The anatomical name and Brodmann area (BA) reported for each supratentorial cluster of activation were determined with the Talairach demon, and the location of clusters was carefully cross-checked by visual comparison with the Talairach atlas.
RESULTS
Demographic characteristics and treatment history were compared between survivor groups (Table I). As expected in this historical comparison, years since diagnosis was different between groups (median 15.9 vs. 13.3, p<0.001). No differences were found in gender, race, age at diagnosis, or age at evaluation. The groups did not differ in cumulative exposure to high dose methotrexate, intrathecal therapy, or proportion of survivors with leukoencephalopathy.
Table I. Demographic and treatment characteristics.
| Variables | No Dexamethasone | Dexamethasone | P |
|---|---|---|---|
| Gender – F:M | 10:10 | 6:12 | 0.34 |
| Race – White : Non-White | 19:1 | 15:3 | 0.33 |
| Median (Range) | Median (Range) | ||
| Age @ diagnosis (years) | 8.7 (3.8-16.9) | 11.8 (5.8-18.6) | 0.08 |
| Age @ evaluation (years) | 24.6 (20.4-32.4) | 24.6 (19.7-31.2) | 0.63 |
| Time since diagnosis (years) | 15.9 (14.8-17.9) | 13.3 (12.0-15.1) | <0.001 |
| Leukoencephalopathy (% [N]) | 42% [8] | 50% [9] | 0.75 |
| Cumulative Chemotherapy | |||
| High Dose Methotrexate (mg/m2) | 20,478 (17,352-25,571) | 21,030 (5,207-25,571) | 0.68 |
| IT Methotrexate (ml) | 180 (132-264) | 180 (156-264) | 0.61 |
| IT Hydrocortisone (ml) | 360 (264-528) | 361 (312-528) | 0.58 |
| IT Cytarabine (ml) | 540 (396-792) | 540 (468-792) | 0.58 |
| Number of IT MHA doses (N) | 15 (11-22) | 15 (13-22) | 0.58 |
| Dexamethasone (mg/m2) | --- | 1,596 | N/A |
| Prednisone (mg/m2) | 10,160 | 2,240 | N/A |
Note: No Dexamethasone group n=20, except Leukoencephalopathy where n=19, 1 participant had no structural MRI. Dexamethasone group n=18. IT: Intrathecal. MHA: Methotrexate, hydrocortisone, cytarabine. P: p-value
Performance on neurocognitive assessments are listed in Table II. Survivors treated with dexamethasone performed worse than the population norm on vocabulary (p=0.05), reading (p=0.002), and math (p=0.03). Survivors treated with no dexamethasone performed better than the population norm on delayed memory for stories (p=0.02). Survivors treated with dexamethasone performed worse than those treated with no dexamethasone on vocabulary (p=0.03), reading (p=0.009), math (p=0.006), immediate word selective reminding (p=0.04), immediate memory for stories (p=0.01), and delayed memory for stories (p=0.02). The effect sizes were large and generally in the range of 0.70 to 0.90, reflecting differences of 3/4 to 1 standard deviation between groups. Only those neurocognitive measures on which performance differed between groups were used for subsequent analyses.
Table II. Total sample and treatment group performance on task performance.
| Total Sample All TOTXIII patients |
Group Comparison | ||||
|---|---|---|---|---|---|
| No Dexamethasone | Dexamethasone | ||||
|
| |||||
| Task Performance | Median (Range) | P | Median (Range) | Median (Range) | P |
| Intelligence | |||||
| Vocabulary | −0.1 (−2.7:1.7) | 0.62 | 0.2 (−2.2:1.7) | −0.6 (−2.7:1.7) | 0.03 |
| Matrices | 0.2 (−1.3:1.6) | 0.04 | 0.2 (−1.3:1.6) | 0.25 (−0.7:1.0) | 0.48 |
| Academics | |||||
| Reading | −0.2 (−3.2:1.0) | 0.03 | −0.1 (−0.8:1.0) | −0.5 (−3.2:0.5) | 0.009 |
| Math | −0.3 (−2.9:1.7) | 0.31 | 0.1 (−1.1:1.3) | −0.9 (−2.9:1.7) | 0.006 |
| Short-Term Memory | |||||
| Word Selective Reminding | −0.3 (−2.7:1.3) | 0.39 | 0.2 (−1.3:1.3) | −0.7 (−2.7:0.7) | 0.04 |
| Visual Selective Reminding | 0.0 (−3.0:1.3) | 0.86 | 0.0 (−3.0:1.3) | −0.3 (−2.7:1.0) | 0.35 |
| Memory for Stories | 0.0 (−2.0:2.3) | 1.00 | 0.3 (−1.3:2.3) | −0.3 (−2.0:1.0) | 0.01 |
| Paired Recall | 0.3 (−2.3:1.3) | 0.15 | 0.7 (−2.3:1.3) | 0.2 (−2.3:1.0) | 0.55 |
| Facial Memory | 0.0 (−1.7:2.3) | 0.46 | 0.3 (−1.3:2.3) | 0.0 (−1.7:1.3) | 0.16 |
| Long-Term Memory | |||||
| Delayed Memory for Stories | 0.3 (−1.7:1.7) | 0.31 | 0.7 (−1.0:1.7) | −0.3 (−1.7:1.0) | 0.02 |
| Delayed Word Selective Reminding | 0.0 (−2.0:1.0) | 0.86 | 0.3 (−2.0:1.0) | −0.2 (−2.0:1.0) | 0.09 |
| fMRI Tasks | |||||
| Word Recognition (out of 20) | 16 (12:20) | <0.001 | 16 (12:20) | 17 (12:20) | 0.60 |
| Reaction Time for New Words (s) | 2.4 (1.7:3.0) | - | 2.3 (1.7:2.9) | 2.5 (1.9:3.0) | 0.15 |
| Reaction Time for Old Words (s) | 1.3 (0.8:3.0) | - | 1.3 (0.8:2.6) | 1.3 (1.0:3.0) | 0.93 |
| Face Recognition (out of 20) | 15 (9:19) | <0.001 | 15 (9:19) | 15 (11:17) | 0.93 |
| Reaction Time for New Faces (s) | 1.0 (0.6:1.3) | - | 0.9 (0.6:1.2) | 1.0 (0.7:1.3) | 0.10 |
| Reaction Time for Old Faces (s) | 0.8 (0.5:1.1) | --- | 0.7 (0.5:1.1) | 0.8 (0.6:1.1) | 0.14 |
Note. No Dexamethasone group n=20, except for vocabulary, matrices, reading, and math where n=19. Dexamethasone group n=18. No population data for fMRI task performance is available, so word and face recognition was compared to chance (mu=10). P: p-value, s: seconds
There was a difference (p=0.009) in survivor education as the median (range) years of education was 12 (8-18) for survivors treated with dexamethasone compared to 14 (10-20) for survivors treated with no dexamethasone. The employment rate for survivors treated with no dexamethasone was 80% compared to 56% for survivors treated with dexamethasone (p=0.12).
Task performance during fMRI for all survivors was above chance for word recognition (p<0.001) and face recognition (p<0.001; see Table II), suggesting good task engagement. Survivors took longer to react to new images as compared to old images in both face and word recognition tasks (both p<0.001). There was no difference in any measure of fMRI task performance between survivors treated with or without dexamethasone (p>0.05).
All participants exhibited activation in response to novel versus old images in brain regions known to be activated by this paradigm [17], including: hippocampus, parahippocampal gyrus, entorhinal cortex, prefrontal cortex, visual cortices, and cerebellum. Activation maps averaged from all survivors were constructed using random effects analysis. The primary brain areas that displayed more activation (p<0.05, Family-Wise Error (FWE) corrected for multiple comparisons) for novel than repeated words include: left parahippocampal gyrus, left superior and inferior frontal gyri, left lingual gyrus and bilateral superior parietal lobule (Figure 1A). Brain areas that were more active (p<0.05, FWE) during novel versus repeated faces include: right parahippocampal gyrus, bilateral occipital gyri, and the bilateral inferior frontal gyri (Figure 1B). Brain activation unique to verbal stimuli was lateralized to the left hemisphere (Figure 1C), whereas, brain activation unique to facial stimuli was lateralized to the right hemisphere (Figure 1D).
Figure 1. Patterns of activation during fMRI tasks of novel versus repeated stimuli in all survivors.
Row A indicates areas more active during blocks of novel words compared to blocks of previously seen words. Row B illustrates areas more active during blocks of novel faces compared to blocks of previously seen faces. Row C is a subtraction of activity in row A from row B to highlight areas distinct to processing the word recognition task. Row D identifies areas unique to conducting the facial recognition task compared to word recognition task. The activation signal threshold was p < 0.05, Family-Wise Error (FWE) corrected for multiple comparisons.
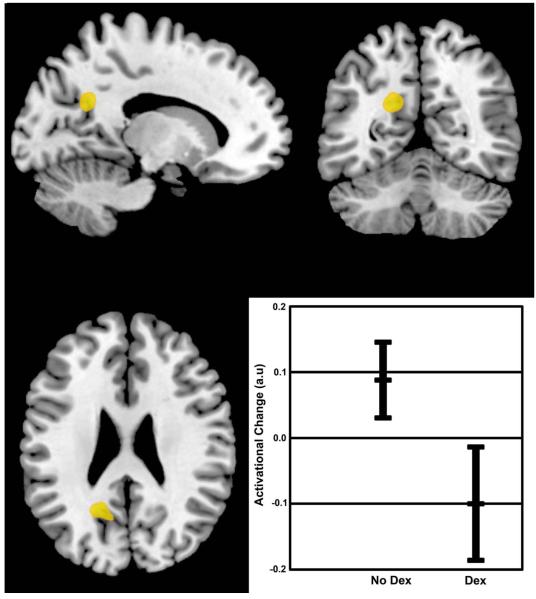
Functional-MRI brain activation during the word recognition task is shown in Figure 2. Survivors treated with dexamethasone demonstrated altered brain activation in regions important for memory function, including decreased activity within the retrosplenial region while processing novel words (p<0.001, uncorrected; p=0.08 cluster-wise corrected when compared to those treated without dexamethasone). The effect size for the activational difference in the retrosplenial region was large at 1.3.
Figure 2. Differences in activational change between survivors treated with dexamethasone or no dexamethasone.
Non-parametric permutation testing identified a group difference in an area (peak: −18, −56, 26) corresponding to the left retrosplenial region (p<0.0001, uncorrected; p=0.08, cluster-wise corrected for multiple comparisons, effect size=1.3). The graph represents the group means and 95% confidence intervals for the change in activation between new and old words
Performance on the fMRI word recognition task was correlated with performance on math (r=0.33, p=0.04), immediate memory for stories (r=0.35, p=0.03) and delayed memory for stories (r=0.36, p=0.03; Table III). Performance of the fMRI face recognition task was correlated with vocabulary (r=0.57, p<0.001) and reading (r=0.41, p=0.01). Brain activity during the word recognition task correlated with task performance. Activity within the left inferior frontal gyrus was positively correlated with recognition of word pairs during the fMRI post-test (Figure 3A). Activity within the left insula during the word recognition task was negatively correlated with clinical assessment of delayed story memory (Figure 3B).
Table III. Spearman correlation between fMRI task performance and neurocognitive tasks.
| Neurocognitive Task | Word Recognition | Face Recognition | ||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| Vocabulary | 0.31 | 0.06 | 0.57 | <0.001 |
| Reading | 0.31 | 0.06 | 0.41 | 0.01 |
| Math | 0.33 | 0.04 | 0.25 | 0.14 |
| Immediate Word Selective Reminding | 0.24 | 0.14 | 0.13 | 0.43 |
| Immediate Memory For Stories | 0.35 | 0.03 | 0.12 | 0.49 |
| Delayed Memory For Stories | 0.36 | 0.03 | 0.26 | 0.11 |
ρ: Spearman’s rho, P: p-value
Figure 3. Activational change related to performance measures.
Panel A shows an area (peak: −40, 22, −4) corresponding to the left inferior frontal gyrus where brain activation by new words was positively correlated to performance on a post-test (p=0.007, cluster-wise corrected for multiple comparisons). Panel B identifies an area (peak: −42, 6, 8) corresponding to the left insula where activation on the word recognition task was negatively correlated to the clinical measure for Delayed Memory for Stories (p=0.029, FWE corrected for multiple comparisons). In both graphs open circles represent survivors treated with dexamethasone; solid circles represent survivors treated with no dexamethasone.
DISCUSSION
A recent fMRI study found that adult survivors of childhood ALL treated with cranial radiation therapy (CRT) demonstrated poorer recognition memory, hippocampal atrophy, and altered hippocampal function compared to healthy controls [18]. Evidence suggests survivors of childhood ALL are at risk for memory deficits, even when treated without CRT [19, 20]. This pilot study is the first to examine brain activation correlates of memory problems in adult survivors of childhood ALL treated with different glucocorticoids. These data suggest survivors of childhood ALL treated with dexamethasone are at greater risk for memory problems compared to those treated with only prednisone.
Survivors treated with dexamethasone performed worse than the population norm in vocabulary, academic learning, and verbal memory. In comparison, survivors treated with no dexamethasone performed at or above the population norm in all neurocognitive domains. The neurocognitive problems seen in survivors exposed to dexamethasone is consistent with long-term verbal memory deficits. Vocabulary and academic learning develop over the course of childhood and are contingent upon the ability to retain newly learned information over time. Reduced performance on these remote memory-based cognitive tasks, combined with difficulties with direct assessment of immediate and delayed recall of newly learned information, suggests the dexamethasone group has experienced reduced ability to learn and retain verbal information for some time. Children treated with ALL, even without CRT, are at risk for academic difficulties [21-24]. Our results demonstrate reduced educational attainment in survivors treated with dexamethasone compared to no dexamethasone. Although the size of our sample that completed fMRI was relatively small, the pattern of educational attainment is consistent with our larger survivor cohort. As research has shown early onset neurocognitive impairment can influence later educational outcomes [25], difficulties with long-term verbal memory seen in survivors treated with dexamethasone may have contributed to their lower educational attainment. It is important that the treatment group performed at the expected mean on visuospatial tasks such as: matrices; visual selective reminding; and facial memory, reinforcing the notion of a specific learning deficit of verbal memory. Selective impairment in verbal memory suggests that developing early interventions focused on improving verbal memory may be useful to help ameliorate the effects seen in survivors treated with dexamethasone. The differences found between those treated with or without dexamethasone are supported by a recent study that demonstrated that among survivors treated only with chemotherapy (n=227) dexamethasone exposure was associated with increased risk for neurocognitive impairment [26].
Survivors treated with dexamethasone demonstrated altered brain activation in regions important for memory function. Specifically, decreased brain activity within the left retrosplenial region was observed during processing of novel words. This brain region has dense reciprocal connections with the hippocampus and projects to medial temporal structures, such as the parahippocampal cortex (PHC) [27-29] and is involved in a variety of functions including new memory formation [27, 30-32]. Since survivors treated with dexamethasone perform worse on verbal memory tasks, it’s interesting to note that previous fMRI studies have implicated the retrosplenium in verbal memory [27, 30, 33, 34]. fMRI studies indicate that the retrosplenium, along with the PHC, is important for successful contextual memory of an event, such as story memory [32, 35, 36]. Together these data suggest that disrupted activity within the retrosplenium may contribute to the difficulties in verbal memory seen in survivors treated with dexamethasone.
Neuroimaging analysis demonstrated activation of several brain regions during recognition memory tasks. The activation patterns elicited in our participants were similar to patterns observed by others using this research paradigm [17]. Many of the regions activated in our participants shared commonalities with those reported in adult survivors of ALL treated with CRT despite using a different type of memory paradigm [18]. These regions include, but are not limited to: inferior frontal gyrus; insula; putamen; and middle occipital gyrus. Brain activity was primarily lateralized to the left hemisphere during the word recognition task. Similar task-specific activation patterns are observed in the general population during verbal-mediated tasks [37]. The primary region unique to the verbal task was the superior frontal gyrus, though activation within the left inferior frontal gyrus was also noted. Activity in this inferior frontal region was positively correlated with task performance during word recognition. This is consistent with research that implicated the left inferior frontal gyrus in successful completion of word recognition tasks.
Brain activity during the word recognition task was negatively correlated with clinical neurocognitive assessment (i.e. delayed memory for stories). Both of these tasks are dependent on long-term verbal memory. The negative correlation with brain activity was found in the left insular region. The insular cortex is a complex structure involved in a variety of functions including mediating verbal memory and regulation of emotional states through balance of the sympathetic and parasympathetic nervous system [38]. The association between increased activation and memory problems may suggest over arousal or emotional interference with performance during challenging tasks.
The increased risk for memory deficits in survivors treated with dexamethasone might be due to relative differences in CNS exposure to glucocorticoids. Dexamethasone penetrates more readily into the CNS and has a longer half-life than prednisone [39]. Studies have shown the relative cytotoxicity of dexamethasone is not fully explained by the conventional 6:1 to 7:1 ratio of glucocorticoid activity [39, 40]. In vitro assays of cytotoxicity indicate that dexamethasone is 6 to 16 times more potent against ALL blast cells than prednisone [41, 42]. In this study, survivors who were treated with no dexamethasone received a cumulative dose of 10,160mg/m2 of prednisone. In comparison, survivors treated with dexamethasone received a cumulative dose of 1,596mg/m2 of dexamethasone as well as 2,240mg/m2 of prednisone. Assuming a conservative 6:1 ratio, the no dexamethasone group received 14% less cytotoxic glucocorticoid than the dexamethasone group. Therefore, the current results might be due to relative differences in exposure to glucocorticoids.
Differences in glucocorticoid exposure may also partially explain apparent discrepancies between studies examining the impact of glucocorticoid treatment. Kadan-Lottick et al. [6] reported that 10-year survivors treated with dexamethasone performed worse in word reading, but not in memory or academic tasks compared to those with prednisone. However, these survivors were treated with less dexamethasone than those survivors who participated in the current study and in the study by Waber et al. [5], both of which found that the dexamethasone group performed worse in word reading, memory, and academic tasks. Along with differences in glucocorticoid exposure, discrepancies between these three studies may be due to differences in neurocognitive assessment procedures and time to follow-up.
The current investigation has several limitations. As a historical comparison study, it is impossible to control for time since diagnosis. However, as late-effects are typically more readily detected in survivors further from diagnosis [43] and the group with greater memory deficits had a shorter time since diagnosis; it seems unlikely that this variable is contributing to the differences found between groups. There was also a trend (p=0.08) for survivors treated with dexamethasone to be older than those treated with no dexamethasone at the time of diagnosis. A recent study demonstrated a differential effect of glucocorticoid treatment for patients diagnosed at 3 years of age or older compared to those diagnosed younger than 3 [6]. In the current study, the average age of diagnosis for all participants was 10 years and no participant was diagnosed before 3. Finally, although this study used stratified random recruitment and obtained a participation rate of 76% among those invited, interpretations are limited by small sample size and limited power to detect statistically significant associations and potentially important covariates, such as: gender and family of origin factors.
In conclusion, these results suggest that adult survivors of childhood ALL treated with dexamethasone are at greater risk for long-term memory problems and altered brain activity associated with memory circuitry. Specifically, survivors treated with dexamethasone have particular difficulties with tasks dependent on verbal memory. This pilot study is the first to examine differences in brain activation within adult survivors of childhood ALL treated with different glucocorticoids. We suggest that fMRI is a valuable tool to examine how brain function may differ due to potentially neurotoxic treatment exposures. The current findings warrant a larger investigation into the late effects of dexamethasone treatment on memory function.
Supplementary Material
Acknowledgements
This work was supported by the Cancer Center Support (CORE) grant CA21765 from the National Cancer Institute and by ALSAC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of Interest Statement
All authors report no conflicts of interest.
References
- 1.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009. 2012 [Google Scholar]
- 2.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26:4138–4143. doi: 10.1200/JCO.2008.16.8864. [DOI] [PubMed] [Google Scholar]
- 4.Reddick WE, Glass JO, Helton KJ, et al. A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation. AJNR Am J Neuroradiol. 2005;26:2371–2377. [PMC free article] [PubMed] [Google Scholar]
- 5.Waber DP, Carpentieri SC, Klar N, et al. Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatr Hematol Oncol. 2000;22:206–213. doi: 10.1097/00043426-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Kadan-Lottick NS, Brouwers P, Breiger D, et al. A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood. 2009;114:1746–1752. doi: 10.1182/blood-2008-12-186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barsegyan A, Mackenzie SM, Kurose BD, et al. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci U S A. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajek T, Kopecek M, Preiss M, et al. Prospective study of hippocampal volume and function in human subjects treated with corticosteroids. Eur Psychiatry. 2006;21:123–128. doi: 10.1016/j.eurpsy.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Newcomer JW, Craft S, Hershey T, et al. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oei NY, Elzinga BM, Wolf OT, et al. Glucocorticoids Decrease Hippocampal and Prefrontal Activation during Declarative Memory Retrieval in Young Men. Brain Imaging Behav. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 15.Woodcock RW, McGrew KS, Mather N, Woodcock-Johnson III. Tests of Achievement. Riverside; Itasca, IL: 2001. [Google Scholar]
- 16.Reynolds CR, Voress JK. Test of Memory and Learning. Second Edition PRO-ED; Austin, TX: 2007. [Google Scholar]
- 17.Golby AJ, Poldrack RA, Illes J, et al. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002;43:855–863. doi: 10.1046/j.1528-1157.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- 18.Monje M, Thomason ME, Rigolo L, et al. Functional and structural differences in the hippocampus associated with memory deficits in adult survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:293–300. doi: 10.1002/pbc.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashford J, Schoffstall C, Reddick WE, et al. Attention and working memory abilities in children treated for acute lymphoblastic leukemia. Cancer. 2010;116:4638–4645. doi: 10.1002/cncr.25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montour-Proulx I, Kuehn SM, Keene DL, et al. Cognitive changes in children treated for acute lymphoblastic leukemia with chemotherapy only according to the Pediatric Oncology Group 9605 protocol. J Child Neurol. 2005;20:129–133. doi: 10.1177/08830738050200020901. [DOI] [PubMed] [Google Scholar]
- 21.Peterson CC, Johnson CE, Ramirez LY, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- 22.Ochs J, Mulhern R, Fairclough D, et al. Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: a prospective study. J Clin Oncol. 1991;9:145–151. doi: 10.1200/JCO.1991.9.1.145. [DOI] [PubMed] [Google Scholar]
- 23.Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, Veerman AJ. Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer. 2006;106:2067–2075. doi: 10.1002/cncr.21820. [DOI] [PubMed] [Google Scholar]
- 24.Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: a critical review of the literature. Pediatr Blood Cancer. 2009;52:447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 25.Lleras C. Do skills and behaviors in high school matter? The contribution of noncognitive factors in explaining differences in educational attainment and earnings. Social Science Research. 2008;37:888–902. [Google Scholar]
- 26.Krull KR, Brinkman TM, Li C, et al. Neurocognitive Outcomes Decades after Treatment for Childhood Acute Lymphoblastic Leukemia: A report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.48.2315. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenstein E, Bowers D, Verfaellie M, et al. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 30.Grasby PM, Frith CD, Friston KJ, et al. Functional mapping of brain areas implicated in auditory--verbal memory function. Brain. 1993;116(Pt 1):1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 32.Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 33.Shallice T, Fletcher P, Frith CD, et al. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Park KY, Seo SW, et al. Reversible verbal and visual memory deficits after left retrosplenial infarction. J Clin Neurol. 2007;3:62–66. doi: 10.3988/jcn.2007.3.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- 36.Spaniol J, Davidson PS, Kim AS, et al. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Golby AJ, Poldrack RA, Brewer JB, et al. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- 38.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 39.Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11:1096–1106. doi: 10.1016/S1470-2045(10)70114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaynon PS, Lustig RH. The use of glucocorticoids in acute lymphoblastic leukemia of childhood. Molecular, cellular, and clinical considerations. J Pediatr Hematol Oncol. 1995;17:1–12. doi: 10.1097/00043426-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Ito C, Evans WE, McNinch L, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. J Clin Oncol. 1996;14:2370–2376. doi: 10.1200/JCO.1996.14.8.2370. [DOI] [PubMed] [Google Scholar]
- 42.Kaspers GJ, Veerman AJ, Popp-Snijders C, et al. Comparison of the antileukemic activity in vitro of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;27:114–121. doi: 10.1002/(SICI)1096-911X(199608)27:2<114::AID-MPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.