Abstract
There are no effective and approved therapies against devastating ocular injuries caused by vesicating chemical agents sulfur mustard (SM) and nitrogen mustard (NM). Herein, studies were carried out in rabbit corneal cultures to establish relevant ocular injury biomarkers with NM for screening potential efficacious agents in laboratory settings. NM (100 nmol) exposure of the corneas for 2 h (cultured for 24 h), showed increases in epithelial thickness, ulceration, apoptotic cell death, epithelial detachment microbullae formation, and the levels of VEGF, cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9). Employing these biomarkers, efficacy studies were performed with agent treatments 2 h and every 4 h thereafter, for 24 h following NM exposure. Three agents were evaluated, including prescription drugs dexamethasone (0.1%; anti-inflammatory steroid) and doxycycline (100 nmol; antibiotic and MMP inhibitor) that have been studied earlier for treating vesicant-induced eye injuries. We also examined silibinin (100 µg), a non-toxic natural flavanone found to be effective in treating SM analog-induced skin injuries in our earlier studies. Treatments of doxycycline + dexamethasone, and silibinin were more effective than doxycycline or dexamethasone alone in reversing NM-induced epithelial thickening, microbullae formation, apoptotic cell death, and MMP-9 elevation. However, dexamethasone and silibinin alone were more effective in reversing NM-induced VEGF levels. Doxycycline, dexamethasone and silibinin were all effective in reversing NM-induced COX-2 levels. Apart from therapeutic efficacy of doxycycline and dexamethasone, these results show strong multifunctional efficacy of silibinin in reversing NM-induced ocular injuries, which could help develop effective and safe therapeutics against ocular injuries by vesicants.
Keywords: Nitrogen mustard, rabbit eye corneal culture, dexamethasone, doxycycline, silibinin, microbullae
Introduction
Vesicating agents could be a potential threat as both chemical warfare agents and terrorist weapons (Noort et al., 2002; Smith and Skelton, 2003; Saladi et al., 2006; Ganesan et al., 2010). Sulfur mustard (SM; 2,2-dichloroethyl sulfide) is the most widely used alkylating warfare agent, but nitrogen mustard (NM; bis 2-chloroethyl methylamine) stockpiled since World War II, also poses a similar threat (Olajos et al., 1998; Wang and Xia, 2007; Sharma et al., 2010). Studies in Iranian survivors exposed to SM during Iran-Iraq war in the 1980’s revealed that eye tissue is ~10 times more sensitive to vesicant exposure than skin and airway irritation, with devastating short-and long-term injuries (up to 40 years) affecting up to 90% of those exposed (Banin et al., 2003; Javadi et al., 2007; Milhorn et al., 2010; Javadi et al., 2011). Though immediate eye irritation can occur, ocular injury symptoms start 1–12 h post-SM exposure that include eyelid burns, inflammation, pain and visual deterioration, prolonged conjunctivitis, corneal opacity, corneal ulceration and blindness in severe cases (Javadi et al., 2005; Naderi et al., 2010; Javadi et al., 2011). Despite the imminent threat and devastating ocular injuries by exposure to vesicating agents, effective therapies suitable for deployment in the case of a mass casualty have not been established.
Limited published reports on the attempts to develop countermeasures against vesicants-induced ocular injury have shown the therapeutic potential of antibiotics doxycycline and Ilomastat, glucocorticoid dexamethasone and non-steroidal anti-inflammatory drug (NSAID) diclofenac (Amir et al., 2000; Banin et al., 2003; Morad et al., 2005; Kadar et al., 2009; Gordon et al., 2010). However, these agents have side effects and their efficacy has been studied in a limited manner only on some of the NM/SM ocular injury symptoms; associated mechanisms of efficacy have not been examined in detail. Therefore, mechanistic aspects of the vesicant-induced ocular injury need to be assessed in order to tailor effective mechanism-driven therapies. Both NM and SM are bi-functional alkylating agents. They are similar in causing severe ocular toxicity and histopathological features, suggesting commonalities in their injury-associated properties and mechanisms (Petrali et al., 2000; Kadar et al., 2001; Banin et al., 2003; Morad et al., 2005; Kadar et al., 2009; Gordon et al., 2010) including DNA damage either directly or via GSH depletion and formation of reactive oxygen and nitrogen (ROS and RNS) species (Matijasevic et al., 2001; Kehe and Szinicz, 2005; Korkmaz et al., 2006; Paromov et al., 2007; Kehe et al., 2008; Brimfield et al., 2009; Tewari-Singh et al., 2010a; Tewari-Singh et al., 2011). Hence, studies were conducted using NM to develop an efficient ocular injury model suitable for histopathological and mechanistic studies in the laboratory, and to enable us to establish some useful ocular injury biomarkers for screening effective therapeutic agents.
For efficacy studies herein, we employed dexamethasone (immunomodulatory glucocorticoid with anti-inflammatory effects) and doxycycline (tetracycline derivative and MMP inhibitor with potent anti-inflammatory effects), both of which have been examined earlier for reducing vesicant-induced and other ocular injuries (Amir et al., 2000; Morad et al., 2005; Kadar et al., 2009; Gordon et al., 2010). We further compared their effect with silibinin (strong antioxidant with anti-inflammatory properties), which was found to be significantly effective in treating SM analog-2-chloroethyl ethyl sulfide (CEES)- and NM-induced skin injuries in our recently completed studies (Tewari-Singh et al., 2010b). Silibinin, a polyphenolic flavanone isolated from milk thistle (Silybum maryanum) seeds, has pleiotropic effects, and an extensive history use in humans for its strong hepatoprotective activity against broad-range of liver toxicities (Pares et al., 1998; Singh and Agarwal, 2005; Pradhan and Girish, 2006; Singh and Agarwal, 2006; Deep and Agarwal, 2010). Silibinin is well tolerated following acute and chronic administration in both animals and humans, is efficacious against various cancers in animal models, and is currently being studied in clinical trials for prostate and skin cancers (Flaig et al., 2007; Cheung et al., 2010; Rajamanickam et al., 2010; Velmurugan et al., 2010; Ramasamy et al., 2011). To test the hypothesis that silibinin will be effective in the treatment of vesicant-induced ocular injuries, we examined the efficacy of silibinin, as well as its formulation, in treating NM-induced ocular injury. Since the eye is a sensitive tissue and topical drug bioavailability is poor due to multiple barriers, development of an appropriate topical formulation that can be effectively administered to the ocular tissue is imperative (Kompella et al., 2010).
In addition to the sclera and limbal region of the eye, the cornea is particularly sensitive to vesicant exposure (Javadi et al., 2007; Gordon et al., 2010; Milhorn et al., 2010). It has been reported that the gross pathology of SM-related rabbit eye lesions closely resembles lesions in humans following battlefield exposures (Petrali et al., 2000). Hence, we sought to establish quantifiable biomarkers and to conduct efficacy studies using the rabbit corneal organ culture model as a substitute for the in vivo rabbit corneas model reported earlier (Gordon et al., 2010). The data obtained from this study shows therapeutic efficacy of dexamethasone, doxycycline and silibinin against ocular injury by NM, and suggests that flavanone silibinin has potential to be developed as an effective and safe therapeutic agent for medical countermeasure against vesicant-induced ocular injury.
Materials and methods
Chemicals and reagents
New Zealand white rabbit eyes were purchased from Pel-Freez (Atlanta, GA). Dulbecco’s modified eagle’s medium(DMEM), 100× minimum essential medium-non essential amino acids (MEM-NEAA), 1× RPMI 1640 vitamin solution were obtained from Gibco BRL (Grand Island, NY). Ascorbic acid, ciprofloaxin, NM (mechlorethamine hydrochloride; 98%), doxycycline hyclate, dexamethasone (water-soluble), silibinin were purchased from Sigma-Aldrich Chemical Co. (St. Louis., MO). COX-2 antibody was from Cayman Chemicals (Ann Arbor, MI) and MMP-9 and VEGF antibodies were from Abcam Inc. (Canbridge, MA).
Corneal organ cultures and exposure
Corneas with scleral rim were dissected from the eyes of New Zealand white rabbits (8–12 weeks old; Pel-Freez) stored overnight in DMEM and antibiotics during transit to the laboratory. Dissected corneas were placed anterior side down in a glass spot plate containing DMEM, and their endothelial side filled with melted 0.75% agar (50°C). After the agar solidified, corneas were turned over and cultured at 37°C in a humidified 5% CO2 incubator in a 12 well plate using DMEM (high glucose) containing 1× MEM NEAA, 1× RPMI 1640 vitamins, 0.1 mg/ml ascorbic acid and 0.01 mg/ml ciprofloaxin antibiotic, and placed as reported earlier (Gordon et al., 2010). To induce corneal injury, the anterior surface was moistened with DMEM medium and exposed to 100 nmol NM (10 µl in the growth media drop wise on the central cornea) for 2 h, and then washed and cultured for 24 h. At the end of each exposure at 24 h study time point, the cornea was cut into four equal sections and one section fixed in 10% phosphate-buffered formalin, other sections were snap frozen in liquid nitrogen for other studies.
Treatments
The dissected rabbit corneas in culture were either untreated (UC), or treated with agents, added drop wise in 10 µl aliquots onto the central cornea after 2 h and every 4 h thereafter, for 24 h. The agents employed were 100 nmol doxycycline (in growth media), 0.1% dexamethasone (in growth media) alone or doxycycline+dexamethasone (in combination), or with 100 µg silibinin formulation or silibinin in DMSO alone. For the silibinin formulation , silibinin (5mg), Tween 80 (4µl), and hydroxyl propyl β cyclodextrin (HPβCD, 3.25 mg) were added to 100 µl of Tris base buffer (pH 8.0; vehicle) and kept overnight (14 h) under stirring at 37°C in a shaker incubator. At the end of 14 h, Tris base buffer (400µl) was added to the silibinin, tween 80 and HPβCD dispersion and vortexed for 10 min. The dispersion was further bath sonicated for 15 min. The final pH of the formulation was adjusted between 7.5 and 8.0.
Histopathological evaluation of corneal sections and measurement of epithelial thickness
At the end of each exposure or treatment (24 h study time point), cornea tissues were fixed in 10% phosphate-buffered formalin, then embedded in paraffin to produce 5 µm microtome sections. These were stained with haematoxylin and eosin (H&E) as detailed earlier (Tewari-Singh et al., 2009) and evaluated microscopically for epidermal thickness, microbullae formation, epidermal necrosis, and infiltration of inflammatory cells. The epidermal thickness (µm) was measured randomly in at least five fields per tissue sample from two sets of H&E stained slides (x400 magnification). The incidence of distinct microbullae (epithelial-stromal separations) formation at the junction of epithelial layer and dermis were measured per 6 cm area of the corneal section analyzed under 400× magnification and area measured using Axiovision Rel 4.5 software to classify into small (vesicle like; less than 50 µm2) or large (more than 50 µm2) epithelial-stromal separations.
Apoptotic cell detection via TUNEL staining
To detect and quantify apoptotic cell death in the untreated, exposed or treated corneas, we used the Dead-End Colorimetric terminal deoxynucleotidyl transferase (tdt)-mediated dUTP-biotin nick end labeling (TUNEL) system following the manufacturer’s protocol with some modifications as reported earlier (Tewari-Singh et al., 2009; Jain et al., 2011b). The brown colored TUNEL positive cells were quantified in 10 randomly selected fields at 400× magnification, and an apoptotic cell index was calculated as the number of apoptotic cells ×100 divided by total number of cells.
Immunohistochemistry for vascular endothelial growth factor (VEGF)
Paraffin embedded corneal sections (5 µm) were subjected to antigen retrieval and blocking of endogenous peroxidase activity as detailed earlier (Tewari-Singh et al., 2009; Jain et al., 2011b). The sections were then incubated with mouse monoclonal VEGF antibody in PBS for 2 h at 37°C in humidity chamber. The N-Universal negative control rabbit IgG antibody (DAKO) was used as a negative control. After washings in PBS, the sections were incubated with the appropriate biotinylated secondary antibody for 1 h followed by incubation with HRP conjugated streptavidin (DAKO) in PBS for 30 min at RT in a humidity chamber. The sections were then incubated in DAB working solution for 10 min at RT and counterstained with diluted hematoxylin for 2 min followed by dehydration and mounting for microscopic observation. The brown colored cytoplasmic staining for VEGF was scored as positivity score in 10 randomly selected fields (x400 magnification). This intensity of immunoreactivity seen as brown color was scored as 0 (no staining), +1 (weak staining), +2 (moderate staining), +3 (strong staining), and +4 (very strong staining).
Western Immunoblotting
Protein was isolated from the frozen corneal tissue and quantified using the Lowry method (Bio-Rad DC protein assay kit, Bio-Rad laboratories, Hercules, CA). For Western blots, 80 µg protein was denatured and subjected to SDS-PAGE, followed by transfer to nitrocellulose membranes as reported earlier (Pal et al., 2009; Tewari-Singh et al., 2010a). Membranes were blocked with 5% nonfat milk powder (w/v) or odyssey blocking buffer for 1 h at RT and probed with primary antibodies overnight at 4°C followed by peroxidase/ IRDye® 800CW conjugated appropriate secondary antibody for 1 h at room temperature. Membranes were then subjected to enhanced chemiluminescence (Amersham, Piscataway, NJ) detection or visualized using Odyssey™ Infrared Imager (LI-COR Biosciences Lincoln, NE). Protein loading was confirmed by stripping and re-probing the membranes with β-actin antibody. All the autoradiogram/bands were scanned using Adobe Photoshop 6.0 (Adobe Systems, Inc., San Jose, CA) and densitometric analysis of the protein bands was done by measuring the integrated density using the Scion Image Program (NIH, Bethesda, MD).
2.10. Microscopic and statistical analyses
All the H&E and IHC stained slides were observed under Zeiss Axioscop2 microscope (Carl Zeiss, Inc., Germany), and image analyses were done by using Carl Zeiss Axiovision Rel 4.5 software. The data were analyzed and all statistical calculations were done using SigmaStat software version 2.03 (Jandal Scientific Corp., San Raphael, CA). Data are expressed as mean ± SEM and were analyzed via one way ANOVA followed by the Bonferroni t-test for multiple comparisons. P< 0.05 was considered statistically significant.
Results
NM induces inflammation-related changes in the epithelium, microbullae formation, apoptotic cell death, increase in VEGF levels, and COX-2 and MMP-9 expression in the cultured rabbit eye cornea
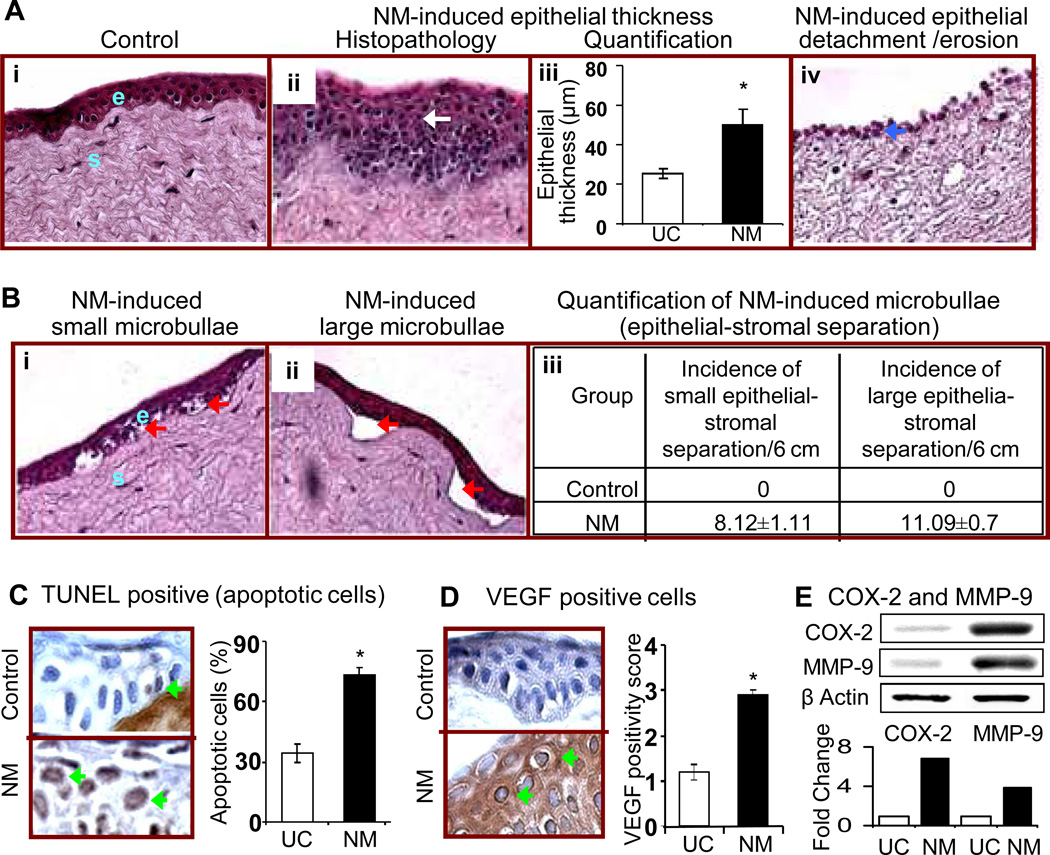
Vesicating agents are reported to cause clinical and histopathological changes related to inflammation and vesication in both eye and skin tissue (Javadi et al., 2007; Gordon et al., 2009; Tewari-Singh et al., 2009; Black et al., 2010; Gordon et al., 2010; Milhorn et al., 2010; Jain et al., 2011b; Joseph et al., 2011). The ocular lesions by vesicating agents mainly involve the cornea (Javadi et al., 2007; Gordon et al., 2009; Gordon et al., 2010; Milhorn et al., 2010). Hence, to investigate the toxic effects of NM on the rabbit corneal tissue, excised rabbit cornea in organ cultures were exposed to 100 nmol of NM for 2 h, washed and cultured for 24 h, H&E stained and examined for histopathological changes. Representative H&E stained corneal sections showed that NM induced a range of injury responses that varied across the corneal sections (Fig. 1). Results from the exposed animals included an increase in a) epithelial layer thickness (observed in 29 % of the epithelial layer; Fig. 1A, panel i), b) ulceration, epithelial detachment/denuding erosion (observed in 33 % of the epithelial layer; Fig. 1A, panels ii and iv), and c) microbullae formation [small (vesicle like; less than 50 µm2) or large (more than 50 µm2) separation of epithelial (e) and stromal (s) layers as observed in 35 % of the epithelial layer; Fig. 1B, panels i and ii, red arrows)]. Our earlier studies have established epidermal thickness and vesication as biomarkers of vesicating agent-induced skin injury (Tewari-Singh et al., 2010a; Jain et al., 2011b). In addition, histopathological examination detailed above evidenced these consequences following NM exposure in the cornea; hence, we quantified these events to establish quantifiable biomarkers of ocular injury. NM exposure doubled the epithelial thickness of the cornea from 25.46 ± 2.36 µm in the untreated control group to 50.28 ± 7.6 µm in the NM exposed corneas (Fig. 1A, panel iii). NM exposure also resulted in 8.12 ± 1.1 incidences of small (less than 50 µm2) and 11.09 ± 0.69 incidences of large (more than 50 µm2) microbullae formations per 6 cm area of the corneal section analyzed under 400× magnification (Fig. 1B, panel iii).
Figure 1.
Effect of topical application of NM on histopathological and molecular responses related to inflammation, vesication and neovascularization in cultured rabbit eye cornea. Corneas with scleral rim were dissected from the eyes of New Zealand white rabbits (8–12 weeks old) and cultured O/N as detailed under ‘Materials and Methods’. The excised rabbit corneas in organ cultures were exposed to 100 nmol of NM for 2 h, washed and cultured for 24 h, fixed, and five µm sections were either H&E stained (A and B), TUNEL stained (C), or subjected to IHC staining for VEGF (D). H&E stained sections from the cornea were evaluated for the histopathological changes. Representative H&E stained corneal sections are shown in A (panels i, ii and iv) and B (panels i and ii). The epithelial thickness (µm) was measured (400× magnification; A, panel iii) and incidence of microbullae formation were measured per 6 cm epithelial section at 400× magnification and classified into small (vesicle like; less than 50 µm2) or large (more than 50 µm2) separations (B, panel ii) as detailed under ‘Materials and Methods’. Percent TUNEL positive cells were calculated from the TUNEL stained corneal sections seen in the representative pictures as detailed under ‘Materials and Methods’ in 10 randomly selected fields (×400 magnification; C). The brown colored cytoplasmic staining for VEGF was scored from the stained corneal sections seen in the representative pictures as detailed under ‘Materials and Methods’ in 10 randomly selected fields (×400 magnification; D). Following 100 nmol NM exposure for the above mentioned duration, equal amount of protein from the frozen corneal tissue as detailed under ‘Materials and Methods’ was subjected to western immunoblotting for COX-2 and MMP-9, and membranes were then stripped and reprobed for β actin as protein loading control. All the autoradiogram/bands were scanned using Adobe Photoshop and fold change compared to the control treatment was calculated from the integrated density of the protein bands, which was done as described in the ‘Materials and Methods’ (E). Data presented are mean ± SEM (n=3). *, p<0.05 as compared to respective control group. White arrow, epithelial thickness; red arrows, microbullae formation; blue arrows, epithelial necrosis, ulceration and loss of the epithelial layer; green arrows, TUNEL and VEGF positive cells (brown stained).
Since vesicating agents are reported to induce apoptotic cell death, which plays an important role in inflammation and wound healing process (Paromov et al., 2007; Tewari-Singh et al., 2010a; Jain et al., 2011b; Joseph et al., 2011), we sought to quantify this effect of NM on the epithelial layer of the cornea. Compared to 35% apoptotic cells in the untreated control epithelial layer of the cornea, NM exposure resulted in 73% TUNEL positive apoptotic epithelial cells (Fig. 1C). Apoptosis was also observed in stromal fibroblasts (keratocytes); however, dark staining of stromal fibrous layer masked its quantification (data not shown).
The ocular manifestation of SM includes neovascularization of the cornea, a major consequence that may enhance scaring, edema and inflammation of the cornea (Banin et al., 2003; Gordon et al., 2009; Kadar et al., 2009) . VEGF is known to play an important role in corneal neovascularization (Chang et al., 2001); however, neovascularization cannot be observed in the cultured corneas; therefore, we next examined the effect of NM on VEGF levels in the epithelial cells. The IHC analysis of the corneal sections showed that NM exposure resulted in increased VEFG levels recording VEGF positivity score of 2.9 ± 0.1 as compared to 1.2 in the untreated control corneas (Fig. 1D).
We observed an increased corneal inflammatory response and epithelial stromal separation following NM exposure that are also significant effects of NM exposure in the skin injury by vesicants (Cheung et al., 2010). Therefore, we next evaluated the effect of NM on the expression of COX-2 (key inflammatory mediator in vesicant skin injury involved in prostaglandin synthesis) and MMP-9 (epithelial-stromal separations in vesicant skin injury are mainly due to the degradation of the basement membrane components mediated by MMPs) (Shakarjian et al., 2010; Jain et al., 2011a). In the current study, western immunoblotting of the cultured rabbit corneal tissue exposed to 100 nmol NM exposure showed a 6.8 and 3.8 fold increase in the expression levels of COX-2 and MMP-9, respectively (Fig. 1E).
Effect of doxycycline, dexamethasone alone or in combination with doxycycline, and silibinin on NM-induced biomarkers of ocular injury in cultured rabbit eye cornea
Study of the biological and molecular consequences of NM exposure in the rabbit corneal cultures described above showed increased epithelial thickness, epithelial-stromal detachments (microbullae formation), epithelial cell death, increase in the VEGF levels, and increase in the COX-2 and MMP-9 expression. Employing these quantifiable biomarkers, we next conducted efficacy studies with the prescription drugs namely dexamethasone and doxycycline, both of which have shown potential in the protection and treatment of vesicant-induced ocular injuries (Amir et al., 2000; Gordon et al., 2010). In addition, we also examined the therapeutic efficacy of silibinin, a natural flavanone with anti-inflammatory and antioxidant properties. The efficacy studies were carried out in the dissected rabbit corneas in culture that were either untreated, or treated with 100 nmol doxycycline, 0.1% dexamethasone alone or in combination, or with 100 µg silibinin formulation or silibinin (in DMSO) alone, after 2 h and every 4 h thereafter, for 24 h following 100 nmol NM exposure.
Epithelial thickness and incidence of microbullae formation
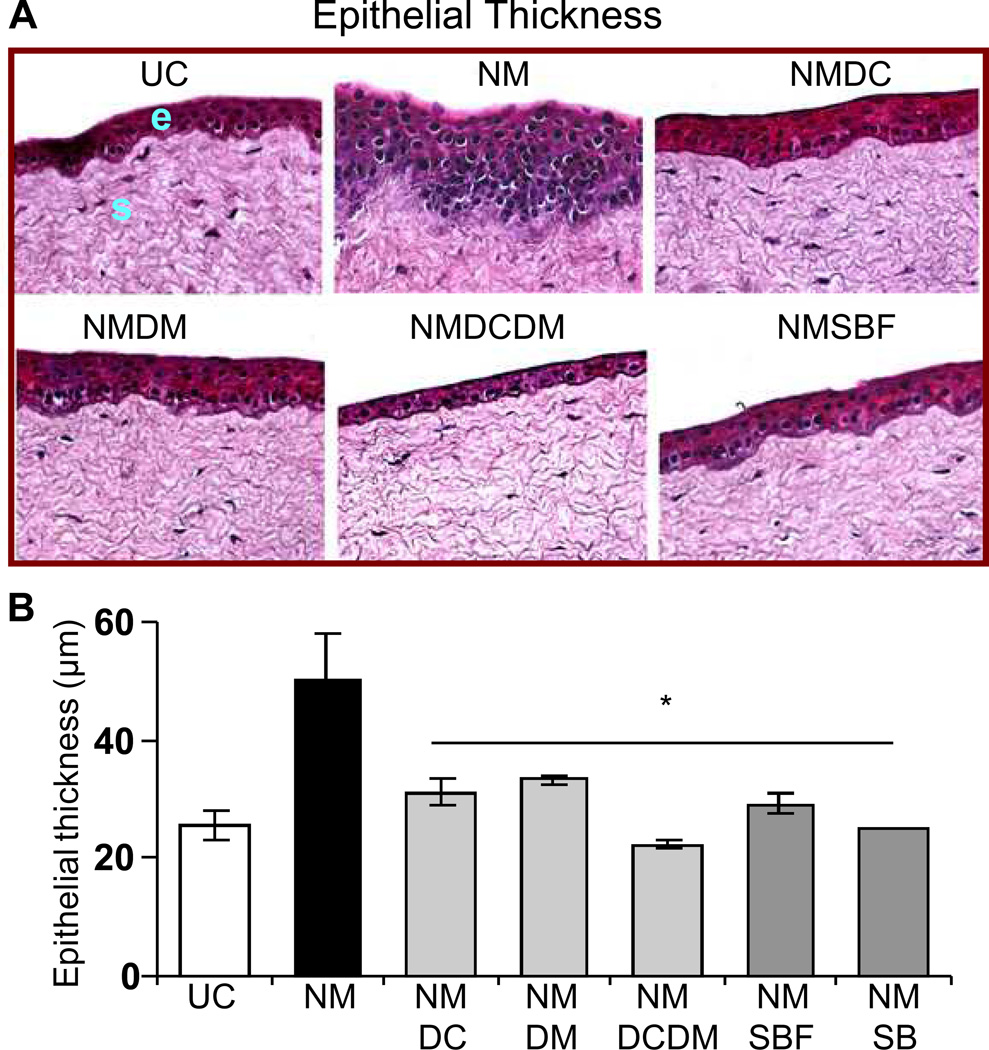
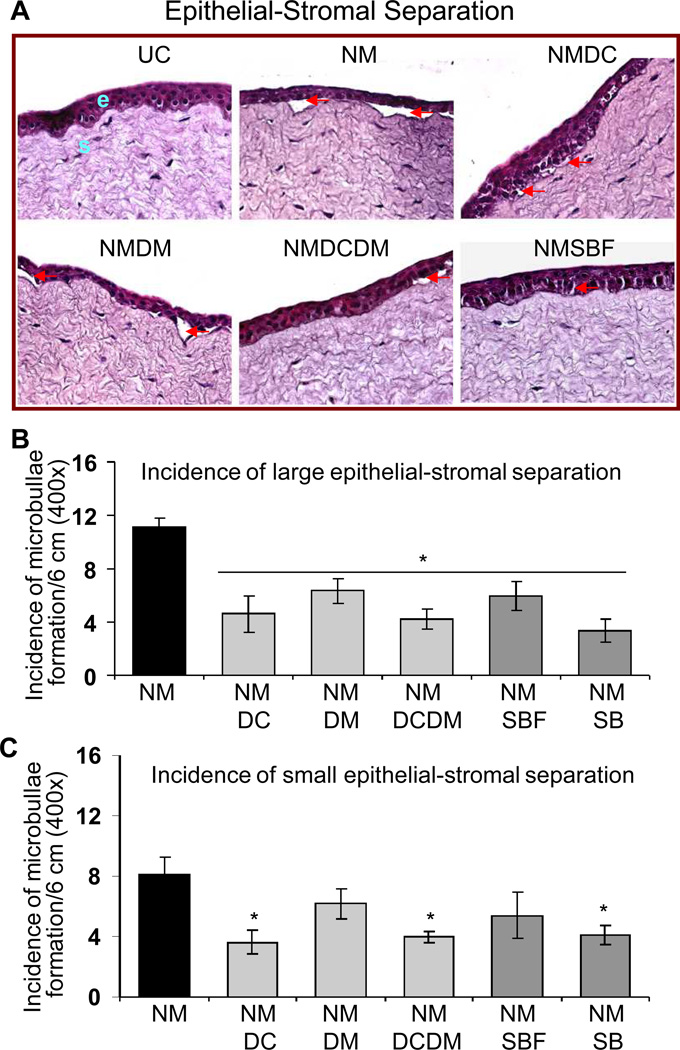
As seen in the representative corneal sections, all the treatments resulted in amelioration of NM-induced epithelial thickness (Fig. 2A). Epithelial measurements evidenced over 50% reversal (p<0.05) in NM-induced increase in epithelial thickness with all the treatments; however, complete reversal was observed with combination treatment of doxycycline + dexamethasone and silibinin alone (Fig. 2B). The representative H&E stained pictures show that treatments following NM exposure resulted in reduction of incidence of NM-induced microbullae formation (Fig. 3A). However, treatments of doxycycline, doxycycline + dexamethasone and silibinin were more effective and showed 50% (p<0.05) or more reversal in NM-induced incidences of large and small epithelial-stromal separations (Fig. 3). Moreover, only these three treatments were also significant (p<0.05) in reversing the NM-induced small epithelial-stromal separations (Fig. 3B). Silibinin alone here was more effective than its formulation in the reversal of both epithelial thickness and microbullae formation (Figs. 2 and 3).
Figure 2.
Effect of doxycycline, dexamethasone treatments alone or in combination, and silibinin or silibinin formulation treatments on NM-induced epithelial thickness in cultured rabbit eye cornea. The dissected rabbit corneas in culture were either untreated (UC), or treated with 10 µl of the agents drop wise on the central cornea after 2 h and every 4 h thereafter, for 24 h following 100 nmol NM exposure as detailed under ‘Materials and Methods’. Following the NM exposure and treatments, the corneas were fixed, five µm sections were H&E stained and evaluated for the histopathological changes. Representative H&E stained sections are shown in A. The epithelial thickness (µm) was measured randomly in at least five fields per tissue sample from two sets of H&E stained slides (×400 magnification; B) as detailed under ‘Materials and Methods’. Data presented are mean ± SEM (n=3). *, p<0.05 as compared to respective NM exposed group. e, epithelial layer; s, stroma; UC, untreated control; NM, nitrogen mustard; NMDC, nitrogen mustard+doxycycline; NMDM, nitrogen mustard+dexamethasone; NMDCDM, nitrogen mustard+doxycycline +dexamethasone; NMSBF, nitrogen mustard+silibinin formulation; NMSB, nitrogen mustard+silibinin in DMSO.
Figure 3.
Effect of doxycycline, dexamethasone treatments alone or in combination, and silibinin or silibinin formulation treatments on NM-induced microbullae formation (epithelial-stromal separation) in cultured rabbit eye cornea. The dissected rabbit corneas in culture were either untreated (UC), or treated with 10 µl of the agents drop wise on the central cornea after 2 h and every 4 h thereafter, for 24 h following 100 nmol NM exposure as detailed under ‘Materials and Methods’. Following the NM exposure and treatments, the corneas were fixed, five µm sections were H&E stained and evaluated for the histopathological changes. Representative H&E stained sections are shown in A. Incidence of microbullae formation were measured per 6 cm epithelial section at 400× magnification and classified into large (more than 50 µm2; B) or small (vesicle like; less than 50 µm2; C) separations as detailed under ‘Materials and Methods’. Data presented are mean ± SEM (n=3). *, p<0.05 as compared to respective NM exposed group. e, epithelial layer; s, stroma; UC, untreated control; NM, nitrogen mustard; NMDC, nitrogen mustard+doxycycline; NMDM, nitrogen mustard+dexamethasone; NMDCDM, nitrogen mustard+doxycycline +dexamethasone; NMSBF, nitrogen mustard+silibinin formulation; NMSB, nitrogen mustard+silibinin in DMSO.
Apoptotic cell death and VEGF expression
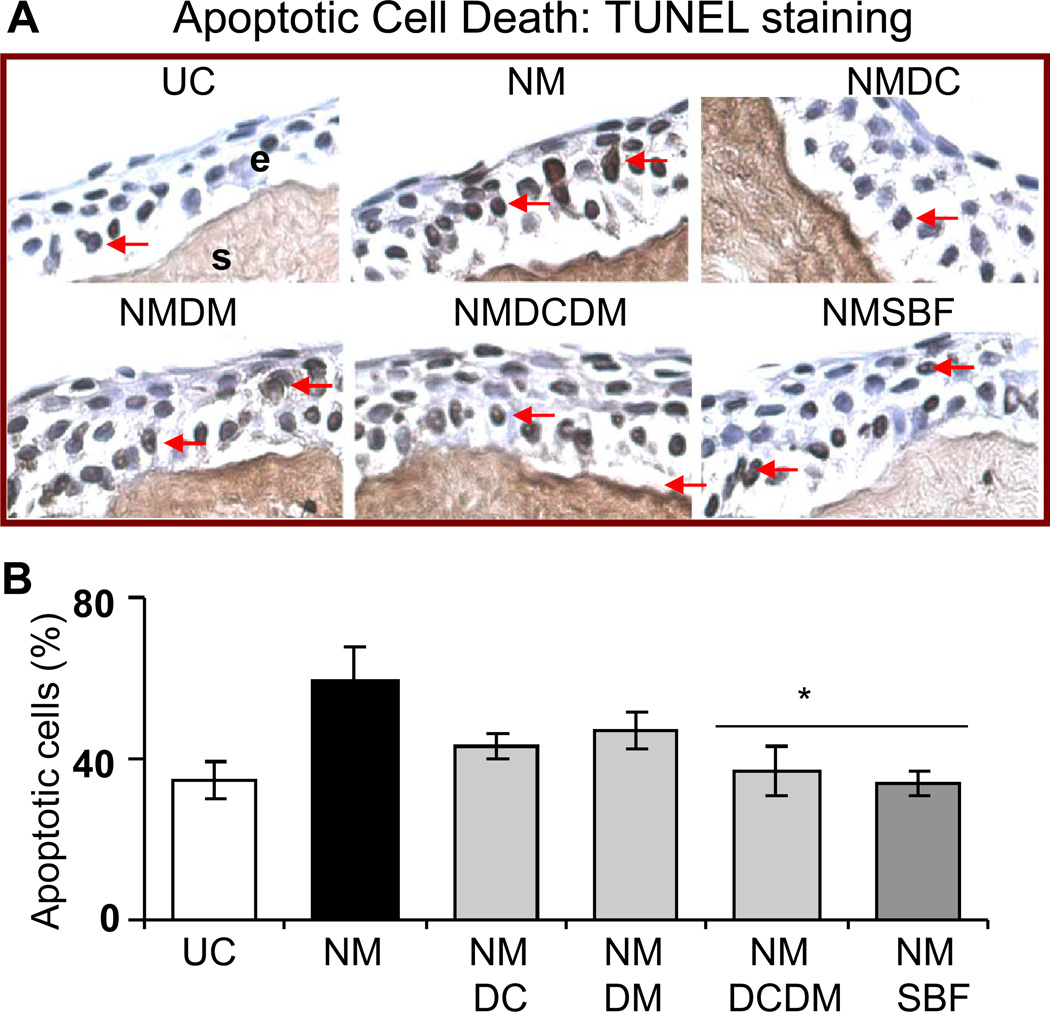
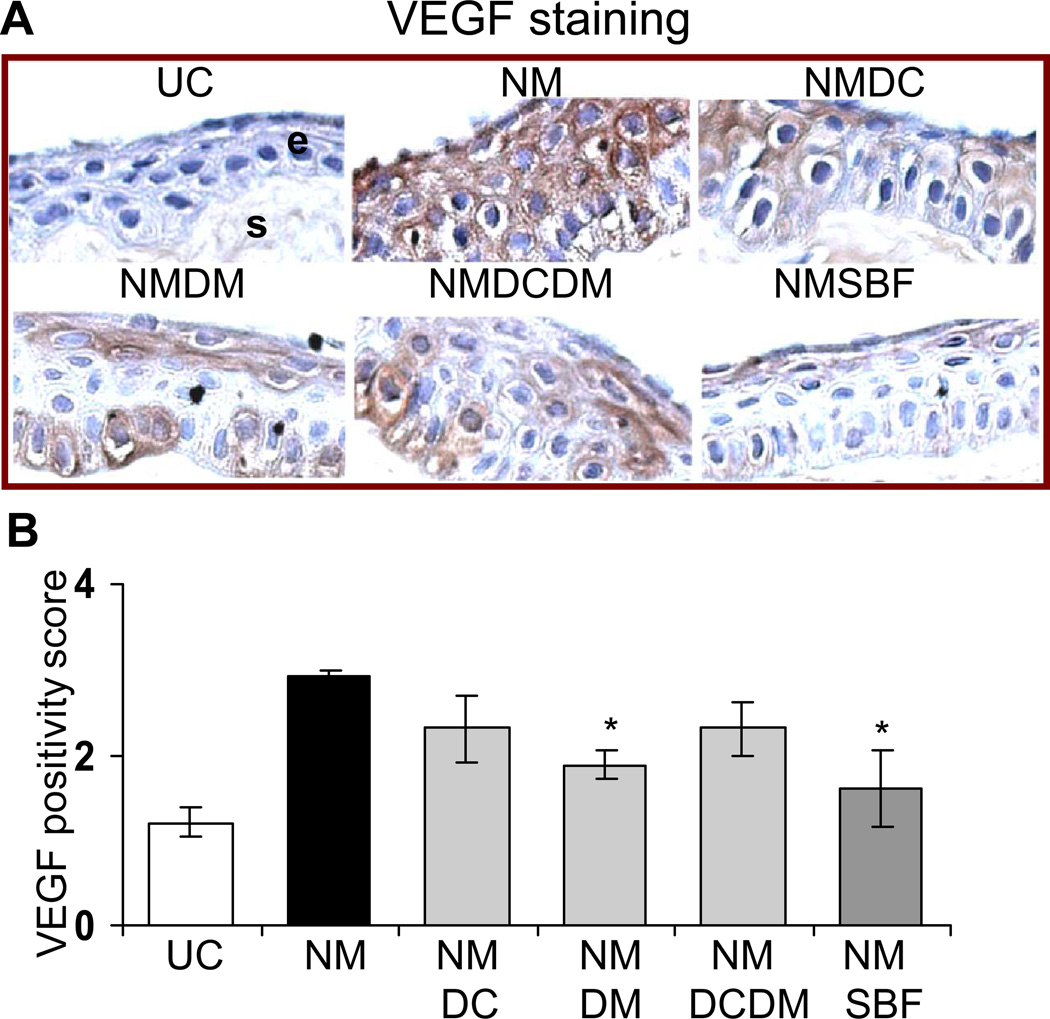
Quantification of the TUNEL stained corneal sections shown in the representative pictures (Fig. 4A) demonstrated 93% (p<0.05) and complete reversal in NM-induced apoptotic cell death with doxycycline + dexamethasone and silibinin treatments, respectively (Fig. 4B). The VEGF staining showed a decrease in NM-induced VEGF levels in the corneal epithelium following agent treatments (Fig. 5A). The quantification of the VEGF score in the corneal epithelial cells showed a significant 60 and 76% (p<0.05) reversal in NM-caused increase in VEGF levels with dexamethasone and silibinin formulation treatments, respectively (Fig. 5B). A significant (p<0.05) difference between the silibinin alone treatment and silibinin formulation treatment was not observed in the reversal of NM-induced apoptotic cell death and VEGF expression, therefore results from the silibinin formulation treatments are shown in Figs. 4 and 5. .
Figure 4.
Effect of doxycycline, dexamethasone treatments alone or in combination, and silibinin formulation treatment on NM-induced apoptotic cell death in cultured rabbit eye corneal epithelium. The dissected rabbit corneas in culture were either untreated (UC), or treated with 10 µl of the agents drop wise on the central cornea after 2 h and every 4 h thereafter, for 24 h following 100 nmol NM exposure as detailed under ‘Materials and Methods’. Following the NM exposure and treatments, the corneas were fixed, five µm sections were TUNEL stained and evaluated for the apoptotic cell death. Representative TUNEL stained corneal sections are shown in A. Percent TUNEL positive cells were calculated from the TUNEL stained corneal sections seen in the representative pictures as detailed under ‘Materials and Methods’ in 10 randomly selected fields (×400 magnification; B). Data presented are mean ± SEM (n=3). *, p<0.05 as compared to respective NM exposed group. e, epithelial layer; s, stroma; UC, untreated control; NM, nitrogen mustard; NMDC, nitrogen mustard+doxycycline; NMDM, nitrogen mustard+dexamethasone; NMDCDM, nitrogen mustard+doxycycline +dexamethasone; NMSBF, nitrogen mustard+silibinin formulation.
Figure 5.
Effect of doxycycline, dexamethasone treatments alone or in combination, and silibinin formulation treatment on NM-induced VEGF expression in cultured rabbit eye corneal epithelium. The dissected rabbit corneas in culture were either untreated (UC), or treated with 10 µl of the agents drop wise on the central cornea after 2 h and every 4 h thereafter, for 24 h following 100 nmol NM exposure as detailed under ‘Materials and Methods’. Following the NM exposure and treatments, the corneas were fixed, five µm sections were IHC stained with the VEGF antibody and evaluated for the cytoplasmic VEGF staining. Representative VEGF stained corneal sections are shown in A. The brown colored cytoplasmic staining for VEGF was scored from the stained corneal sections seen in the representative pictures as detailed under ‘Materials and Methods’ in 10 randomly selected fields (×400 magnification; B). Data presented are mean ± SEM (n=3). *, p<0.05 as compared to respective NM exposed group. e, epithelial layer; s, stroma; UC, untreated control; NM, nitrogen mustard; NMDC, nitrogen mustard+doxycycline; NMDM, nitrogen mustard+dexamethasone; NMDCDM, nitrogen mustard+doxycycline +dexamethasone; NMSBF, nitrogen mustard+silibinin formulation.
COX-2 and MMP-9 levels
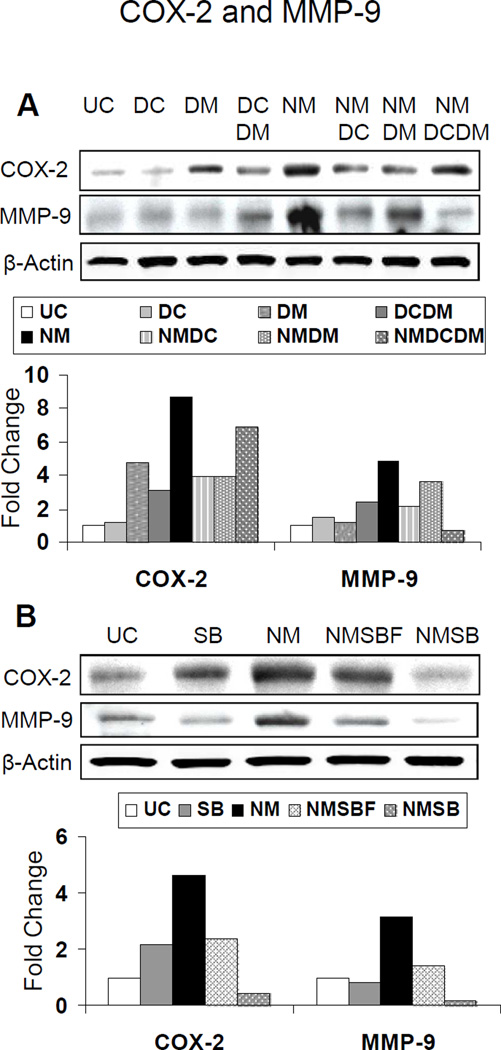
Treatments with doxycycline and dexamethasone alone, compared to their combination, were more effective resulting in over 60% reversal of NM-induced COX-2 levels. However, dexamethasone + doxycycline treatment was more effective and caused complete reversal of NM-induced MMP-9 levels than treatment with each of these alone (Fig. 6A). Treatment with doxycycline, an MMP inhibitor, resulted in 69% reversal in NM-induced MMP-9 levels as compared to dexamethasone alone, which caused only 30% reversal of this NM-induced effect (Fig. 6A). Silibinin treatment was also effective and resulted in complete reversal of NM-induced COX-2 levels whereas silibinin formulation caused 62% reversal (Fig. 6B). Similarly, both silibinin alone and its formulation treatments resulted in 82% and complete reversal of NM-induced MMP-9 levels, respectively (Fig. 6B).
Figure 6.
Effect of doxycycline, dexamethasone treatments alone or in combination (A), and silibinin or silibinin formulation treatments (B) on NM-induced protein expression of COX-2 and MMP-9 in cultured rabbit eye cornea. The dissected rabbit corneas in culture were either untreated (UC), or treated with 10 µl of the agents dropwise on the central cornea after 2 h and every 4 h thereafter, for 24 h following 100 nmol NM exposure as detailed under ‘Materials and Methods’. Following 100 nmol NM exposure for the above mentioned duration, equal amount of protein from the frozen corneal tissue as detailed under ‘Materials and Methods’ was subjected to western immunoblotting for COX-2 and MMP-9, and membranes were then stripped and reprobed for β actin as protein control. All the autoradiogram/bands were scanned using Adobe Photoshop and fold change compared to the control treatment was calculated from the integrated density of the protein bands, which was done as described in the ‘Materials and Methods’ (E). UC, untreated control; DC, doxycycline; DM, dexamethasone; DCDM, doxycycline +dexamethasone; NM, nitrogen mustard; NMDC, nitrogen mustard+doxycycline; NMDM, nitrogen mustard+dexamethasone; NMDCDM, nitrogen mustard+doxycycline +dexamethasone; SB, silibinin; NMSBF, nitrogen mustard+silibinin formulation; NMSB, nitrogen mustard+silibinin in DMSO.
In all these studies, doxycycline, dexamethasone or silibinin alone did not show any injury effects, and treatment with silibinin vehicles (formulation vehicle and DMSO) alone after NM did not cause measurable reversal in NM-induced injury endpoints (data not shown).
Discussion
There has been limited research to identify and validate effective therapies to minimize pain and interrupt permanent tissue damage resulting from mustard agent-induced ocular injuries following a large scale attack (Amir et al., 2000; Banin et al., 2003; Gordon et al., 2010; Milhorn et al., 2010; Javadi et al., 2011). Current countermeasures available to rescue ocular injuries by vesicants are mainly symptomatic and supportive, and use of most of these agents are marred with some degree of side effects (Banin et al., 2003; Petersen et al., 2008; Kadar et al., 2009; Gordon et al., 2010). Hence, to identify effective and safe therapies against vesicant-induced ocular injuries, we established ocular injury biomarkers in rabbit corneal organ culture with NM. Employing this ocular injury model, our studies show the therapeutic efficacy of doxycycline and dexamethasone and confirm previous observations that these agents reduce some histopathological changes in cornea tissue exposed to vesicating agents. We now demonstrate that the polyphenolic flavanone silibinin is effective in reversing all NM-induced injury endpoints related to inflammation, vesication and neovascularization, suggesting some potential for future therapeutic use of this compound against ocular injury by vesicants.
Because of a good correlation between human and animal eye, especially rabbit eye exposure to vesicants, these studies offer a valuable tool for identifying therapies against ocular injuries by vesicants (Gordon et al., 2009). Rabbit corneal organ cultures are used for corneal wound healing studies, and injuries in corneal organ cultures resemble those occurring in vivo in the corneas of rabbit eyes (Foreman et al., 1996; Gordon et al., 2010). As demonstrated in our current study and reported earlier, this ocular injury model could serve as an ideal cost effective in vitro system in laboratory settings to establish injury, and evaluate and optimize therapeutic agents before testing in SM ocular injury model (Gordon et al., 2010). Both NM and SM are bi-functional alkylating agents that cause severe ocular toxicity with delayed vesicating effects and show similar histopathological changes as well as clinical manifestations (Smith et al., 1998; Banin et al., 2003; Morad et al., 2005). This signifies the fact that with the caveat of SM being not feasible for laboratory settings, NM is the ideal primary vesicant for studies in laboratory setting. A study with vesicating agents in rabbit corneal cultures has been reported with detailed histologic examination showing comparable NM- and SM-induced ocular injuries (Gordon et al., 2010); however, quantitative data on biological changes and related mechanistic aspects are not available. As reported earlier by Gordon et al.(Gordon et al., 2010), the current study also shows that NM-induced injury is variable with areas in the cornea that have evident microbullae with epithelial depressions into the stroma (like microvesication observed in the skin tissue), epithelium completely separated/detached or denuded from the stromal region and regions with epithelial thickness. Our findings with NM in the ocular tissue also corroborate with the other reports on SM exposures that show histopathological changes of corneal thinning and ulceration, keratocyte death, inflammation, stromal neovascularization, and vesication seen as microbullae formation (Amir et al., 2000; Javadi et al., 2005; Javadi et al., 2007; Gordon et al., 2010; Milhorn et al., 2010). Since, inflammation and vesication are key events that occur several hours following vesicant exposure, our earlier studies in the skin tissue established epidermal thickness, apoptotic cell death, microvesication as well as induction of inflammatory mediators COX-2 and vesication-related MMP-9 as quantifiable biomarkers following vesicant exposure (Tewari-Singh et al., 2009; Jain et al., 2011a; Jain et al., 2011b). Similar to our studies in the skin tissue, inflammation and microbullae formation have been reported following NM and SM exposure in the ocular tissue. The current study for the first time established quantifiable biomarkers related to these events following NM exposure in corneal organ cultures, namely a) epithelial thickness, b) microbullae formation, c) apoptotic cell death, and d) expression of COX-2 and MMP-9. In addition, we quantified the angiogenic factor VEGF (that promotes ocular neovascularization) (Chang et al., 2001; Gordon et al., 2009) as an injury biomarker following NM exposure. This factor could serve as an important biomarker since corneal neovascularization is the chronic and delayed onset of vesicant ocular injury reported in several studies (Kadar et al., 2001; Richter et al., 2006; Gordon et al., 2009) but cannot be studied in the corneal cultures.
Similar to the inflammatory response in the skin tissue, studies in the ocular tissue herein demonstrate a role of COX-2 in vesicant-mediated ocular injury, which is a key protein involved in the inflammatory and cytotoxic responses and mediates the generation of prostaglandins (Kehe et al., 2009; Missel et al., 2010; Shakarjian et al., 2010; Jain et al., 2011a). MMPs (gelatinases), mainly MMP-9 and MMP-2, implicated in several blistering diseases and vesicant injury play a major role in the degradation of the basement membrane components causing disruption of the epidermal-dermal junction in the skin (Kehe and Szinicz, 2005; Kehe et al., 2009; Shakarjian et al., 2010; Jain et al., 2011a). In agreement with the earlier reports, the current study shows that MMP-9 plays a key role in the NM-induced separation of epithelial-stromal junction in the ocular tissues (Gordon et al., 2010). Based on the reported cutaneous and ocular studies as well as results from the studies herein, it is evident that inflammation and vesication related pathways play a key role in the vesicating agent-induced ocular injury. Hence, we chose to use dexamethasone and doxycycline, which have been earlier studied and shown to reduce injury symptoms by the vesicating agents, NM and SM (Amir et al., 2000; Banin et al., 2003; Kadar et al., 2009; Gordon et al., 2010). Dexamethasone is an approved drug used to treat corneal burns and is reported to exert immunosuppressive and anti-inflammatory effects (Chung et al., 1999; Kimura et al., 2011) and doxycycline is a tetracycline derivative that is a broad-spectrum antibiotic can chelate metal ions, and has the ability to inhibit MMPs and synthesis of MMPs and IL-1(Solomon et al., 2000; Smith and Cook, 2004). The studies herein demonstrate that treatments combining doxycycline and dexamethasone were more effective than doxycycline or dexamethasone alone in reversing most of the NM-induced injury markers such as epithelial thickness, microbullae formation, apoptotic cell death, and MMP-9 levels. This could be due to the combined effects of these two drugs where dexamethasone is reported to exhibit anti-inflammatory and immunosuppressive effects (Kimura et al., 2011), and MMP inhibitor doxycycline is shown to possess anti-inflammatory, anti-apoptotic and antioxidant properties (Yeh et al., 2007). Also, combination of these two drugs is beneficial as doxycycline is an antibiotic that might help in the treatment of any secondary infections, which could arise due to vesicant ocular injury. The decrease in the vesicant-induced MMP-9 expression in corneal organ and mouse cultures following doxycycline is reported; however, this study presents quantitative efficacy data with doxycycline and expression levels of MMP-9. Although, the role of doxycycline in the inhibition of angiogenesis is reported (Cox et al., 2010; Gordon et al., 2010), the current study shows that dexamethasone alone was more effective in reversing NM-induced VEGF expression.
Although, our studies show therapeutic effects of dexamethasone and doxycycline in NM-induced ocular injury, the use of these drugs is accompanied by some side effects. Therefore, to identify the efficacy of a non-toxic natural agent that can target multiple pathways involved in the vesicant ocular injury, we tested and compared the efficacy of silibinin, which was found to be effective in the treatment of vesicant-induced skin injury in our earlier studies (Tewari-Singh et al., 2010b) (unpublished data). Silibinin, a polyphenolic, non-toxic plant flavonoid possesses strong anti-inflammatory, antioxidant, and anti-angiogenic properties (Singh and Agarwal, 2005; Singh and Agarwal, 2009; Deep and Agarwal, 2010). It is known to target various signaling pathways including MAPKs, Akt, AP-1, NF-κB, COX-2, iNOS, MMP-9 that could lead to tissue damage and inflammatory responses (Singh and Agarwal, 2005; Deep and Agarwal, 2010). Our recent studies show that silibinin treatment significantly reverses vesicant-induced oxidative stress, vesication, inflammatory responses (including skin bi-fold and epidermal thickness, apoptotic cell death and neutrophil infiltration), iNOS, COX-2 and MMP-9 induction, activation of NF-κB and AP-1, and oxidative DNA damage in mouse skin tissue (unpublished data). Overall, these are critical events in vesicant-induced injuries, and these complex pathways directly or via oxidative stress are found to be involved in CEES-induced skin injury in our earlier studies (Pal et al., 2009; Inturi et al., 2011; Jain et al., 2011a; Tewari-Singh et al., 2011), and could be involved in the vesicant-induced ocular injuries. Limited earlier studies suggest that inflammatory cytokines, chemokines, MMPs, oxidative stress, and angiogenic pathways could play an important role in ocular injuries by vesicants (Banin et al., 2003; Ruff and Dillman, 2007; Rebholz et al., 2008; Pal et al., 2009; Gordon et al., 2010; Shakarjian et al., 2010; Javadi et al., 2011). Since, silibinin possesses pleiotropic properties, studies herein show strong therapeutic efficacy of silibinin in reversing NM-induced injurious effects related to inflammation, vesication and neovascularization, further indicating that skin and ocular tissue injuries by vesicating agents involve somewhat similar signaling pathways. In these studies, silibinin alone in DMSO was more effective than its formulation in reversing NM-induced epithelial thickness and microbullae formation as well as the COX-2 and MMP-9 expression. Silibinin is a poorly water-soluble drug and cannot be delivered in organic solvents like DMSO used in this study. Earlier reports and studies suggest that drug-cyclodextrin complexation can increase ocular drug bioavailability and increase drug solubility, which has been obtained for dexamethasone, cyclosporine etc.(Loftsson et al., 1994; Kristinsson et al., 1996; Bary et al., 2000). Therefore, use of cyclodextrin in this study for an appropriate formulation development is a useful method to increase the aqueous solubility as well as bioavailability of silibinin. However, further optimization of this formulation or added nanaoparticle approach (Kompella et al., 2010) for silibinin is required to obtain an effective, safe topical treatment with optimal bioavailability and drug solubility for its enhanced therapeutic effect against vesicant ocular injuries.
Together, these results show strong efficacy of dexamethasone and doxycycline, and flavanone silibinin in reversing various attributes of NM-caused ocular injuries. Noticeably, since the drugs tested so far have other adverse effects in humans, these studies demonstrate strong potential of silibinin to be further optimized and developed as a safe, effective and easily administrable therapeutics against ocular injuries by vesicants. This is the first accelerated attempt to develop an ocular silibinin formulation, and detailed studies are needed in in vivo animal models to develop a safe and effective formulation of silibinin for treating vesicant-induced ocular injuries.
Acknowledgements
This work was supported by supplemental grant from the Department of Pharmaceutical Sciences, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. The study sponsor had no involvement in the study design; collection, analysis and interpretation of data; the writing of the manuscript; and the decision to submit the manuscript for publication
Abbreviations
- CEES
2-chloroethyl ethyl sulfide
- COX-2
cyclooxygenase-2
- DMEM
Dulbecco’s modified eagle’s medium
- H&E
haematoxylin and eosin
- iNOS
inducible nitric oxide
- MEM-NEAA
minimum essential medium-non essential amino acids
- MMP-9
matrix metalloproteinase-9
- NM
nitrogen mustard (bis 2-chloroethyl methylamine)
- NSAID
non-steroidal anti-inflammatory drug
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SM
Sulfur mustard (2,2,dichloroethyl sulfide)
- TUNEL
terminal deoxynucleotidyl transferase (tdt)-mediated dUTP-biotin nick end labeling
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Contributor Information
Neera Tewari-Singh, Email: Neera.Tewari-Singh@ucdenver.edu.
Anil K Jain, Email: Anil.Jain@ucdenver.edu.
Swetha Inturi, Email: Swetha.Inturi@ucdenver.edu.
David A Ammar, Email: David.Ammar@ucdenver.edu.
Chapla Agarwal, Email: Chapla.Agarwal@ucdenver.edu.
Puneet Tyagi, Email: Puneet.Tyagi@ucdenver.edu.
Uday B Kompella, Email: Uday.Kompella@ucdenver.edu.
Robert W Enzenauer, Email: Robert.Enzenauer@ucdenver.edu.
J Mark Petrash, Email: Mark.Petrash@ucdenver.edu.
References
- Amir A, Turetz J, Chapman S, Fishbeine E, Meshulam J, Sahar R, Liani H, Gilat E, Frishman G, Kadar T. Beneficial effects of topical anti-inflammatory drugs against sulfur mustard-induced ocular lesions in rabbits. Journal of applied toxicology : JAT. 2000;20(Suppl 1):S109–S114. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat669>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Banin E, Morad Y, Berenshtein E, Obolensky A, Yahalom C, Goldich J, Adibelli FM, Zuniga G, DeAnda M, Pe'er J, Chevion M. Injury induced by chemical warfare agents: characterization and treatment of ocular tissues exposed to nitrogen mustard. Investigative ophthalmology & visual science. 2003;44:2966–2972. doi: 10.1167/iovs.02-1164. [DOI] [PubMed] [Google Scholar]
- Bary AR, Tucker IG, Davies NM. Considerations in the use of hydroxypropyl-beta-cyclodextrin in the formulation of aqueous ophthalmic solutions of hydrocortisone. Eur J Pharm Biopharm. 2000;50:237–244. doi: 10.1016/s0939-6411(00)00108-9. [DOI] [PubMed] [Google Scholar]
- Black AT, Hayden PJ, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicology and applied pharmacology. 2010;249:178–187. doi: 10.1016/j.taap.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimfield AA, Mancebo AM, Mason RP, Jiang JJ, Siraki AG, Novak MJ. Free radical production from the interaction of 2-chloroethyl vesicants (mustard gas) with pyridine nucleotide-driven flavoprotein electron transport systems. Toxicology and applied pharmacology. 2009;234:128–134. doi: 10.1016/j.taap.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Current opinion in ophthalmology. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin--a promising new treatment for cancer. Anti-cancer agents in medicinal chemistry. 2010;10:186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- Chung JH, Paek SM, Choi JJ, Park YK, Lee JS, Kim WK. Effect of topically applied 0.1% dexamethasone on endothelial healing and aqueous composition during the repair process of rabbit corneal alkali wounds. Current eye research. 1999;18:110–116. doi: 10.1076/ceyr.18.2.110.5375. [DOI] [PubMed] [Google Scholar]
- Cox CA, Amaral J, Salloum R, Guedez L, Reid TW, Jaworski C, John-Aryankalayil M, Freedman KA, Campos MM, Martinez A, Becerra SP, Carper DA. Doxycycline's effect on ocular angiogenesis: an in vivo analysis. Ophthalmology. 2010;117:1782–1791. doi: 10.1016/j.ophtha.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer metastasis reviews. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glode LM. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investigational new drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- Foreman DM, Pancholi S, Jarvis-Evans J, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Experimental eye research. 1996;62:555–564. doi: 10.1006/exer.1996.0065. [DOI] [PubMed] [Google Scholar]
- Ganesan K, Raza SK, Vijayaraghavan R. Chemical warfare agents. Journal of pharmacy and bioallied sciences. 2010;2:166–178. doi: 10.4103/0975-7406.68498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, Anumolu SS, Schlager JJ, Gallo MA, Gerecke DR, Heindel ND, Svoboda KK, Babin MC, Sinko PJ. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Enzenauer RW, Babin MC. Ocular toxicity of Sulfur Mustard. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. USA: Elsevier; 2009. [Google Scholar]
- Inturi S, Tewari-Singh N, Gu M, Shrotriya S, Gomez J, Agarwal C, White CW, Agarwal R. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free radical biology & medicine. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Gu M, Inturi S, White CW, Agarwal R. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicology letters. 2011a;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Orlicky DJ, White CW, Agarwal R. 2-Chloroethyl ethyl sulfide causes microvesication and inflammation-related histopathological changes in male hairless mouse skin. Toxicology. 2011b;282:129–138. doi: 10.1016/j.tox.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi MA, Jafarinasab MR, Feizi S, Karimian F, Negahban K. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmology. 2011;118:1272–1281. doi: 10.1016/j.ophtha.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Javadi MA, Yazdani S, Kanavi MR, Mohammadpour M, Baradaran-Rafiee A, Jafarinasab MR, Einollahi B, Karimian F, Zare M, Naderi M, Rabei HM. Long-term outcomes of penetrating keratoplasty in chronic and delayed mustard gas keratitis. Cornea. 2007;26:1074–1078. doi: 10.1097/ICO.0b013e3181334752. [DOI] [PubMed] [Google Scholar]
- Javadi MA, Yazdani S, Sajjadi H, Jadidi K, Karimian F, Einollahi B, Ja'farinasab MR, Zare M. Chronic and delayed-onset mustard gas keratitis: report of 48 patients and review of literature. Ophthalmology. 2005;112:617–625. doi: 10.1016/j.ophtha.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Joseph LB, Gerecke DR, Heck DE, Black AT, Sinko PJ, Cervelli JA, Casillas RP, Babin MC, Laskin DL, Laskin JD. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Experimental and molecular pathology. 2011;91:515–527. doi: 10.1016/j.yexmp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, Turetz J, Gutman H, Buch H, Brandeis R, Horwitz V, Solomon A, Amir A. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Kadar T, Turetz J, Fishbine E, Sahar R, Chapman S, Amir A. Characterization of acute and delayed ocular lesions induced by sulfur mustard in rabbits. Current eye research. 2001;22:42–53. doi: 10.1076/ceyr.22.1.42.6975. [DOI] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kehe K, Raithel K, Kreppel H, Jochum M, Worek F, Thiermann H. Inhibition of poly(ADP-ribose) polymerase (PARP) influences the mode of sulfur mustard (SM)-induced cell death in HaCaT cells. Archives of toxicology. 2008;82:461–470. doi: 10.1007/s00204-007-0265-7. [DOI] [PubMed] [Google Scholar]
- Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kimura K, Teranishi S, Kawamoto K, Nishida T. Protective effect of dexamethasone against hypoxia-induced disruption of barrier function in human corneal epithelial cells. Experimental eye research. 2011;92:388–393. doi: 10.1016/j.exer.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Therapeutic delivery. 2010;1:435–456. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz A, Yaren H, Topal T, Oter S. Molecular targets against mustard toxicity: implication of cell surface receptors, peroxynitrite production, and PARP activation. Archives of toxicology. 2006;80:662–670. doi: 10.1007/s00204-006-0089-x. [DOI] [PubMed] [Google Scholar]
- Kristinsson JK, Fridriksdottir H, Thorisdottir S, Sigurdardottir AM, Stefansson E, Loftsson T. Dexamethasone-cyclodextrin-polymer co-complexes in aqueous eye drops. Aqueous humor pharmacokinetics in humans. Invest Ophthalmol Vis Sci. 1996;37:1199–1203. [PubMed] [Google Scholar]
- Loftsson T, Frithriksdottir H, Stefansson E, Thorisdottir S, Guthmundsson O, Sigthorsson T. Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits. J Pharm Pharmacol. 1994;46:503–504. doi: 10.1111/j.2042-7158.1994.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Matijasevic Z, Precopio ML, Snyder JE, Ludlum DB. Repair of sulfur mustard-induced DNA damage in mammalian cells measured by a host cell reactivation assay. Carcinogenesis. 2001;22:661–664. doi: 10.1093/carcin/22.4.661. [DOI] [PubMed] [Google Scholar]
- Milhorn D, Hamilton T, Nelson M, McNutt P. Progression of ocular sulfur mustard injury: development of a model system. Annals of the New York Academy of Sciences. 2010;1194:72–80. doi: 10.1111/j.1749-6632.2010.05491.x. [DOI] [PubMed] [Google Scholar]
- Missel P, Chastain J, Mitra A, Kompella U, Kansara V, Duvvuri S, Amrite A, Cheruvu N. In vitro transport and partitioning of AL-4940, active metabolite of angiostatic agent anecortave acetate, in ocular tissues of the posterior segment. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:137–146. doi: 10.1089/jop.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad Y, Banin E, Averbukh E, Berenshtein E, Obolensky A, Chevion M. Treatment of ocular tissues exposed to nitrogen mustard: beneficial effect of zinc desferrioxamine combined with steroids. Investigative ophthalmology & visual science. 2005;46:1640–1646. doi: 10.1167/iovs.04-1165. [DOI] [PubMed] [Google Scholar]
- Naderi M, Jadidi K, Falahati F, Alavi SA. The effect of sulfur mustard and nitrogen mustard on corneal collagen degradation induced by the enzyme collagenase. Cutaneous and ocular toxicology. 2010;29:234–240. doi: 10.3109/15569527.2010.491102. [DOI] [PubMed] [Google Scholar]
- Noort D, Benschop HP, Black RM. Biomonitoring of exposure to chemical warfare agents: a review. Toxicology and applied pharmacology. 2002;184:116–126. [PubMed] [Google Scholar]
- Olajos EJ, Morgan EW, Renne RA, Salem H, McVeety B, Johnson R, Phelps RL. Acute inhalation toxicity of neutralized chemical agent identification sets (CAIS) containing agent in chloroform. Journal of applied toxicology : JAT. 1998;18:363–371. doi: 10.1002/(sici)1099-1263(1998090)18:5<363::aid-jat521>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pal A, Tewari-Singh N, Gu M, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free radical biology & medicine. 2009;47:1640–1651. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, Panes J, Rigau J, Santos J, Rodes J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- Paromov V, Suntres Z, Smith M, Stone WL. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. Journal of burns and wounds. 2007;7:e7. [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Carlsson T, Karlsson JO, Jonhede S, Zetterberg M. Effects of dexamethasone on human lens epithelial cells in culture. Molecular vision. 2008;14:1344–1352. [PMC free article] [PubMed] [Google Scholar]
- Petrali JP, Dick EJ, Brozetti JJ, Hamilton TA, Finger AV. Acute ocular effects of mustard gas: ultrastructural pathology and immunohistopathology of exposed rabbit cornea. Journal of applied toxicology : JAT. 2000;20(Suppl 1):S173–S175. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat679>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer research. 2010;70:2368–2378. doi: 10.1158/0008-5472.CAN-09-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy K, Dwyer-Nield LD, Serkova NJ, Hasebroock KM, Tyagi A, Raina K, Singh RP, Malkinson AM, Agarwal R. Silibinin prevents lung tumorigenesis in wild-type but not in iNOS−/− mice: potential of real-time micro-CT in lung cancer chemoprevention studies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:753–761. doi: 10.1158/1078-0432.CCR-10-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebholz B, Kehe K, Ruzicka T, Rupec RA. Role of NF-kappaB/RelA and MAPK pathways in keratinocytes in response to sulfur mustard. The Journal of investigative dermatology. 2008;128:1626–1632. doi: 10.1038/sj.jid.5701234. [DOI] [PubMed] [Google Scholar]
- Richter MN, Wachtlin J, Bechrakis NE, Hoffmann F. Keratoplasty after mustard gas injury: clinical outcome and histology. Cornea. 2006;25:467–469. doi: 10.1097/01.ico.0000183491.23754.44. [DOI] [PubMed] [Google Scholar]
- Ruff AL, Dillman JF. Signaling molecules in sulfur mustard-induced cutaneous injury. Eplasty. 2007;8:e2. [PMC free article] [PubMed] [Google Scholar]
- Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clinical and experimental dermatology. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Vijayaraghavan R, Agrawal OP. Comparative toxic effect of nitrogen mustards (HN-1, HN-2, and HN-3) and sulfur mustard on hematological and biochemical variables and their protection by DRDE-07 and its analogues. International journal of toxicology. 2010;29:391–401. doi: 10.1177/1091581810365730. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocrine-related cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Cosmeceuticals and silibinin. Clinics in dermatology. 2009;27:479–484. doi: 10.1016/j.clindermatol.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Skelton H. Chemical warfare agents: their past and continuing threat and evolving therapies. Part I of II. Skinmed. 2003;2:215–221. doi: 10.1111/j.1540-9740.2003.02509.x. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Smith WJ, Hamilton T, Skelton HG, Graham JS, Okerberg C, Moeller R, Hackley BE., Jr Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard. The American Journal of dermatopathology. 1998;20:22–28. doi: 10.1097/00000372-199802000-00005. [DOI] [PubMed] [Google Scholar]
- Smith VA, Cook SD. Doxycycline-a role in ocular surface repair. The British journal of ophthalmology. 2004;88:619–625. doi: 10.1136/bjo.2003.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Li D, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. Doxycycline inhibition of interleukin-1 in the corneal epithelium. American journal of ophthalmology. 2000;130:688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. The Journal of pharmacology and experimental therapeutics. 2011;336:450–459. doi: 10.1124/jpet.110.173708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Gu M, Agarwal C, White CW, Agarwal R. Biological and molecular mechanisms of sulfur mustard analogue-induced toxicity in JB6 and HaCaT cells: possible role of ataxia telangiectasia-mutated/ataxia telangiectasia-Rad3-related cell cycle checkpoint pathway. Chemical research in toxicology. 2010a;23:1034–1044. doi: 10.1021/tx100038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, White CW, Agarwal R. 49th Annulal Meeting of the Societ of Toxicoloy. Salt Lake City, Utah: Society of Toxicology; 2010b. Therapeutic efficacy of silibinin, a natural flavanone, in sulfur mustard analog-induced skin toxicity; p. 108. [Google Scholar]
- Tewari-Singh N, Rana S, Gu M, Pal A, Orlicky DJ, White CW, Agarwal R. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicological sciences : an official journal of the Society of Toxicology. 2009;108:194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan B, Gangar SC, Kaur M, Tyagi A, Deep G, Agarwal R. Silibinin exerts sustained growth suppressive effect against human colon carcinoma SW480 xenograft by targeting multiple signaling molecules. Pharmaceutical research. 2010;27:2085–2097. doi: 10.1007/s11095-010-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GQ, Xia ZF. Tissue injury by hot fluid containing nitrogen mustard. Burns : journal of the International Society for Burn Injuries. 2007;33:923–926. doi: 10.1016/j.burns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Yeh YC, Lai HC, Ting CT, Lee WL, Wang LC, Wang KY, Liu TJ. Protection by doxycycline against doxorubicin-induced oxidative stress and apoptosis in mouse testes. Biochemical pharmacology. 2007;74:969–980. doi: 10.1016/j.bcp.2007.06.031. [DOI] [PubMed] [Google Scholar]