Abstract
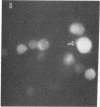
The covalently binding fluorescent probe 5-dimethylamino-1-naphthalenesulfonyl (dansyl) chloride was affixed directly to the plasma membrane of viable human peripheral blood lymphocytes via a solid phase transfer method utilizing Sephadex G-10 as the transfer vehicle. After dansylation, lymphocytes retain maximal short-term viability. Dansyl, as the protein conjugate or as the free acid, does not appear to penetrate the cells to any significant extent. Dansylated mixed lymphocyte cultures respond to lectin mitogen stimulation for at least 72 hr. Furthermore, differential response of dansylated lymphocytes in culture to three plant lectin mitogens provides a clue to the binding loci of concanavalin A with respect to phytohemagglutinin and pokeweed mitogen on the lymphocyte surface receptors for these lectins. The ability to sustain functionally responsive dansylated lymphocytes for several days in culture suggest that such probe-tagged cells may be useful in elucidating aspects of the plasma membrane in the regulation of cell behavior.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A. The application of fluorescent probes in membrane studies. Q Rev Biophys. 1975 May;8(2):237–316. doi: 10.1017/s0033583500001803. [DOI] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Demus H. Subcellular fractionation of human lymphocytes. Isolation of two plasma membrane fractions and comparison of the protein components of the various lymphocytic organelles. Biochim Biophys Acta. 1973 Jan 2;291(1):93–106. doi: 10.1016/0005-2736(73)90064-3. [DOI] [PubMed] [Google Scholar]
- Ferber E., Resch K., Wallach D. F., Imm W. Isolation and characterization of lymphocyte plasma membranes. Biochim Biophys Acta. 1972 May 9;266(2):494–504. doi: 10.1016/0005-2736(72)90105-8. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Rotational relaxation time of concanavalin A bound to the surface membrane of normal and malignant transformed cells. J Mol Biol. 1973 Dec 5;81(2):245–253. doi: 10.1016/0022-2836(73)90192-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lalezari P., Nehlsen S. L., Novodoff J., Lalezari I. Role of amino groups in formation of human lymphocyte-xenogeneic erythrocyte rosettes; a proposed mechanism for antigen recognition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):697–700. doi: 10.1073/pnas.72.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl-Kiessling K. Mechanism of phytohemagglutinin (PHA) action. V. PHA compared with concanavalin A (Con A). Exp Cell Res. 1972 Jan;70(1):17–26. doi: 10.1016/0014-4827(72)90176-0. [DOI] [PubMed] [Google Scholar]
- Melera P. W., Cronin-Sheridan A. P. Investigation of human lymphocyte plasma membrane associated nucleic acid. Biochim Biophys Acta. 1976 May 19;432(3):300–311. doi: 10.1016/0005-2787(76)90139-8. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Membrane site modified on induction of the transformation of lymphocytes by periodate. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3207–3210. doi: 10.1073/pnas.69.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M., Sachs L. Rotational diffusion of lectins bound to the surface membrane of normal lymphocytes. FEBS Lett. 1973 Aug 15;34(2):247–250. doi: 10.1016/0014-5793(73)80804-x. [DOI] [PubMed] [Google Scholar]
- Toyoshima S., Fukuda M., Osawa T. Chemical nature of the receptor site for various phytomitogens. Biochemistry. 1972 Oct 10;11(21):4000–4005. doi: 10.1021/bi00771a025. [DOI] [PubMed] [Google Scholar]