Abstract
Importance
Thirty-six percent of US adults are obese and many cannot lose sufficient weight to improve health with lifestyle interventions alone.
Objective
Conduct a systematic review of medications currently approved in the US for obesity treatment in adults. We also discuss off-label use of medications studied for obesity and provide considerations for obesity medication use in clinical practice.
Evidence Acquisition
A PubMed search from inception through September, 2013 was performed to find meta-analyses, systematic reviews, and randomized, placebo-controlled trials for currently-approved obesity medications lasting ≥1y, that had a primary or secondary outcome of body weight, included ≥50 participants per group, reported ≥50% retention, and reported results on an intention-to-treat basis. Studies of medications approved for other purposes but tested for obesity treatment were also reviewed.
Results
Obesity medications approved for long-term use, when prescribed with lifestyle interventions, produce additional weight loss relative to placebo ranging from approximately 3% of initial weight for orlistat and lorcaserin to 9% for top-dose (15/92mg) phentermine/topiramate-ER at 1y. The proportion of patients achieving clinically-meaningful (≥5%) weight loss ranges from 37–47% for lorcaserin, 35–73% for orlistat, and 67–70% for top-dose phentermine/topiramate-ER. All three produce greater improvements in many cardiometabolic risk factors than placebo, but no obesity medication has been shown to reduce cardiovascular morbidity or mortality. Most prescriptions are for noradrenergic medications, despite their approval only for short-term use and limited data for their long-term safety and efficacy.
Conclusions/Relevance
Medications approved for long-term obesity treatment, when used as an adjunct to lifestyle intervention, lead to greater mean weight loss and an increased likelihood of achieving clinically-meaningful 1-year weight loss relative to placebo. By discontinuing medication in patients who do not respond with weight loss ≥5%, clinicians can decrease their patients' exposure to the risks and costs of drug treatment when there is little prospect of long-term benefit.
INTRODUCTION
Obesity (body mass index [BMI] ≥30 kg/m2) is highly prevalent in the United States; 36%, more than 78 million, of US adults are estimated to be obese.1 Almost all health professionals in the US treat patients with obesity and are well aware of its medical consequences.
Weight loss of 5–10% of initial weight, achieved through intensive lifestyle intervention, reduces cardiovascular disease (CVD) risk factors, prevents or delays the development of type 2 diabetes, and improves other health consequences of obesity.2, 3 Although improvements in some CVD risk factors can be seen with sustained weight loss as small as 3%, weight loss ≥5% is generally considered to be clinically meaningful.4, 5 Even larger weight losses produce greater reductions in cardiometabolic risk.6
With intensive lifestyle treatments, a majority of obese participants in clinical trials lose 7–10% of their initial weight at one year.5 However, results from these efficacy trials are far better than those attained by patients in primary care settings, where studies using low intensity counseling have not consistently demonstrated clinically meaningful mean weight loss.7 Regardless of initial weight loss success, longer-term weight maintenance is difficult. With continued lifestyle treatment, weight regain can be ameliorated but not eliminated.8 The need for constant vigilance to sustain behavior changes in the face of biologic and environmental pressures to regain weight emphasizes the challenges faced by even the most motivated patinets who have achieved weight loss. Thus, there is a need for adjunctive therapies that can help patients who are not able to lose or sustain sufficient weight loss to improve health with lifestyle interventions alone.
This article systematically reviews the literature for long-term use of medications currently approved by the US Food and Drug Administration (FDA) for obesity treatment in adults (Table 1). We also discuss off-label use of medications approved for other purposes that have been studied for obesity treatment or drug-induced weight gain, and provide considerations for use of obesity medications in clinical practice.
Table 1.
Drugs with an FDA-approved indication for obesity. Only orlistat, lorcaserin, and phentermine/topiramate-ER are FDA-approved for long-term use; the others are approved only for short-term use (i.e., a few weeks).
| Generic Name | Trade Name(s) | Mechanism of action | Dosage | Whole-sale price per mo.* | Mean weight change relative to placebo at 1y, kg** | Interactions | Contraindications& | Common Adverse Events& | Cautions and Warnings& |
|---|---|---|---|---|---|---|---|---|---|
| Phenterminea | Adipex-P, Fastin, Oby-Cap, lonamin, Others |
Noradrenergic causing appetite suppression |
15– 37.5mg/d |
$6– $45 |
Not Available |
Guanethidine, CNS stimulants, alcohol, tricyclic antidepressants; requirements for insulin or oral hypoglycemic medications may be altered |
Pregnancy or nursing, advanced cardiovascular disease, uncontrolled hypertension, hyperthyroidism, glaucoma, agitated states, history of drug abuse, MAOIs |
Insomnia, elevation in heart rate, dry mouth, taste alterations, dizziness, tremors, headache, diarrhea, constipation, vomiting, gastro- intestinal distress, anxiety, and restlessness. |
Do not increase beyond recommended dose if tolerance to the anorexiant effect develops. Caution prescribing to patients with even mild hypertension. Caution for patients using alcohol or other CNS active drugs or engaging in hazardous activity. |
| Diethylpropiona | Tenuate, Tenuate Dospan, Tepanil |
Noradrenergic causing appetite suppression |
25mg 3 times/d or 75mg sustained- release/d |
$47– $120 |
Not Available |
Same as phentermine |
Same as phentermine |
Same as phentermine |
Same as phentermine |
| Phendimetrazineb | Bontril | Noradrenergic | 17.5–70mg 2–3 times/d or 105mg sustained- release/d |
$6– $20 |
Not Available |
Same as phentermine |
Same as phentermine |
Same as phentermine |
Same as phentermine |
| Benzphetamineb | Didrex | Noradrenergic causing appetite suppression |
25–50mg 1–3 times/d |
$20– $50 |
Not Available |
Same as phentermine |
Same as phentermine |
Same as phentermine |
Same as phentermine |
| Orlistatc | Xenical, Alli |
Lipase inhibitor causing excretion of ~30% of ingested triglycerides in stool |
60 or 120mg 3 times/d within 1 hr of a fat- containing meals, plus a daily multi- vitamin |
For 60mg TID: $45 For 120mg TID: $207 |
For 60mg TID:−2.5 kg (−1.5 to −3.5) For 120mg TID: −3.4 kg (−3.2 to −3.6) |
Decreased drug concentrations of cyclosporine and levothyroxine. Doses should be temporally separated from orlistat. Fat soluble vitamin absorption is decreased by orlistat |
Pregnancy, chronic malabsorption syndromes, cholestasis |
Oily Spotting, Flatus with Discharge, Fecal Urgency, Fatty/Oily Stool, Increased Defecation, Fecal Incontinence |
Use with caution in those at risk for renal insufficiency, since treatment may increase urinary oxalate. Cholelithiasis and, rarely, severe liver injury including hepatocellular necrosis and acute hepatic failure leading to death, have been reported |
| Lorcaserina | Belviq | Highly selective serotonergic 5-HT2C receptor agonist causing appetite suppression |
10mg two times/d |
$240 | −3.2 kg (−2.7 to −3.8) |
Triptans, MAOIs including linezolid, SSRIs, SNRIs, dextro- methorphan, tricyclic antidepressants, bupropion, lithium, tramadol, tryptophan, and St. John's Wort |
Pregnancy | Headache, dizziness, fatigue, nausea, dry mouth, cough, and constipation, and back pain, cough and hypoglycemia in patients with type 2 diabetes. |
Risk for Serotonin Syndrome or Neuroleptic Malignant Syndrome-like Reactions. Evaluate patients for signs or symptoms of valvular heart disease. Euphoria, hallucination, and dissociation have been seen with supra- therapeutic doses. Use with caution in men at risk for priapism |
| Phentermine / Topiramate-ERa |
Qsymia | Noradrenergic + GABA- receptor activator, kainite/AMPA glutamate receptor inhibitor causing appetite suppression |
3.75/23mg /d for 2 weeks, then 7.5/46mg/ d, escalating to a maximum of 15/92mg/d |
$140 – $195 |
For 7.5/46mgd: −6.7 kg (−5.9 to −7.5) For 15/92mg/d −8.9 kg (−8.3 to −9.4) |
Oral contraceptives, alcohol and other CNS depressants, non-potassium- sparing diuretics |
Pregnancy, Glaucoma, Hyperthyroidism, MAOIs |
Paresthesias dizziness, taste alterations, insomnia, constipation, dry mouth, elevation in heart rate, memory or cognitive changes |
Metabolic acidosis, hypokalemia, and elevated creatinine have been reported, and periodic monitoring is advised. Increased risk of nephrolithiasis. Advise patients to report changes in mood/suicidalit y. Abrupt withdrawal of topiramate may cause seizures; taper over 1 week for patients using 15/92mg phentermine- topiramate-ER. There is an increased risk of oral clefts in offspring of women who become pregnant while taking topiramate. |
MAOI: monoamine oxidase inhibitor, CNS: central nervous system, SSRI: selective serotonin-reuptake inhibitors, SNRI: selective serotonin-norepinephrine reuptake inhibitors.
Reference prices found on March 8, 2013.99
Weight change relative to placebo (95 percentile confidence interval) using intent-to-treat analyses for each medication at 1 year. No studies for older noradrenergic agents (phentermine, diethylpropion, phendimetrazine, and benzphetamine) met inclusion criteria for length of treatment, sample size, and attrition.
Medications listed on Drug Enforcement Administration Schedule IV are associated with a lower risk of abuse than
medications on Schedule III;
Orlistat is a non-Drug Enforcement Administration scheduled drug.
Common adverse events for noradrenergic agents include those listed as common in the NIDDK Weight-control Information Network Fact Sheet “Prescription Medications for the Treatment of Obesity”100 as adverse event frequency is not available in the drug package inserts for these agents. For orlistat, lorcaserin, and phentermine/topiramate ER, common adverse events are those listed in the drug package inserts48,52,54 that are reported to occur more frequently than placebo and with more than 5% prevalence.
See full prescribing information for all adverse effects, cautions, and contraindications.
METHODS
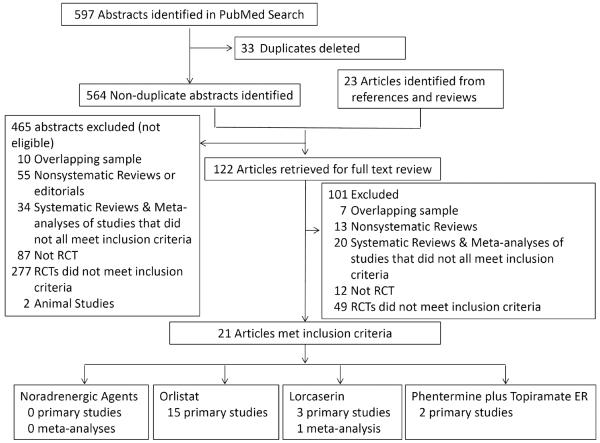
A PubMed search was conducted from inception to September 15, 2013 to find long-term studies investigating drugs currently approved alone or in combination for an obesity or weight management indication utilizing the keywords `obesity,' `appetite' or `satiety' and `drug' or `pharmacotherapy', and `orlistat,' `phentermine,' `diethylpropion,' `phendimetrazine,' `benzphetamine,' `topiramate,' `Qsymia,' `Qnexa,' `lorcaserin,' or `Belviq,' and `clinical trial' or `meta-analysis'. Searches were restricted to human studies in English. The primary search resulted in 564 articles (Figure 1). Automated searches were supplemented by examination of expert recommendation reports and bibliographic references from included research studies, and searches of www.clinicaltrials.gov for each identified medication. Studies identified underwent review of the title, abstract, or both by each Author to discard clearly nonrelevant articles as well as reports describing drugs that have been withdrawn from use (e.g. sibutramine) or for which further development for an obesity indication has been abandoned (e.g. fluoxetine). To be included, studies had to report randomized placebo-controlled clinical trials lasting a minimum of 1 year with a primary or secondary outcome of body weight, study at least 50 participants per group at baseline, report at least 50% retention, and report results on an intention-to-treat basis. Results for randomized controlled trials meeting our inclusion criteria are reported in Table 2.
Figure 1. Identification of manuscripts for systematic review.
A PubMed search was conducted from inception to September 15, 2013 to find long-term (≥1 y) placebo-controlled randomized clinical trials and meta-analyses investigating drugs currently FDA-approved alone or in combination for an obesity or weight management indication.
Table 2.
Studies included in systematic review for long-term pharmacotherapy of obesity. Attrition for each study was calculated from the total number of participants who were randomized to treatments. Results for weight change are reported from intention to treat analyses, generally with the last observation carried forward. Some results reported in the studies (e.g. for follow-up intervals other than 1 year) are not included in the table.
| Drug | Study | Subjects (sites for trial) | Groups (number randomized) | Lifestyle intervention | Attrition (%) | Drug change in weight at 1y (kg) | Placebo change in weight at 1y (kg) | Drug change in weight relative to baseline weight at 1y (%) | Placebo change in weight relative to baseline weight at 1y (%) | Drug percent losing ≥5% of baseline weight at 1y (%) | Placebo percent losing ≥5% of baseline weight at 1y (%) | Drug percent losing ≥10% of baseline weight at 1y (%) | Placebo percent losing ≥10% of baseline weight at 1y (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orlistat | Hollander et al9 |
Adults (49% female) with type 2 diabetes and BMI 28–40 kg/m2who were clinically stable on oral sulfonylureas only and treatment compliance of 70% during placebo run- in. Those with recurrent nephrolithiasis or symptomatic cholelithiasis were excluded (USA) |
Orlistat 120mg TID (n=163); Placebo TID (n=159) |
500 kcal/day caloric reduction |
21 | −6.2 | −4.3 | −6.2 | −4.3 | 48.8 | 22.6 | 17.9 | 8.8 |
| Orlistat | Sjostrom et al10 |
Adults (83% female) with BMI 28–47 kg/m2 and treatment compliance of 75% during placebo run- in. Those with uncontrolled hypertension and drug-treated diabetes were excluded (Europe) |
Orlistat 120mg TID (n=345); Placebo TID (n=343) |
600–900 kcal/day caloric reduction |
21 | −10.3 | −6.1 | −10.2 | −6.1 | 68.5 | 49.2 | 38.8 | 17.7 |
| Orlistat | Davidson et al11 |
Adults (84% female) with BMI 30–43 kg/m2 and treatment compliance of 75% during placebo run- in. Those with drug- treated diabetes were excluded (USA) |
Orlistat 120mg TID (n=668); Placebo TID (n=224) |
500–900 kcal/day caloric reduction; behavior modification program involving exercise counseling, food diary |
34 | −8.8 | −5.8 | −8.8 | −5.8 | 65.7 | 43.6 | 38.9 | 24.8 |
| Orlistat | Finer et al12 |
Adults (88% female) with BMI 30 – 43 kg/m2 and treatment compliance of 70% during placebo run- in. Those with diabetes or uncontrolled hypertension (UK) |
Orlistat 120mg TID (n=114); Placebo TID (n=114) |
600–900 kcal/day caloric reduction |
39 | −3.3 | −1.3 | −8.5 | −5.4 | 35 | 21 | 28 | 17 |
| Orlistat | Hauptman et al13 |
Adults (78% female) with BMI 30–44 kg/m2 and treatment compliance of 75% during placebo run- in. Those with uncontrolled hypertension were excluded (USA) |
Orlistat 60mg TID (n=213); Orlistat 120mg TID (n=210); Placebo TID (n=212) |
1200–1500 kcal/day diet; exercise; food diary; educational video |
33 | For 60mg: −7.1 For 120mg: −7.9 |
−4.1 | For 60mg: −7.1 For 120mg: −7.9 |
−4.2 | For 60mg: 48.8 For 120mg: 50.5 |
30.7 | For 60mg: 24.4 For 120mg: 28.6 |
11.3 |
| Orlistat | Lindgarde et al14 |
Adults (64% female) with BMI 28–38 kg m2) with type 2 diabetes treated only with metformin or sulfonylurea, hypercholesterolem ia and/or hypertension who completed a placebo run-in (Sweden) |
Orlistat 120mg TID (n=190); Placebo TID (n=186) |
600–900 kcal/day caloric reduction; exercise; self-help weight control educational package |
14 | −5.6 | −4.3 | −5.9 | −4.6 | 54.2 | 40.9 | 19.2 | 14.6 |
| Orlistat | Rossner et al15 |
Adults (82% female) with BMI 28–43 kg/m2 and treatment compliance of 75% during placebo run- in. Those with uncontrolled hypertension, drug- treated diabetes mellitus, or history or presence of symptomatic cholelithiasis were excluded (Europe) |
Orlistat 60mg TID (n=242); Orlistat 120mg TID (n=244); Placebo TID (n=243) |
600 kcal/day caloric reduction; food diaries, counseling by dietitian |
28 | For 60mg: −8.5 For 120mg: −9.4 |
−6.4 | For 60mg: −8.6 For 120mg: − 9.7 |
−6.6 | For 60mg: N/A For 120mg: N/A |
N/A | For 60mg: 31.2 For 120mg: 38.3 |
18.8 |
| Orlistat | Broom et al16 |
Adults (78% female) with BMI ≥28 kg/m2 and untreated hypertension, impaired glucose tolerance, or dyslipidemia who were eligible after a 2-week placebo run-in. If compliance with medication was <60%, subjects were withdrawn (UK) |
Orlistat 120mg TID (n=265); Placebo TID (n=266) |
600–900 kcal/day caloric reduction; food diary |
35 | −5.8 | −2.3 | −5.8 | −2.3 | 55.6 | 24.3 | 19.7 | 11 |
| Orlistat | Hanefield and Sachse17 |
Adults (51% female) with BMI ≥28 kg/m2 with diabetes and HA1C 6.5–11% treated with diet alone or sulfonylurea who were eligible after a 4-week placebo run-in. Those with uncontrolled hypertension were excluded. If compliance with medication was <75%, subjects were withdrawn (Germany) |
Orlistat 120mg TID (n=195); Placebo TID (n=188) |
600 kcal/day caloric reduction, diet diary |
31 | −5.3 | −3.4 | −5.4 | −3.6 | 51.3 | 31.6 | N/A | N/A |
| Orlistat | Miles et al19 |
Adults (48% female) with BMI 28 – 43 kg/m2 and type 2 diabetes with HA1C 7.5–12% receiving oral hypoglycemic who were eligible after a 2-week screening phase. Those with poorly controlled hypertension or treated with insulin, thiazolidinediones, or alpha- glucosidase inhibitors were excluded (USA and Canada) |
Orlistat 120mg TID (n=255); Placebo TID (n=261) |
600 kcal/day caloric reduction and exercise counseling |
40 | −4.7 | −1.8 | −4.6 | −1.7 | 39.0 | 15.7 | 14.1 | 3.9 |
| Orlistat | Krempf et al18 |
Adults (86% female) with BMI ≥28 kg/m2 without diabetes or other significant medical condition who were eligible after a 2- week placebo run- in (France) |
Orlistat 120mg TID (n=346); Placebo TID (n=350) |
20% energy reduced diet, increased by 10% if weight stable; food diary |
≤39* | −6.3 | −3.3 | −6.3 | −3.6 | 65.9 | 46.4 | 32.9 | 24.5 |
| Orlistat | Torgerson et al20 |
Adults (55% female) age 30–60y with BMI ≥30 kg/m2. Patients were required to have nondiabetic glucose tolerance. Those with ongoing and active cardiovascular or gastrointestinal disease were excluded (Sweden) |
Orlistat 120mg TID (n=1650); Placebo TID (n=1655) |
800 kcal/day caloric reduction; lifestyle intervention program |
16 | −10.6 | −6.2 | N/A | N/A | 72.8 | 45.1 | 41 | 20.8 |
| Orlistat | Berne et al21 |
Adults (45% female) age 30–75y with BMI 28–40 kg/m2 and type 2 diabetes and HA1C 6.5–10% treated only with metformin or sulfonylurea who were eligible after a 2-week diet run-in (Sweden) |
Orlistat 120mg TID (n=111); Placebo TID (n=109) 1 subject unclear allocation |
600 kcal/day caloric reduction; diet and exercise counseling; self- management educational package |
14 | N/A | N/A | −5.0 | −1.8 | 45.9 | 11 | 13.5 | 2.8 |
| Orlistat | Swinburn et al22 |
Adults (57% female) age 40–70y with BMI 30–50 kg/m2 and type 2 diabetes treated only with oral agents and HA1C 6.5–10%, hypercholesterolem ia and/or hypertension who completed a placebo run-in. Those with uncontrolled hypertension were excluded (Australia and New Zealand) |
Orlistat 120mg TID (n=170); Placebo TID (n=169) |
Reduced fat diet and exercise counseling |
21 | −4.7 | −0.9 | N/A | N/A | N/A | N/A | N/A | N/A |
| Orlistat | Derosa et al23 |
Adults (49% female) with BMI ≥30 kg/m2 with type 2 diabetes and HA1C >8.0% (Italy) |
Orlistat 120mg TID (n=126); Placebo TID (n=128) |
600 kcal/day caloric reduction, behavior modification program, exercise counseling |
8 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lorcaserin | Smith et al31 |
Adults (83% female) with BMI 30–45 kg/m2 or 27– 29.9 kg/m2 with an obesity-related comorbid condition. Those with cardiac valvulopathy, type 2 diabetes, and uncontrolled hypertension were excluded (USA) |
Lorcaserin 10mg BID (n=1595; Placebo BID (n=1587) |
600 kcal/day caloric reduction; Standardize d nutritional and exercise counseling |
50 | −5.8 | −2.2 | −5.8 | −2.2 | 47.5 | 20.3 | 22.6 | 7.7 |
| Lorcaserin | Fidler et al32 |
Adults (80% female) with BMI 30–45 kg/m2 or 27– 29.9 kg/m2 with an obesity-related comorbid condition. Those with type 2 diabetes, and uncontrolled hypertension or dyslipidemia were excluded (USA) |
Lorcaserin 10mg qD (n=801) Lorcaserin 10mg BID (n=1602); Placebo (n=1601) |
600 kcal/day caloric reduction and exercise counseling, diet diary |
45 | For 10mg qD: −4.7 For 10mg BID: −5.8 |
−2.9 | For 10mg qD: −4.7 For 10mg BID: −5.8 |
−2.8 | For 10mg qD: 40.2 For 10mg BID: 47.2 |
25.0 | For 10mg qD: 17.4 For 10mg BID: 22.6 |
9.7 |
| Lorcaserin | O'Neil et al30 |
Adults (54% female) with BMI 27–45 kg/m2 and type 2 diabetes treated with metformin or sulfonylurea and HA1C 7–10%. Those with severe cardiovascular disorders were excluded (USA) |
Lorcaserin 10mg qD (n=95); Lorcaserin 10mg BID (n=256); Placebo (n=252) |
600 kcal/day caloric reduction; nutritional and exercise counseling |
34 | For 10mg qD: −5 For 10mg BID: −4.7 |
−1.6 | For 10mg qD: −5 For 10mg BID: −4.5 |
−1.5 | For 10mg qD: 44.7 For 10mg BID: 37.5 |
16.1 | For 10mg qD: 18.1 For 10mg BID: 16.3 |
4.4 |
| Phentermine / Topiramate ER |
Allison et al.33 |
Adults (83% female) with BMI ≥35 kg/m2 with fasting glucose ≤110mg/dl, triglycerides ≤200 mg/dl with ≤1 lipid- lowering medications, BP ≤140/90 mm Hg with ≤2 antihypertensive medications. (USA) |
Phentermine/ topiramate ER 3.75/23mg (starting dose) (n=241); Phentermine/ topiramate- ER 15/92mg (top dose) (n=512), Placebo (n=514) |
Self-help weight control manual, 500 kcal/day caloric reduction, monthly progress reviews |
40 | N/A | N/A | For 3.75/23mg :−5.1 For 15/92mg: −10.9 |
−1.6 | For 3.75/23m g: 44.9 For 15/92mg: 66.7 |
17.3 | For 3.75/23mg: 18.8 For 15/92mg: 47.2 |
7.4 |
| Phentermine / Topiramate ER |
Gadde et al34 |
Adults (70% female) with BMI 27–45 kg/m2 and 2 or more weight- related comorbidities. No lower BMI limit for participants with type 2 diabetes (16% of cohort). Those with uncontrolled diabetes, hypertension, or hypertriglyceridemi a, or who used antidiabetic drugs other than metformin were excluded (USA) |
Phentermine/ topiramate ER 7.5/46mg (recommend er dose) (n=498) Phentermine/ topiramate- ER 15/92mg (top dose) (n=995) Placebo (n=994) |
Standardize d lifestyle counseling, 500 kcal/day caloric reduction |
31 | For 7.5/46mg : −8.1 For 15/92mg: −10.2 |
−1.4 | For 7.5/46mg: −7.8 For 15/92mg:- −9.8 |
−1.2 | For 7.5/46mg: 62; For 15/92mg: 70 |
21 | For 7.5/46mg: 37 For 15/92mg: 48 |
7 |
HA1C: Hemoglobin A1C; N/A: Not available.
Attrition available only at 18mos.
Medications approved for other purposes but tested for at least 52 weeks for obesity prevention or treatment were also reviewed non-systematically.
Results
Included studies are reported in Table 2. No studies for older noradrenergic agents (phentermine, diethylpropion, phendimetrazine, and benzphetamine) met inclusion criteria for length of treatment, sample size and/or attrition. Fifteen trials9–23 reporting intention-to-treat data from 5006 orlistat-treated and 4555 placebo-treated adults appeared to meet study entry criteria for orlistat, although data for absolute weight change were not ascertainable from two.21, 23 Multiple meta-analyses24–28 for orlistat 120mg have been carried out that included most of these identified studies and found similar pooled 1-year weight loss results, but none met all of the criteria for study inclusion/exclusion. Pooled, sample size-weighted, estimates and confidence intervals for weight loss at 1 year were calculated from the primary studies. There were no meta-analyses identified for orlistat 60mg. Pooled, sample size-weighted, estimates and confidence intervals for weight loss at 1 year were calculated from the primary studies of 452 orlistat-treated and 449 placebo-treated adults reported in the two primary studies13, 15 that met criteria for inclusion. One meta-analysis29 reported results from 3350 lorcaserin 10mg BID-treated and 3288 placebo-treated adults reported in the three papers30–32 meeting study criteria for lorcaserin; results are reported from this meta-analysis. There were no meta-analyses identified for phentermine/topiramate-ER. However, pooled data from 488 phentermine/topiramate-ER 7.5/46mg-treated, 1479 phentermine/topiramate-ER 15/92-mg treated, and 1477 placebo-treated adults in the phase III studies for change in weight that met inclusion criteria33, 34 were obtained from the Integrated Summary of Efficacy submitted by Vivus, Inc. to the Food and Drug Administration as part of their New Drug Application.35, 36 No studies, and therefore no meta-analyses, for any of the noradrenergic medications met inclusion criteria. Results from a quantitative analysis of the extant clinical trials by Haddock et al37 are reported in the text. Primary studies meeting inclusion criteria for each medication were also reviewed for their impact on health outcomes other than weight loss.
Medications currently approved by the FDA for obesity treatment are listed in Table 1. All are considered indicated for adults with BMI ≥30 kg/m2 and all but benzphetamine and diethylpropion are also approved for patients with BMI ≥27 kg/m2 plus at least one weight-related comorbidity, such as hypertension or type 2 diabetes.
Noradrenergic activation
Four centrally-acting noradrenergic agents (phentermine, diethylpropion, phendimetrazine, benzphetamine) are FDA-approved for the “short-term” (usually considered ≤12 weeks) management of obesity. All were approved before the necessity of long-term treatment for obesity was established. In addition, none were required to meet the current efficacy benchmarks for weight loss relative to placebo (mean weight loss ≥5% more than that of the placebo group or proportion of drug-treated subjects who lose ≥5% of initial weight is ≥35% and approximately double the proportion who lose ≥5% in the placebo group).38 Limited treatment duration for these noradrenergic agents was a requirement added in 1973 because of concerns about abuse potential and transient efficacy.38 No trials of these four medications met our criteria for inclusion (duration, size, and attrition), although a meta-analysis with shorter-term outcomes has been published.37 These centrally-acting agents reduce appetite by increasing activation of adrenergic and dopaminergic receptors.39
Phentermine, despite its approval by the FDA for short term use, is frequently prescribed off-label for longer periods.40, 41 Phentermine is by far the most widely prescribed obesity medication in the US, with 25.3 million prescriptions dispensed to an estimated 6.2 million users between 2008–2011.41 Although it has a long history of use, there are few controlled trials of phentermine monotherapy for six months or more, and studies describing the effect of phentermine monotherapy on weight and cardiovascular disease risk factors for more than one year are limited to case reports and case series. A meta-analysis of 6 studies ranging from 2 to 24 weeks37 found that patients using 15–30mg/d phentermine had a mean additional weight loss relative to placebo of 3.6 kg, with mean total weight loss of 6.3 kg. The longest published placebo-controlled trial of phentermine lasted 36 weeks in 108 obese women treated with phentermine 30mg/day either continuously or intermittently (alternating months) and found similar weight loss in the continuous (12.2 kg) and intermittent (13.0 kg) arms vs. 4.8 kg with placebo. However, attrition was 41%, and data were presented only for completers, which is likely to overstate efficacy.42 Among completers, transient symptoms of central nervous system stimulation such as insomnia, irritability, and anxiety did not differ between those receiving continuous (24%) vs. intermittent (27%) therapy, compared with 8% for those taking placebo. Several short-term placebo-controlled studies of phentermine have shown elevations in pulse or smaller decreases in pulse and/or blood pressure than would be expected given the degree of weight loss.3
Diethylpropion has a similar adverse-effect and weight loss profile to phentermine, but is much less frequently prescribed, with approximately 1 million prescriptions dispensed between 2008–2011.41 A meta-analysis of 9 small studies ranging from 6–52 weeks37 found that patients using diethylpropion 75mg/d had a mean additional weight loss relative to placebo of 3.0 kg, with a mean total weight loss of 6.5 kg.
Phendimetrazine, despite the paucity of randomized controlled trials37 is prescribed three times more frequently than diethylpropion for obesity treatment, with more than 3 million phendimetrazine prescriptions estimated to have been filled between 2008–2011.41 In the completer's analyses from two small 12-week trials,43, 44 it appears to have similar weight loss to other noradrenergic drugs.
Benzphetamine is less commonly prescribed for obesity treatment than the other noradrenergic drugs,41 and there are few data from controlled trials evaluating its safety or efficacy.37
Common adverse effects of noradrenergic drugs are shown in Table 1. Because these medications were approved prior to the requirements for long-term trials with adequate power to ascertain clinical endpoints, an adverse effect of noradrenergic obesity drugs on cardiovascular disease events cannot be excluded, and is of concern given their known effect on heart rate and blood pressure.
Gastrointestinal lipase inhibition
Orlistat is a gastrointestinal lipase inhibitor which, when taken three times a day during or up to 1 hour after meals, leads to the excretion of approximately 30% of ingested fat. It is available both in prescription (120mg) and over-the-counter (60mg) strength. Orlistat 120mg is FDA-approved for use in adults and adolescents age 12–16y. The mean weight reduction attributable to orlistat 120mg TID at 12 months is modest: among adults participating in behavioral weight control programs and prescribed a lower fat diet (~30% of calories from fat), orlistat-treated patients lost on average 3.4 kg (~3.1% of initial weight) more than placebo-treated participants (Table 1). Two trials13, 15 of orlistat 60mg TID met study criteria for inclusion; the pooled estimate from these studies indicates 2.5 kg greater weight loss than placebo at 52 weeks. Among the orlistat 120mg trials examined (Table 2), the percentage of orlistat 120mg-treated participants who achieved clinically-meaningful (≥5%) weight loss at 1 year varied from 35–73% and the proportion losing ≥10% varied from 14–41%, with both ≥5% and ≥10% weight loss at 1 year significantly greater for orlistat-treated than for placebo-treated participants. At the end of a second year of treatment when a weight-maintenance diet was prescribed, orlistat 120mg-treated participants had lost approximately 3.3 kg (~3.3% of initial weight) more and orlistat 60mg-treated participants had lost approximately 2.5 kg (~2.5% of initial weight) more than those given placebo (Table 1).10, 11, 13, 15 Because of its weight-loss related and weight-loss independent45 actions, orlistat 120mg treatment is associated with significant improvements in cardiovascular risk factors including decreases in total- and LDL- cholesterol, fasting glucose, and systolic and diastolic blood pressures after 1 year of treatment.46, 47 Data from the XENDOS trial of 3,305 patients treated for up to 4 years (attrition at 4 years: 48% for orlistat-treated and 66% for placebo-treated) found, in an intention-to-treat approach, that orlistat use decreased body weight over 4 years by 2.7 kg (approximately 2.4% of initial body weight) more than placebo and significantly decreased risk for developing type 2 diabetes from 9.0% with placebo to 6.2% with orlistat.20 Because orlistat leads to obligate increases in undigested stool triglycerides, it may cause considerable gastrointestinal adverse effects (Table 1)48 that may be decreased by co-administration of fiber-containing supplements.49 These adverse effects may cause patients who do not reduce their fat intake to discontinue therapy. Indeed, despite being FDA-approved in 1999 for indefinite treatment of obesity, among those prescribed orlistat 120mg clinically, fewer than 10% take it for at least 1y and <2% of patients use the medication for 2y.41, 50
Serotonin receptor activation
Lorcaserin is a selective serotonin 2C (5HT2c) receptor agonist that was anticipated to recapitulate the weight loss effects of fenfluramine without its adverse cardiac effects.51 Lorcaserin 10mg BID was FDA-approved in 2012 on the basis of two large randomized, placebo-controlled trials in nondiabetic patients (BLOOM,31 n=3182, 50% attrition; BLOSSOM,32 n=4004, 45% attrition) along with a third, smaller trial in adults with type 2 diabetes (BLOOM-DM,30 n=603, 34% attrition). In these trials, participants received low-intensity nutritional and exercise counseling. Lorcaserin decreased body weight modestly, by about 3.2 kg (~3.2% of initial body weight) more than placebo.29 However, significantly more patients treated with lorcaserin 10mg bid than placebo lost ≥5% (BLOOM: 47 vs. 20%, BLOSSOM: 47 vs. 25%, BLOOM-DM: 37 vs. 16%) or ≥10% (BLOOM: 23 vs. 8%, BLOSSOM: 23 vs. 10%, BLOOM-DM: 16 vs. 4%) of their initial weight. Reduction in body weight below baseline in the one study31 with data from participants who took lorcaserin for 2 years had average weight loss of 5.6 kg, versus 2.4 kg among placebo-treated participants. Blood pressure, total cholesterol, LDL-cholesterol, and triglycerides also decreased significantly more in lorcaserin-treated participants.52 Among patients with diabetes, lorcaserin treatment led to lower body weight and improved glycated hemoglobin concentrations.30 Adverse effects (Table 1) include headache, nausea, fatigue, and dizziness.52 Although neither incidence of valvulopathy nor hypertension was statistically greater during lorcaserin than placebo treatment, both were numerically somewhat more prevalent and the FDA has requested that a post-approval trial to assess the long-term cardiovascular effects of lorcaserin be conducted.53
Combination therapy
Phentermine/topiramate-Extended Release (ER) is the first FDA-approved combination drug for obesity, combining low-dose phentermine with a non-standard dose of the antiepileptic medication topiramate-ER (Table 1). Phentermine/topiramate-ER, is administered as a once-daily capsule in 4 fixed-dose combinations: 3.75mg phentermine/23mg topiramate (starting dose); 7.5mg phentermine/46mg topiramate (recommended dose); 11.25mg phentermine/69mg topiramate (titration dose); and 15mg phentermine/92mg topiramate (top dose). Dosage is increased over 14 days to 7.5mg phentermine/46mg topiramate, with additional titration to the top dose if weight loss is inadequate.54
Phentermine/topiramate-ER was recommended for approval based largely on 2 one-year Phase 3 clinical trials (EQUIP,33 n=1267; CONQUER,34 n=2487). All groups received a low-intensity lifestyle program. All underwent dose titration over 4 weeks to assigned dose followed by 52 weeks on drug or placebo. EQUIP33 randomized adults without diabetes and with BMI ≥35 kg/m2 to placebo, phentermine/topiramate-ER 3.75/23mg (starting dose), or 15/92mg (top dose). 40% of participants withdrew. At the top dose, mean 1y weight loss was 10.9% vs. 1.6% of initial weight for placebo. 67% of patients given the top dose lost ≥5% of initial weight and 47% lost ≥10% of initial weight, compared with 17% and 7%, respectively for placebo. CONQUER34 randomized a higher-risk sample of adults with BMI 27–45 kg/m2 and ≥2 obesity-associated comorbid conditions, to placebo or phentermine/topiramate-ER. 31% of participants withdrew. One year weight loss was 8.1 kg (7.8%) with the recommended dose and 10.2 kg (9.8%) with the top dose, vs. 1.4 kg (1.2%) with placebo. In addition, 62% (recommended dose) and 70% (top dose) lost ≥5% of initial weight vs. 21% for placebo, with 37%, 48%, and 7% respectively losing ≥10% of initial weight. Many CVD risk factors improved with active drug treatment at recommended- or top-dose.55 SEQUEL56 an extension to CONQUER, followed 78% of CONQUER participants at sites selected for high enrollment and retention and who had completed the initial 56-week trial for a total of 108 weeks. 84% completed their second year of treatment with sustained weight loss of 9.3% and 10.5% at the recommended and top doses, respectively, vs. 1.8% for placebo, and continued differences in many CVD risk factors. In addition, there was a significantly lower incidence of progression to type 2 diabetes in the top-dose group (0.9%) vs. placebo (3.7%).
An area of considerable concern, given that most users of obesity medications are women of reproductive age, is the potential for oral clefts in the offspring of women who become pregnant while taking topiramate (Table 1).57 A risk evaluation and mitigation strategy (REMS) was developed to minimize the likelihood of pregnancy in women with reproductive potential that includes provider training, dispensing only via certified pharmacies, and supplying patient information regarding risks and the necessity of using effective contraception.58 Women with childbearing potential should have a negative pregnancy test prior to starting phentermine/topiramate-ER and monthly thereafter.58 A small increase in resting heart rate has been observed in the clinical trials of phentermine/topiramate-ER at higher doses, with more patients on top-dose (56.1%) than placebo (42.1%) having increases of more than 10 beats per minute, leading to some concerns regarding its potential long-term effect on CVD events.59 Phentermine/topiramate-ER was approved with a requirement for a post-marketing trial of to assess long-term cardiovascular safety.53 The labeling recommends against prescription in patients with recent or unstable cardiac or cerebrovascular disease, and suggests regular monitoring of resting heart rate.54
Other medications studied off-label for obesity prevention or treatment
Medications that are FDA-approved for other conditions and found to result in weight loss have been tested as potential obesity treatments. Some, such as fluoxetine, were found to promote weight loss for up to six months, but not longer-term.60Bupropion, a norepinephrine and dopamine reuptake inhibitor, was tested as monotherapy for up to one year as a weight loss medication. A pooled analysis of 3 studies ranging from 6 to 12 months showed additional weight loss relative to placebo of 2.8 kg in patients receiving 400mg/d bupropion, with total weight loss of 4.4 kg.25Metformin, increasing used off-label in prediabetes and other insulin resistant states, produces small sustained weight losses of about 2% relative to placebo.61, 62 Metformin improves insulin sensitivity, has a good safety profile, and long-term clinical experience. As weight loss attributable to metformin is small, its usefulness as monotherapy for obesity treatment is limited, but its salutary effects on body weight make it a good choice when other indications warrant its prescription. Metformin has also been used to prevent or ameliorate weight gain with atypical antipsychotic agents and mood stabilizers. A meta-analysis examining the effect of medications for attenuation of antipsychotic weight gain found an approximate 3 kg additional weight loss relative to placebo attributable to metformin.63Zonisamide, an antiepileptic medication, also induces weight loss. A 12-month RCT of 225 adults, with 97% follow-up found that a 400mg dose led to significantly greater weight loss than placebo (6.8% vs. 3.7%), as well as a greater proportion losing ≥5% and ≥10% of initial weight.64 However, adverse effects were limiting. Pramlintide is a synthetic analogue of human amylin, which is administered subcutaneously at meal times as an adjunct to insulin for patients with type 1 and type 2 diabetes. A meta-analysis65 of 8 studies in patients with type 2 diabetes and obese non-diabetic populations found additional weight loss relative to placebo of about 2.2 kg for both groups. One study66 evaluating pramlintide in combination with phentermine vs. pramlintide alone found significantly greater weight loss with combination therapy, although diastolic blood pressure and heart rate increased despite greater weight loss with the combination.
Drugs in late-phase clinical trials for obesity treatment
A proprietary formulation of naltrexone-SR 32mg plus bupropion-SR 360mg, which was recommended for FDA-approval as an anti-obesity agent in December 2010,67 is currently undergoing late-phase safety trials to assess its cardiovascular consequences.68 Three randomized controlled trials called Contrave Obesity Research trials (COR-1,69 n=1742; COR-II,70 n= 1,496; and COR-BMOD,71 n= 793) suggest efficacy: ~4–5 kg more weight loss with naltrexone-SR plus bupropion-SR 32/360mg than with placebo at 1year, and with 48–66%, versus 16–42% of placebo-treated participants, losing ≥5% of initial body weight and 25–42%, versus 6–20%, losing ≥10% of initial body weight at 1 year, varying with intensity of the lifestyle intervention. The Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA), injectable incretins approved for treatment of type 2 diabetes, are known to produce weight loss. A meta-analysis of the effect of GLP-1RA on body weight found a placebo-subtracted weight reduction of approximately 3% at 6 to 12 months72 and studies in obese patients without diabetes have found additional weight loss relative to placebo at 6 to 12 months of 3.5 to 5.8 kg.73, 74 Both liraglutide75 and exenatide76 are in late phase clinical trials as obesity treatments. A recently completed phase 3 trial77 evaluating liraglutide 3.0mg/d vs. placebo for weight maintenance in 422 non-diabetic overweight and obese patients (72% retention) who successfully lost ≥5% initial weight during a 4–12 week dietary run-in, found that weight decreased an additional 6.2% in the active treatment group over the ensuing 56 weeks, a placebo subtracted-difference of −6.1%. Both groups received face-to-face lifestyle counseling throughout the trial. Those on drug were more likely to both maintain their initial weight loss (81 vs. 49%) and to lose ≥5% (51 vs. 22%) or ≥10% (26 vs. 6%) additional weight than those taking placebo during follow-up, suggesting a potential role for liraglutide in augmenting weight loss or ameliorating regain after initial weight loss achieved through lifestyle intervention. Recently, concerns have emerged regarding an increased risk of pancreatitis and pancreatic cancer with GLP-1RA,78 although additional research is necessary to determine causality and clinical significance.
DISCUSSION
Rational Use of Medications in Obesity Management
The scientific literature on drug treatment for obesity is limited, particularly for studies conducted before the requirement for registration of all clinical trials, by short intervention periods, high attrition, inadequate description of methods, and data analyses that used biased approaches to deal with missing data79 or concentrated on results of those completing the trial.
Orlistat, lorcaserin, and phentermine/topiramate-ER, when used as an adjunct to lifestyle intervention, all increase the likelihood that a patient will achieve a clinically-meaningful (≥5%) 1-year weight loss. Because obesity contributes to many diseases, medications to help patients lose weight and sustain weight loss could potentially lead to improvements in multiple domains. Weight loss achieved through lifestyle modification and bariatric surgery has been shown to result in many such improvements; however, one cannot extrapolate from these studies to assume similar benefits will be attributable to weight loss attained with medications. Once established, obesity, like hypertension or dyslipidemia, requires long-term treatment. Therefore, medications for obesity treatment must be viewed through the lens of long-term use when evaluating their safety and efficacy.
A lesson from the withdrawal of previous anti-obesity drugs is that uncommon but serious adverse effects may become apparent only when a drug is used in larger populations or for longer periods of time than in pre-approval trials.80 Given that more than one-third of the US adult population is obese, there is great potential exposure to any obesity medication. Because weight stigma is prevalent in the population and thinness is valued, misuse of medications for cosmetic purposes is also a concern, particularly among women.81 However, untreated obesity confers risk; thus, the adverse effects of medication must be weighed against the health benefits that may result from successfully treated obesity, including improvements in feeling, functioning, and obesity-related comorbidities.82
Obesity drugs that are approved for long-term use result, on average, in additional weight loss relative to placebo ranging from ~3% for orlistat and lorcaserin to 9% for phentermine/topiramate-ER at one year. Mean total weight loss can be 1–5% greater than these placebo-subtracted values, and varies based on factors including patient population and intensity of concomitant lifestyle intervention. However, it is only for those who lose weight successfully that a drug's benefits might conceivably exceed its risks. Unfortunately, there are few consistent pre-treatment predictors for response to a given medication. Most studies have shown that initial weight loss response at 12 weeks predicts later weight loss at ≥1 year.53, 83, 84 Therefore, if a patient does not lose at least 5% of initial weight after 12 weeks of therapy (after assessment for adherence and, where appropriate, an increase in dosage), that patient is more likely than those achieving this threshold to be exposed to the risks and costs of drug treatment when there is little prospect of long-term benefit. Depending upon the medication used, patient population studied, and intensity of concomitant lifestyle intervention, from 30% to more than 60% of drug-treated patients may not achieve a 5% weight reduction at 12 weeks.83, 85 In such cases, the clinician should assess the balance of benefits and risks, consider discontinuing the medication, and re-evaluate treatment options, including intensification of behavioral strategies, use of a medication with a different mechanism of action, reassessment and management of medical or other contributory factors, or referral for evaluation for bariatric surgery if otherwise appropriate. The recommendation to discontinue drug with insufficient weight loss after an adequate trial is included in the labeling for both lorcaserin and for phentermine-topiramate-ER.52, 54 The FDA labels have a 12-week threshold of <3% weight loss for discontinuation or escalation of recommended-dose phentermine-topiramate-ER (7.5/46mg) and a 12-week threshold of <5% for discontinuation of top-dose phentermine-topiramate-ER (15/92mg) and lorcaserin. No discontinuation recommendations based on weight loss are included in the product labels for orlistat or the noradrenergic drugs, although the latter are approved only for short-term use.
In 2011, approximately 2.74 million patients were estimated to use obesity drugs in the US,41 a small number given the high prevalence of obesity. Barriers to the initiation or sustained use of obesity medications include costs, safety concerns, perception of limited efficacy, and reluctance to view obesity as a disease requiring medical treatment.41, 86 Studies with medications approved for long-term use have demonstrated improvements, compared with placebo, in patients' progression to diabetes and in many cardiovascular disease risk factors. It should be noted, however, that no weight loss medication (or behavioral treatment87) has been shown to have a favorable effect on cardiovascular morbidity and mortality, and the Endocrinologic and Metabolic Drugs Advisory Committee has recommended to the FDA that all new medications reviewed for an obesity indication undergo premarket testing to ensure that they do not increase CVD events.88
As recommended by the US Preventive Services Task Force, physicians should offer or refer their patients with obesity for high-intensity multicomponent behavioral interventions.4 Comprehensive lifestyle interventions not only help patients to make the critical dietary and physical activity changes necessary for successful weight loss, but lead to better weight loss than provision of medication alone.89 It is reasonable to advise patients who, during their lifetimes, have not previously participated in a comprehensive lifestyle intervention program, preferably of high-intensity, to do so prior to initiating obesity medication as a substantial proportion will respond to lifestyle treatment alone with clinically-meaningful weight loss.5 Effective treatment can be provided in primary care settings, specialized weight management clinics, community-based programs, through referral to a nutrition professional, via telephonically- or electronically-delivered interventions, or through commercial programs that are evidence-based.5 Once this criterion has been met, however, there are no data to support requirements for an arbitrary length of treatment failure with behavioral intervention prior to prescription of obesity drugs,90 particularly for patients who have a history of multiple unsuccessful attempts to lose weight or sustain weight loss. Although adding medications as a “rescue strategy” only for patients who do not lose weight after several months of behavioral treatment is attractive in theory, the non-randomized addition of orlistat for non-responders to an intensive lifestyle intervention did not suggest benefit.91 Clinical trials examining the efficacy of medications as rescue therapy are needed. An intermittent strategy for use of obesity drugs (e.g., taking medication during alternating months) has been reported to have efficacy in a few small trials,42, 92, 93 but the benefits from this approach with newer medications and in broader populations are unknown. Similarly, the usefulness of adding obesity medications after successful weight loss achieved through lifestyle intervention in order to help patients improve or sustain their weight loss long-term77, 94 appears promising and deserves further study, including evaluating both continuous and intermittent administration.
The goal of obesity medication use is to improve a patient's health and quality of life. Therefore, clinicians may wish to consider factors other than BMI alone when deciding whether or not to add an obesity medication to a patient's weight management regimen.82, 90, 95 For example, a patient with a BMI of 30 kg/m2 who has prediabetes and knee osteoarthritis may warrant greater consideration of adjunctive obesity medication use; for a patient with a similar BMI but no elevation in cardiometabolic risk or other obesity related conditions, the balance of benefits to risks may be less favorable. It is also possible, however, that obesity medications that elevate pulse and/or blood pressure could actually increase risk in patients at highest risk for cardiovascular disease.80 Initial choice of a specific medication can be influenced by demographic factors such as sex and age, concomitant medications and medical conditions, drug efficacy, response to treatment, adverse effect profile, availability of long-term safety data, and cost. For women with reproductive potential the increased likelihood of weight loss of 10% or more along with improvements in existing comorbid conditions with phentermine/topiramate-ER must be weighed against the teratogenic risk of the topiramate component and the need for monthly pregnancy testing. Similarly, extreme caution should be used when considering prescribing lorcaserin to patients taking a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor due to the potential for serotonin syndrome. Phentermine has the advantage of low cost and many years of clinical experience, but its long-term use is considered off-label, long-term effects on CVD outcomes are unknown, and most use has been a few months or less.41 There are even fewer data for long-term safety and efficacy of the other noradrenergic agents. Orlistat has a reasonably good safety profile, but modest weight loss and unpleasant gastrointestinal adverse effects limit its acceptability to patients. Medications used off-label for weight loss have not been sufficiently tested for long-term safety and efficacy to be recommended outside of clinical trials.
Many patients with obesity take multiple medications, some of which are associated with significant weight gain. It is helpful to evaluate patients' medication regimens for drugs that may be contributing to weight gain and to consider adding or substituting drugs with weight-neutral or weight-loss potential where medically appropriate, such as bupropion for depression or smoking cessation, zonisamide or topiramate for mood stabilization,96 or metformin for diabetes or prediabetes.2 Clinicians should be aware of the need to monitor patients using antihypertensive therapy or taking diabetes medications that can cause hypoglycemia when initiating treatment with drugs that may cause weight loss. Medication adjustment may be necessary to decrease the risks of hypotension or hypoglycemia, particularly during the initial period of more rapid weight loss.
Because combination pharmacotherapy for obesity deploys medications with differing mechanisms of action, it offers the prospect of overcoming the counter-regulatory mechanisms that become manifest in the weight reduced state. Combination therapy may also allow prescription of lower doses of each medication to minimize adverse effects.97 The first combination medication for obesity treatment has been approved, and others are in development.97 Unfortunately there are few studies examining the safety and efficacy of many of the drug combinations for obesity currently being prescribed. A survey of bariatric physicians found that 65% reported prescribing combinations of medications off-label to treat obesity, including 20% who prescribed 5-hydroxytryptophan/carbidopa plus phentermine.98 Use of non-approved drug combinations for obesity treatment should be limited to clinical trials, and patients should be informed when drugs are being used off-label alone or in combination.
Our systematic review was limited to currently-approved medications with at least 1 year of data with relatively large sample sizes, and we did not systematically review drugs used off-label or drugs in development. Even the included studies are frequently limited by their high attrition rates. Many were efficacy, rather than effectiveness trials and thus may not reflect patient outcomes in real-world clinical settings. Finally, there were few longer term data (>2y) from controlled trials of drugs used for obesity treatment to provide information on long-term risks and benefits.
New drugs for obesity treatment provide additional options for weight management. For carefully-selected patients who respond with clinically-meaningful weight loss accompanied by improvements in feeling, functioning, CVD risk factors, or other obesity-related comorbid conditions, obesity drugs may be useful adjuncts to lifestyle treatment. However, no obesity medication has been shown to reduce cardiovascular morbidity or mortality. Additional studies are needed to determine the long-term health effects of obesity medications in large and diverse patient populations.
Acknowledgments
Dr. Susan Yanovski and Dr. Jack Yanovski had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the funding organization: The conduct of this research was supported in part by the Intramural Research Program of NICHD grant 1ZIAHD000641 (to J. Yanovski). The NIDDK and NICHD had no role in the design and conduct of the study, collection, management, analysis, or interpretation of the data, preparation of the manuscript for publication, or decision to submit the manuscript for publication, but did review the manuscript and approve its submission. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the US Public Health Service, the National Institutes of Health, or the US Department of Health and Human Services.
Footnotes
Conflict of Interest Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan DH, Bray GA. Pharmacologic treatment options for obesity: what is old is new again. Curr Hypertens Rep. 2013;15(3):182–189. doi: 10.1007/s11906-013-0343-6. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157(5):373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 5.Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Annals of the New York Academy of Sciences. 2013;1281:191–206. doi: 10.1111/nyas.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadden TA, Volger S, Tsai AG, et al. Managing obesity in primary care practice: an overview with perspective from the POWER-UP study. International journal of obesity. 2013;37(Suppl 1):S3–S11. doi: 10.1038/ijo.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(6):509–517. doi: 10.1111/j.1467-789X.2011.00972.x. [DOI] [PubMed] [Google Scholar]
- 9.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care. 1998;21(8):1288–1294. doi: 10.2337/diacare.21.8.1288. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 11.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA : the journal of the American Medical Association. 1999;281(3):235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 12.Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(3):306–313. doi: 10.1038/sj.ijo.0801128. [DOI] [PubMed] [Google Scholar]
- 13.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Archives of family medicine. 2000;9(2):160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 14.Lindgarde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish Multimorbidity Study. J Intern Med. 2000;248(3):245–254. doi: 10.1046/j.1365-2796.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 15.Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res. 2000;8(1):49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- 16.Broom I, Wilding J, Stott P, Myers N. Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. International journal of clinical practice. 2002;56(7):494–499. [PubMed] [Google Scholar]
- 17.Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes, obesity & metabolism. 2002;4(6):415–423. doi: 10.1046/j.1463-1326.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- 18.Krempf M, Louvet JP, Allanic H, Miloradovich T, Joubert JM, Attali JR. Weight reduction and long-term maintenance after 18 months treatment with orlistat for obesity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(5):591–597. doi: 10.1038/sj.ijo.0802281. [DOI] [PubMed] [Google Scholar]
- 19.Miles JM, Leiter L, Hollander P, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes care. 2002;25(7):1123–1128. doi: 10.2337/diacare.25.7.1123. [DOI] [PubMed] [Google Scholar]
- 20.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Berne C. A randomized study of orlistat in combination with a weight management programme in obese patients with Type 2 diabetes treated with metformin. Diabetic medicine : a journal of the British Diabetic Association. 2005;22(5):612–618. doi: 10.1111/j.1464-5491.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- 22.Swinburn BA, Carey D, Hills AP, et al. Effect of orlistat on cardiovascular disease risk in obese adults. Diabetes, obesity & metabolism. 2005;7(3):254–262. doi: 10.1111/j.1463-1326.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 23.Derosa G, Cicero AF, D'Angelo A, Fogari E, Maffioli P. Effects of 1-year orlistat treatment compared to placebo on insulin resistance parameters in patients with type 2 diabetes. Journal of clinical pharmacy and therapeutics. 2012;37(2):187–195. doi: 10.1111/j.1365-2710.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 24.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(12):1437–1446. doi: 10.1038/sj.ijo.0802475. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142(7):532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 26.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335(7631):1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. The American journal of clinical nutrition. 2004;80(6):1461–1468. doi: 10.1093/ajcn/80.6.1461. [DOI] [PubMed] [Google Scholar]
- 29.Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(5):383–392. doi: 10.1111/obr.12015. [DOI] [PubMed] [Google Scholar]
- 30.O'Neil PM, Smith SR, Weissman NJ, et al. Randomized Placebo-Controlled Clinical Trial of Lorcaserin for Weight Loss in Type 2 Diabetes Mellitus: The BLOOM-DM Study. Obesity (Silver Spring) 2012;20(7):1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 31.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 32.Fidler MC, Sanchez M, Raether B, et al. A One-Year Randomized Trial of Lorcaserin for Weight Loss in Obese and Overweight Adults: The BLOSSOM Trial. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 33.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 35.Vivus, Data on file. New Drug Application 022580: Integrated Summary of Effectiveness. Submitted Oct 17, 2011. Page 209 of 318. Vivus Inc.; 2011. [Google Scholar]
- 36.Roberts MD. US Food and Drug Administration Endocrinologic and Metabolic Drugs Advisory Committee Meeting Clinical Briefing Document. US Food and Drug Administration; Silver Spring, MD: 2012. [Accessed September 21, 2013]. New Drug Application 22580: VI-0521 QNEXA (phentermine/topiramate) Sponsor: VIVUS. February 22, 2012. Table 7; p. 32. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/End ocrinologicandMetabolicDrugsAdvisoryCommittee/UCM292315.pdf. [Google Scholar]
- 37.Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002;26(2):262–273. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- 38.Colman E. Food and Drug Administration's Obesity Drug Guidance Document: a short history. Circulation. 2012;125(17):2156–2164. doi: 10.1161/CIRCULATIONAHA.111.028381. [DOI] [PubMed] [Google Scholar]
- 39.Ioannides-Demos LL, Proietto J, McNeil JJ. Pharmacotherapy for obesity. Drugs. 2005;65(10):1391–1418. doi: 10.2165/00003495-200565100-00006. [DOI] [PubMed] [Google Scholar]
- 40.Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011;19(12):2351–2360. doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- 41.Hampp C, Kang EM, Borders-Hemphill V. Pharmacotherapy. 2013. Use of Prescription Antiobesity Drugs in the United States. doi:10.1002/phar.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1(5588):352–354. doi: 10.1136/bmj.1.5588.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runyan JW., Jr Observations on the use of phendimetrazine, a new anorexigenic agent, in obese diabetics. Current therapeutic research, clinical and experimental. 1962;4:270–275. [PubMed] [Google Scholar]
- 44.Hadler AJ. Sustained-action phendimetrazine in obesity. The Journal of clinical pharmacology and the journal of new drugs. 1968;8(2):113–117. doi: 10.1002/j.1552-4604.1968.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 45.Erdmann J, Lippl F, Klose G, Schusdziarra V. Cholesterol lowering effect of dietary weight loss and orlistat treatment--efficacy and limitations. Aliment Pharmacol Ther. 2004;19(11):1173–1179. doi: 10.1111/j.1365-2036.2004.01966.x. [DOI] [PubMed] [Google Scholar]
- 46.Johansson K, Sundstrom J, Neovius K, Rossner S, Neovius M. Long-term changes in blood pressure following orlistat and sibutramine treatment: a meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11(11):777–791. doi: 10.1111/j.1467-789X.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhou YH, Ma XQ, Wu C, et al. Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2012;7(6):e39062. doi: 10.1371/journal.pone.0039062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche Laboratories, Inc. [Accessed July 2, 2013];XENICAL - orlistat capsule. 1/2009; http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf.
- 49.Cavaliere H, Floriano I, Medeiros-Neto G. Gastrointestinal side effects of orlistat may be prevented by concomitant prescription of natural fibers (psyllium mucilloid) Int J Obes Relat Metab Disord. 2001;25(7):1095–1099. doi: 10.1038/sj.ijo.0801645. [DOI] [PubMed] [Google Scholar]
- 50.Padwal R, Kezouh A, Levine M, Etminan M. Long-term persistence with orlistat and sibutramine in a population-based cohort. International journal of obesity. 2007;31(10):1567–1570. doi: 10.1038/sj.ijo.0803631. [DOI] [PubMed] [Google Scholar]
- 51.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 52.Eisai Inc. [Accessed June 27, 2013];BELVIQ (lorcaserin hydrochloride) tablets, for oral use. 01/04/2013; http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf.
- 53.Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. The FDA's assessment of two drugs for chronic weight management. The New England journal of medicine. 2012;367(17):1577–1579. doi: 10.1056/NEJMp1211277. [DOI] [PubMed] [Google Scholar]
- 54.Vivus Inc. [Accessed June 28, 2013];Qsymia (phentermine and topiramate extended-release) capsules, for oral use. 4/16/2013; http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022580s004lbl.pdf.
- 55.Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index >/=27 kg/m(2) The American journal of cardiology. 2013;111(8):1131–1138. doi: 10.1016/j.amjcard.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 56.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margulis AV, Mitchell AA, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. American journal of obstetrics and gynecology. 2012;207(5):405, e401–407. doi: 10.1016/j.ajog.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vivus Inc. NDA 22580: QSYMIA (phentermine and topiramate extended-release) Capsules. Risk evaluation and mitigation strategy (REMS); [Accessed 7/3/2013]. Reference ID: 3294731. 4/2013; http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM312598.pdf. [Google Scholar]
- 59.Tran PT. [Accessed 7/3/2013];Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting. 2012 Feb 22; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM304401.pdf.
- 60.Goldstein DJ, Rampey AH, Jr, Enas GG, Potvin JH, Fludzinski LA, Levine LR. Fluoxetine: a randomized clinical trial in the treatment of obesity. Int J Obes Relat Metab Disord. 1994;18(3):129–135. [PubMed] [Google Scholar]
- 61.LeBlanc E, O'Connor E, Whitlock EP, Patnode C, Kapka T. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Agency for Healthcare Research and Quality; Rockville (MD): 2011. Screening for and Management of Obesity and Overweight in Adults. Report No.: 11-05159-EF-1. [PubMed] [Google Scholar]
- 62.Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes care. 2012;35(4):731–737. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520–1530. doi: 10.1038/npp.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gadde KM, Franciscy DM, Wagner HR, 2nd, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289(14):1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 65.Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(2):169–180. doi: 10.1111/j.1463-1326.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- 66.Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity (Silver Spring) 2010;18(9):1739–1746. doi: 10.1038/oby.2009.478. [DOI] [PubMed] [Google Scholar]
- 67.Tran PT, Thomas A. Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee. [Accessed July 3, 2013];U.S. Food and Drug Administration Center for Drug Evaluation and Research. 2010 Dec 7; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM241508.pdf.
- 68.Orexigen Therapeutics, Inc. [Accessed June 27, 2012];Orexigen® Announces Agreement From the FDA on a Special Protocol Assessment for the Contrave® Outcomes Trial. 2012 Feb 6; http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1656731&highlight=.
- 69.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 70.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity. 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;2012:672658. doi: 10.1155/2012/672658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. International journal of obesity. 2012;36(6):843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33(6):1173–1175. doi: 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Novo Nordisk [Accessed July 5, 2013];Effect of Liraglutide on Body Weight in Non-diabetic Obese Subjects or Overweight Subjects With Co-morbidities: SCALE™ - Obesity and Pre-diabetes. 2013 Apr 30; http://www.clinicaltrials.gov/ct2/show/NCT01272219.
- 76.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [Accessed July 5, 2013];The Effects of Exenatide (Byetta™) on Energy Expenditure and Weight Loss in Nondiabetic Obese Subjects. 2013 May 21; http://www.clinicaltrials.gov/ct2/show/NCT00856609.
- 77.Wadden T, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie diet-induced weight loss: the SCALE Maintenance randomized study. International journal of obesity. 2013 doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 78.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies--review of the scientific evidence. J Clin Endocrinol Metab. 2011;96(7):2027–2031. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 79.Simons-Morton DG, Obarzanek E, Cutler JA. Obesity research--limitations of methods, measurements, and medications. JAMA : the journal of the American Medical Association. 2006;295(7):826–828. doi: 10.1001/jama.295.7.826. [DOI] [PubMed] [Google Scholar]
- 80.Lauer MS. Lemons for obesity. Ann Intern Med. 2012;157(2):139–140. doi: 10.7326/0003-4819-157-2-201207170-00438. [DOI] [PubMed] [Google Scholar]
- 81.Blanck HM, Khan LK, Serdula MK. Prescription weight loss pill use among Americans: patterns of pill use and lessons learned from the fen-phen market withdrawal. Prev Med. 2004;39(6):1243–1248. doi: 10.1016/j.ypmed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 82.Kahan S, Ferguson C, David S, Divine L. Obesity drug outcome measures: Results of a multi-stakeholder critical dialoque. Current Obesity Reports. 2013;2(2):128–133. [Google Scholar]
- 83.Rissanen A, Lean M, Rossner S, Segal KR, Sjostrom L. Predictive value of early weight loss in obesity management with orlistat: an evidence-based assessment of prescribing guidelines. Int J Obes Relat Metab Disord. 2003;27(1):103–109. doi: 10.1038/sj.ijo.0802165. [DOI] [PubMed] [Google Scholar]
- 84.Finer N, Ryan DH, Renz CL, Hewkin AC. Prediction of response to sibutramine therapy in obese non-diabetic and diabetic patients. Diabetes, obesity & metabolism. 2006;8(2):206–213. doi: 10.1111/j.1463-1326.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 85.Golden J. US Food and Drug Administration Endocrinologic and Metabolic Drugs Advisory Committee Clinical Briefing Document May 10, 2012. Arena Pharmaceuticals, Inc; [Accessed 10/5/2013]. New Drug Application 022529: Lorcaserin hydrochloride tablets 10mg. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM303198.pdf. [Google Scholar]
- 86.Greenway FL, Caruso MK. Safety of obesity drugs. Expert opinion on drug safety. 2005;4(6):1083–1095. doi: 10.1517/14740338.4.6.1083. [DOI] [PubMed] [Google Scholar]
- 87.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran PT, Thomas A. [Accessed July 3, 2013];Summary Minutes of the Endocrinologic and Metabolic Drugs Advisory Committee Meeting March 28 – 29, 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM303352.pdf.
- 89.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 90.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013;19(2):327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 91.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weintraub M, Sundaresan PR, Schuster B, et al. Long-term weight control study. II (weeks 34 to 104). An open-label study of continuous fenfluramine plus phentermine versus targeted intermittent medication as adjuncts to behavior modification, caloric restriction, and exercise. Clin Pharmacol Ther. 1992;51(5):595–601. doi: 10.1038/clpt.1992.70. [DOI] [PubMed] [Google Scholar]
- 93.Silverstone T. Intermittent treatment with anorectic drugs. Practitioner. 1974;213(1274):245–252. [PubMed] [Google Scholar]
- 94.Hill JO, Hauptman J, Anderson JW, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-y study. Am J Clin Nutr. 1999;69(6):1108–1116. doi: 10.1093/ajcn/69.6.1108. [DOI] [PubMed] [Google Scholar]
- 95.Garvey WT. New Tools for Weight Loss Therapy Enable a More Robust Medical Model for Obesity Treatment: Rationale for a Complications-Centric Approach. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013:1–31. doi: 10.4158/EP13263.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 97.Gadde KM, Allison DB. Combination pharmaceutical therapies for obesity. Expert Opin Pharmacother. 2009;10(6):921–925. doi: 10.1517/14656560902824152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17(9):1730–1735. doi: 10.1038/oby.2009.69. [DOI] [PubMed] [Google Scholar]
- 99.Drug ABC List Prices. [Accessed March 8, 2013];Accessed by Richard Decederfelt, B.S.Pharm., M.S.I.S. https://passport.amerisourcebergen.com/irj/portal.
- 100.Prescription Medications for the Treatment of Obesity. [Accessed September 25, 2013, 2013];NIDDK Weight-control Information Network Fact Sheet. NIH Publication No. 07-4191 [April 2013; http://win.niddk.nih.gov/publications/PDFs/Prescription_Medications.pdf.