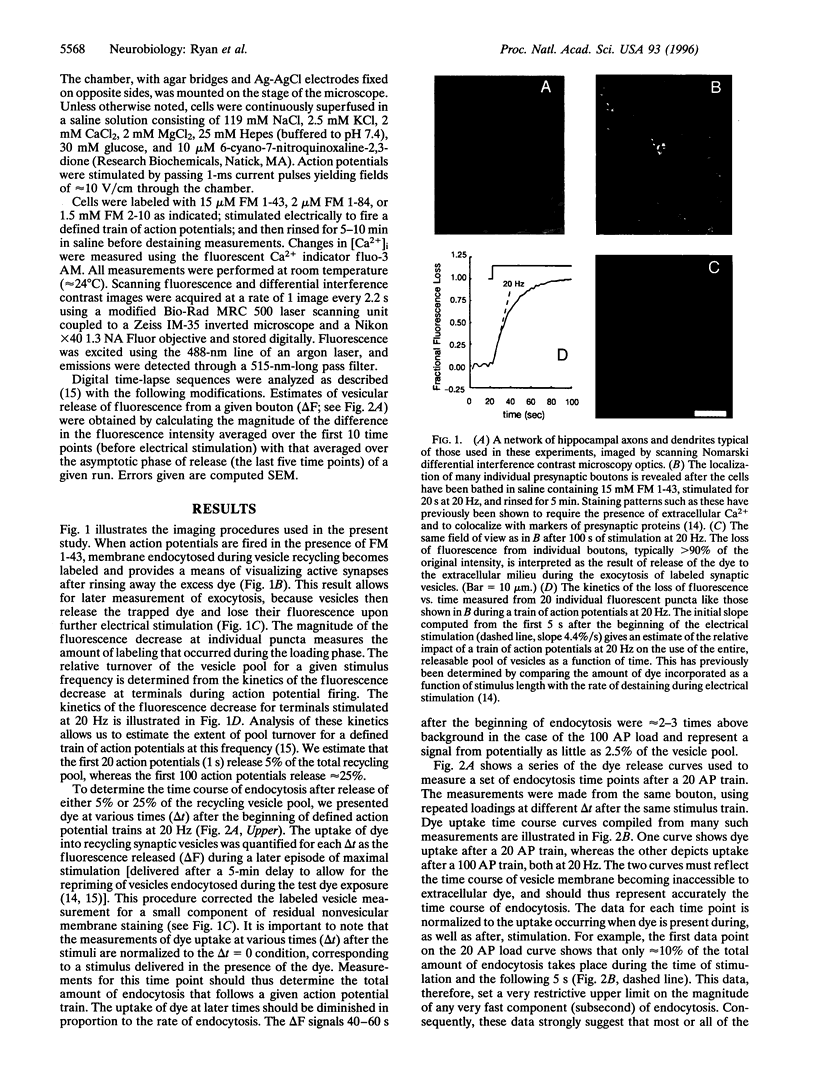
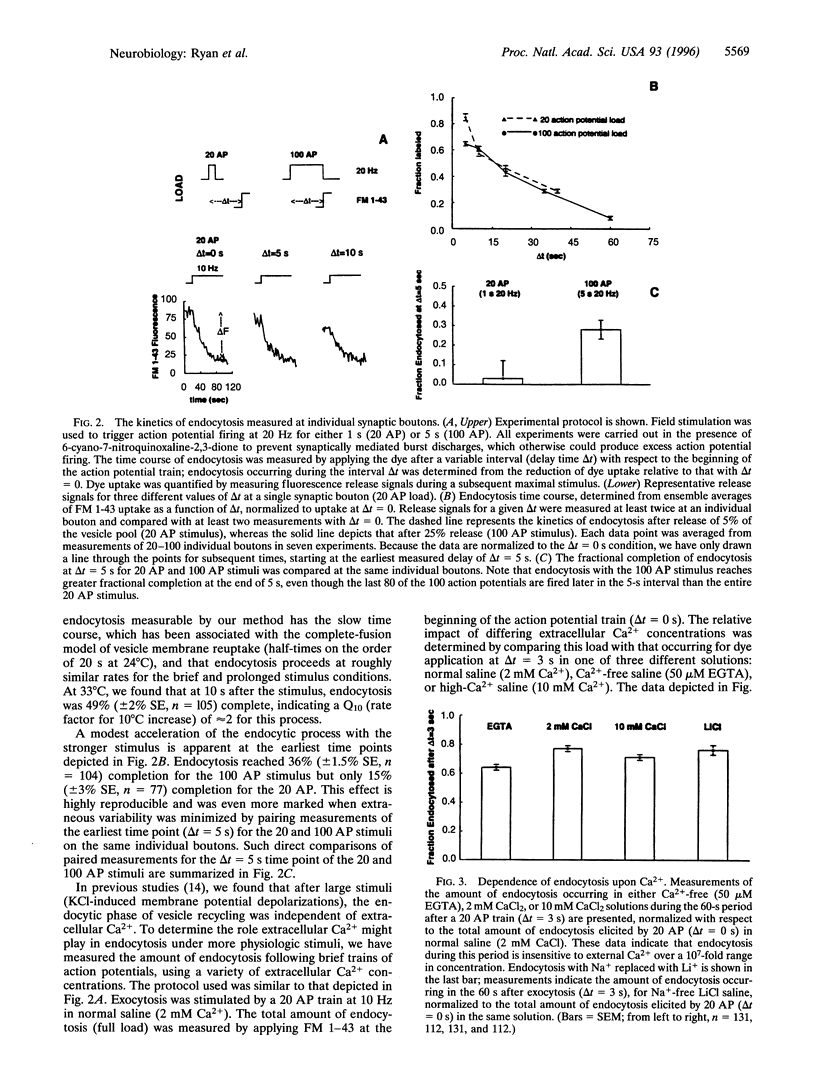
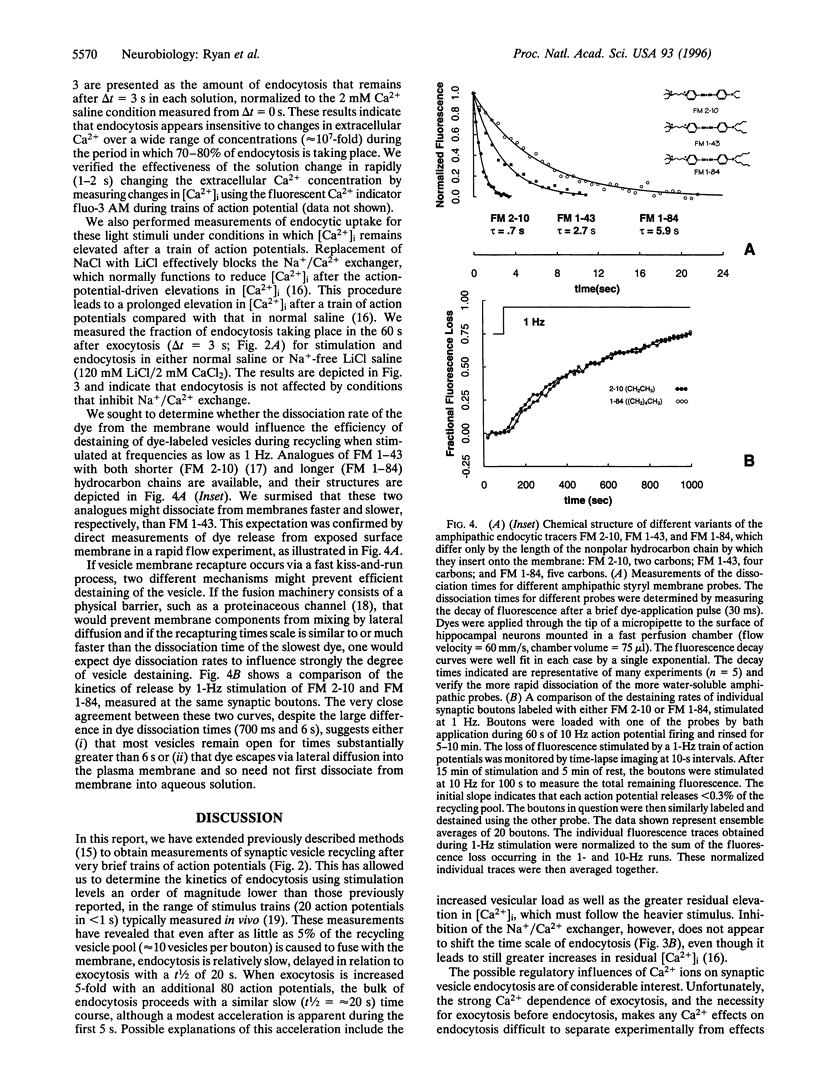
Abstract
Alternative models to describe the endocytosis phase of synaptic vesicle recycling are associated with time scales of vesicle recovery ranging from milliseconds to tens of seconds. There have been suggestions that one of the major models, envisioned as a slow process that occurs only after complete fusion of the vesicle membrane with the neurolemma, might be applicable only under conditions of heavy, nonphysiological stimulation. Using FM 1-43 and similar fluorescent probes to label recycling synaptic vesicles in rat hippocampal neurons, we have measured the kinetics of endocytosis with a wide range of action-potential-driven exocytotic loads. Our results indicate that when either 5% or 25% of the vesicle pool is used, vesicles are recovered with a half-time on the order of 20 s (24 degrees C). This endocytosis rate was not influenced by operations designed to alter intracellular Ca2+ during membrane retrieval, suggesting that residual Ca2+ after strong stimuli probably does not greatly retard endocytosis. Finally, we have shown that vesicle-destaining kinetics are not strongly influenced by the substantially differing rates at which two marker dyes tested dissociate from membranes. This observation suggests that vesicles remain open long enough for essentially complete dissociation of even the slower dye (a few seconds) or, alternatively, that both dyes readily escape vesicle membrane by lateral diffusion through any exocytotic opening. These data seem most consistent with applicability of the slow-endocytosis, complete-fusion model at low as well as high levels of exocytosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Tse F. W. Transmitter release from synapses: does a preassembled fusion pore initiate exocytosis? Neuron. 1990 Jun;4(6):813–818. doi: 10.1016/0896-6273(90)90134-2. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernández-Chacón R., Fernández J. M. Release of secretory products during transient vesicle fusion. Nature. 1993 Jun 10;363(6429):554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Bewick G. S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992 Jan 10;255(5041):200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Bewick G. S. Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction. J Physiol. 1993 Jan;460:287–309. doi: 10.1113/jphysiol.1993.sp019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980 Oct;87(1):297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R., Grohovaz F., Valtorta F., Meldolesi J. Neurotransmitter release: fusion or 'kiss-and-run'? Trends Cell Biol. 1994 Jan;4(1):1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Stevens J. K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989 Aug;9(8):2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P., Chieregatti E., Valtorta F., Haimann C. Alpha-latrotoxin channels in neuroblastoma cells. J Membr Biol. 1994 Feb;138(1):91–102. doi: 10.1007/BF00211072. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Ceccarelli B. Exocytosis and membrane recycling. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):55–65. doi: 10.1098/rstb.1981.0171. [DOI] [PubMed] [Google Scholar]
- Miller T. M., Heuser J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984 Feb;98(2):685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M., Krishnan K. S., Kelly R. B. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994 Aug;13(2):363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Porzig H. Localization and functional significance of the Na+/Ca2+ exchanger in presynaptic boutons of hippocampal cells in culture. Neuron. 1995 Nov;15(5):1077–1084. doi: 10.1016/0896-6273(95)90096-9. [DOI] [PubMed] [Google Scholar]
- Ribchester R. R., Mao F., Betz W. J. Optical measurements of activity-dependent membrane recycling in motor nerve terminals of mammalian skeletal muscle. Proc Biol Sci. 1994 Jan 22;255(1342):61–66. doi: 10.1098/rspb.1994.0009. [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Reuter H., Wendland B., Schweizer F. E., Tsien R. W., Smith S. J. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993 Oct;11(4):713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Smith S. J. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995 May;14(5):983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Thomas P., Lee A. K., Wong J. G., Almers W. A triggered mechanism retrieves membrane in seconds after Ca(2+)-stimulated exocytosis in single pituitary cells. J Cell Biol. 1994 Mar;124(5):667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A., Bragin A., Nádasdy Z., Jandó G., Szabó I., Sik A., Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995 Jan;15(1 Pt 1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994 Feb 24;367(6465):735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994 Aug 25;370(6491):652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]




