Abstract
Dendritic cells (DCs) play an important role in both immunological tolerance and immunity as well as in pro- and anti-inflammatory responses. Upon uptake of apoptotic cells and apoptotic bodies, DCs degrade self antigens from them and induce anti-inflammatory response and T regulatory (Treg) cells resulting in the maintenance of tolerance to self antigens. Ageing is characterized by progressive immunodeficiency, inflammation, and autoimmunity. In this paper we will review the role of DCs in inflammation and autoimmunity in human ageing and will highlight the observations that DCs from aged humans display impairment in their capacity to uptake apoptotic cells, and paradoxically induce proinflammatory response and increased reactivity to self antigen (DNA). DNA from aged is hypomethylated and hypomethylation is associated with increased immunogenecity of self DNA.
Keywords: Apoptosis, cytokines, dendritic cells, DNA methylation, tolerance
Introduction
Human ageing is characterized by progressive decline in immune functions resulting in increased susceptibility to infections and cancer, and increased production of proinflammatory cytokines, and elevated levels of autoantibodies1,2,3,4,5,6,7. Progressive decline in T cell number and functions appears to be due to involution of thymus and increased apoptosis. When cells die by apoptosis, they are taken up by phagocytic cells including dendritic cells (DCs), where self antigens are degraded, and anti-inflammatory response is induced as evident by production of transforming growth factor beta (TGFβ) and interleukin-10 (IL-10) and lack of presence of inflammatory cells. Furthermore, there is an induction of T regulatory (Treg) cells. Together, it results in the induction of tolerance to self antigens.
During the process of differentiation and maturation immature DCs are produced from stem cells. These cells are avidly phagocytic and express only a few surface antigens and induce tolerance. However, following uptake of antigens, DCs begin to express a number of antigens including major histocompatibility complex (MHC) antigens, and co-stimulatory molecules and migrate to lymphoid tissue, and differentiate into mature DCs, which are less phagocytic but interact with T cells, B cells, natural killer (NK) cells, and induce immune responses8.
In this review we briefly discuss our findings in the context of published data regarding DCs functions in ageing.
Apoptosis in human ageing
Apoptosis is a physiological form of programmed cell death that plays an important role in immunological tolerance, lysis of target cells by CD8+ T cells, and NK cells, and immune homeostasis9. There are three major pathways of apoptosis, namely death receptor-mediated, mitochondrial pathway, and the endoplasmic reticulum (ER) stress pathway10,11,12. There are several death receptors that induced apoptosis including CD95 and tumour necrosis factor (TNF) receptors (TNFR). In ageing, there is an increased production of TNF-α and soluble CD95 ligand. We and others have reported increased death-receptor-mediated (CD95 and TNF-R) and endoplasmic reticulum (ER)-stress pathway induced apoptosis of CD4+ and CD8+ T cells, and increased apoptosis is almost exclusively confined to naïve and central memory CD4+ and CD8+ T cells13,14,15,16,17,18,19. Increased apoptosis is also associated with changes in adapter molecules, activation of caspases, and changes in pro-apoptotic and anti-apoptotic genes13,17.
Role of DCs in inflammation in ageing
DCs provide an essential link between innate and adaptive immunity. DCs are composed of several subsets with distinct functions. The two major subpopulations in vivo are myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs)20. The mDCs exist in peripheral tissues, secondary lymphoid organs, and in the circulating blood. pDCs circulate in the blood and enter lymphoid organs through high endothelial venules. Compared to mDCs, they express different sets of Toll like receptors (TLRs)21. pDCs express TLR7 and TLR9 and upon stimulation secrete large amounts of interferon (IFN)-α22. In response to microbial infection, monocytes migrate into inflammatory sites and differentiate into DCs22. In vitro monocytes activated with granulocyte macrophage colony stimulating factor (GM-CSF) and IL-4 differentiates into MDDCs (monocyte-derived DCs).
Numbers and morphology of DCs and their differentiation and maturation
Several studies have reported age-related changes in the number and functions of DCs in ageing. We23 and others24,25,26 have reported normal percentages of circulating mDCs in aged humans. However, Della Bella and associates27 reported a decline in the percentage and numbers of mDCs in aged subjects. Similarly both normal and decreased numbers of pDCs have been reported26,28,29,30. We did not find any significant differences in the proportions of pDCs23. Variability in donor population may account for some of the conflicting data. Our ageing population was of middle and upper middle socio-economic status, and living active independent lives. Further, our subjects were required to discontinue any anti-oxidant or other supplement that might influence immune responses, at least seven days prior to donation of blood samples. DCs generated in vitro from aged human monocytes using GMCSF and IL-4 (MDDc) were similar in numbers and phenotype to MDDCs from young23. Further confirmation was obtained from the Affymetrix data which did not reveal a major difference in the gene expression profile of DC phenotypic markers (unpublished data). Exposure of MDDCs to lipopolysaccharide (LPS) resulted in comparable levels of activation between the aged and young DCs as measured by upregulation of CD40, CD80, CD86 CD83 and HLADR. Lung et al24 have reported similar findings. This would suggest that the differentiation and maturation of MDDCs in vitro does not change during ageing. Therefore, ageing in humans does not affect the numbers and phenotype of mDCs and pDCs, and in vitro differentiation and maturation of MDDCs.
Pro- and anti-inflammatory cytokine production by MDDCs in ageing
In ageing, increased plasma levels of proinflammatory cytokines have been reported2,3,4; however, the cellular sources are not defined. Our study showed that when MDDCs from young and aged humans were stimulated with LPS (via TLR4) and ss-RNA (via TLR7), aged DCs secreted significantly higher levels of IL-6 and TNF-α, whereas IL-10, IL-12p70 and IL-12-p40 secretion was comparable23. However, Lung et al24 observed no difference in proinflammatory cytokine secretion between the young and the aged.
Since LPS stimulate via TLR4 receptors, expression of TLR-4 on mDC was examined and no difference was observed on their expression on MDDCs from young and aged subjects23. This would imply that signaling pathway downstream of TLR-4 and in case of ss-RNA downstream of TLR-7 may be responsible for increased proinflammatory cytokines produced by aged MMDCs.
DCs play an important role in inflammation and clearing of the infections. DCs present at the sites of pathogen entry ‘sample’ their environment by phagocytosis, initiating specific immune responses when they sense microbes or tissue damage. The efficient uptake of pathogens is thus essential for generation of immunity against an infection. Since uptake of apoptotic bodies is associated with anti-inflammatory response, we examined the capacity of young and aged MDDCs to uptake apoptotic cells, Lucifer yellow as an example of micropinocytosis and of FITC (fluorescein isothiocynate)-conjugated dextran as an example of endocytosis23. An impairment of uptake of all three was observed suggesting that there is global defect of uptake of antigens by aged MDDCs rather than just an impairment of phagocytosis of apoptotic cells. We further showed that aged MDDCs produced proinflammatory cytokines instead of anti-inflammatory cytokines following uptake of apoptotic cells23.
PI3 kinase signaling pathways in MDDCs in ageing
To define the mechanism(s) which affect the functioning of DCs in aged, the role for the PI3K signaling pathway was investigated. The phosphoinositide 3-kinases (PI3Ks) are a conserved family of signal transduction enzymes that are involved in regulating cellular activation, inflammatory responses, chemotaxis, and apoptosis. PKB (protein kinase B)/Akt is a downstream target of PI3K and plays a key role in cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration. One of the major findings of our study was that the phosphorylation of AKT in response to LPS was reduced in MDDCs from aged individuals23. AKT is known to play a major role in cytoskeleton motility and survival in DCs. Defective functioning of this pathway is reported to result in decreased phagocytosis and migration of DCs. Recent evidence indicates that the PI3K/Akt signaling pathway may also function as an endogenous negative feedback or compensatory mechanism that serves to limit pro-inflammatory cytokines in response to injurious stimuli31,32. Reports indicate that there is cross-talk between the TLR and PI3K/Akt signaling pathways. Fukao and Koyasu31 reported that PI3K may be an endogenous negative feedback regulator of Toll-like receptor (TLR)-mediated inflammatory responses. Martin and colleagues32 have shown how the TLR and PI3K pathways serve to balance pro-inflammatory and anti-inflammatory responses. Our findings of reduced phosphorylation of AKT and concomitant increased phosphorylation of p38 MAPkinase in DCs from ageing23 are in agreement with above observations regarding a role of PI3K in regulation of inflammatory response. We also showed an impaired migration of LPS-stimulated DCs in aging is impaired in response to MIP-3β and stem cell-derived factor (SDF), which is independent of receptors of chemotactic factors23. We demonstrated that the treatment of MDDCs from young subjects with PI3K inhibitor LY294002 inhibited LPS-induced phosphorylation AKT with concomitant increased p38 phosphorylation, associated with decreased uptake of antigen, decreased migration, and increased cytokine production23. Therefore, an impaired function PI3K signaling pathway can affect multiple DC functions resulting in enhancement of proinflammatory cytokine secretion and simultaneous impairment of antigen uptake and migration of DCs.
PI3Kinase/AKT is regulated by a variety of intracellular and extracellular signals, and phosphatase and tensin homologue (PTEN) has emerged as one of its major negative regulators. Therefore, we examined the expression of PTEN at both RNA and protein levels23. PTEN expression was increased at both message and protein level, which may be responsible for impaired PI3K pathway in aged DCs.
Role of DCs in autoimmunity in ageing
In contrast to progressive decline in immune functions with advancing age, there is an increased reactivity to self and endogenous antigens as evidenced by the presence and increased titres of a variety of autoantibodies33,34, which suggest a loss of peripheral tolerance in ageing. The information regarding mechanisms of impaired tolerance in human ageing is limited. Apoptosis plays an important role in the effector functions and immune homeostasis. One of the critical steps in apoptosis is a rapid uptake of apoptotic cells and apoptotic bodies by neighbouring phagocytic cells, resulting in intracellular degradation of self antigens, and induction of anti-inflammatory response and generation of Treg. In ageing, apoptosis is increased whereas DCs are impaired in their capacity to uptake apoptotic cells23. As a consequence apoptotic cells would undergo secondary necrosis with additional proteolytic degradation of specific autoantigens, which may release endogenous danger signals like nuclear antigens clustered in apoptotic blebs and bodies (e.g. chromatic, DNA, RNA, histones, etc.), resulting in maturation of DCs, and T cell immunity to self antigens, and stimulating autoantibody responses35.
Increased reactivity of aged DCs to self DNA
DCs are unique antigen-presenting cells cause of their capacity to prime naïve T cells, and therefore, function as initiators of T cell immunity. DCs can prime or tolarize T cells. Under physiological conditions, DCs play a role in unresponsiveness to self antigens. DCs are essential for both central and peripheral tolerance. Impaired clearance of apoptotic cells has been implicated in autoimmune diseases like systemic lupus erythematosus36,37. Therefore, we compared priming capacity of young and aged DCs to self DNA. DNA was purified from young humans and delivered intracellularly to MDDCs from young and aged subjects and examined for activation, cytokine production and T cell proliferation38. The expression of co-stimulatory molecules CD80 and CD86 and the maturation molecule (CD83) in the inactivated and activated DCs was measured by flow cytometry. DNA-primed MDDCs from aged subjects upregulated co-stimulatory molecules, and secreted increased levels of IL-6 and IFN-α as compared to young MDDCs. Similar increase in cytokine secretion was observed from aged MDDCs in response to late apoptotic cells. Furthermore, DNA-primed aged MDDCs induced autologous T cell proliferation, whereas DNA-primed young MDDCs did not induce T cell proliferation, suggesting a role of DCs in increased reactivity to self DNA and a loss of tolerance in aged humans. This increased reactivity to DNA is independent of TLR-9. The expression of cytosolic DNA sensor DNA-dependent activator of IFN-regulatory factor (DAI) was comparable between young and aged, suggesting step downstream of cytosolic sensor may be involved in self-ractivity to DNA by aged DCs. Since there are several DNA censors, a possibility of involvement of one of those DNA sensors cannot be excluded. One of the steps downstream of DNA censors is the interferon-responsive factor-3 (IRF-3). We have reported an increased activation of IRF-3 transcription factor in MDDCs from aged in response to intracellular self DNA38. MDDCs from aged display higher basal levels of NF-κB activation, suggesting that DCs from aged are in an activated state.
Epigenetic modifications in ageing DNA increase its immunogenicity
Human DNA undergoes age-associated genetic and epigenetic changes39,40. During ageing cells and tissues become hypomethylated while selected genes becomes progressively hypermethylated41. There is a relationship between genomic instability, DNA damage, and ageing42. DNA repair mechanisms in ageing are impaired resulting in DNA lesions with single and double-stranded breaks43. Oxidative damage to DNA has been implicated in ageing and age-related degenerative disorders44.
We examined whether modifications in DNA (self antigen) play a role in inflammation and autoimmunity by rendering DNA more immunogenic. Human DNA is generally inert and does not stimulate DCs. However, we showed that DNA from aged mononuclear cells when introduced into young MDDCs resulted in upregulation of co-stimulatory molecules CD80 and CD86, and increased secretion of IFN-α, as compared to young DNA, suggesting an increased immunogenicity of aged DNA45. We further showed that DNA from aged subjects was hypomethylated compared to DNA from young subjects. When aged DNA was hypermethylated comparable to methylation of young DNA, aged DCs no longer induced increased levels of IFN-α, demonstrating that immunogenicity of mammalian DNA correlates inversely with DNA methylation. Though DNA oxidative damage is well documented in ageing, the intracellular delivery of oxidative-damaged DNA does not result in the activation of MDDCs. This suggests that DNA damage per se does not increase immunogenicity of aged DNA, and hypomethylation of DNA is responsible for its increased immunogenicity in ageing. The site-specific hypomethylation confer increased immunogenicity to self DNA in ageing remains to be defined.
In summary, DCs from aged humans demonstrate impaired signaling through PI3K pathways and increased reactivity to self antigen (DNA) resulting in increased inflammation, decreased migration and uptake of antigens, and increased reactivity to DNA (autoimmunity), which appears to be alterations in both aged DCs and aged DNA (Figure).
Fig.
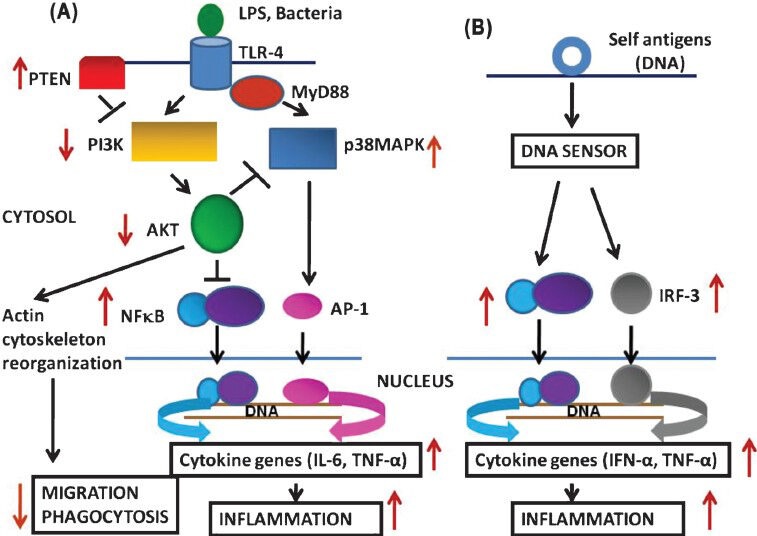
Normal signaling pathways and alterations in aged DCs in response to lipopolysaccharide (LPS)/bacteria (A), and in response to self antigen (DNA). IRF-3: Interferon responsive factor-3. Black arrows show normal responses; red arrows show changes in aged DCs. In ageing, in response to exogenous stimuli (LPS/Bacteria) there is an impairment in P13 kinase signaling pathway that is associated with decreased phosphorylation of AKT resulting in decreased migration and phagocytosis, and increased NF-κB activation resulting increased proinflammatory cytokine production (A). In response to self DNA (B) aged DCs secrete proinflammatory cytokines and T-cell proliferation indicating increased reactivity to self antigen.
Acknowledgment
Part of this work was supported by grants from the National Institutes of Health (AIG 027512), and Ellison Medical Foundation, USA.
References
- 1.Powlec G, Barnett Y, Effros R, Forsey R, Frasca D, Globerson A, et al. T cells and aging. Front Biosci. 2002;7:d1058–83. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- 2.Fagiola U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–8. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 3.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Rink I, Kirchner H. Altered cytokine production in the elderly. Mech. Ageing Develop. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 5.Rowley MJ, Buchanan H, Mackay IR. Reciprocal change with age in antibody to extrinsic and intrinsic antigens. Lancet. 1968;2:24–6. doi: 10.1016/s0140-6736(68)92893-6. [DOI] [PubMed] [Google Scholar]
- 6.Manavalan JS, Kirman I, Zhao K, Weksler ME. Aging and autoimmunity. In: Rose NR, Mackay IR, editors. The autoimmune diseases. San Diego: Academic Press; 1998. pp. 783–94. [Google Scholar]
- 7.Tomer Y, Shoenfeld Y. Aging and autoantibodies. Autoimmunity. 1988;1:145–9. doi: 10.3109/08916938809001927. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Schmitt N, Klechevsky E, Pedroza-Goanzales A, Matsui T, Zurawski G, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S. Molecular steps of cell suicide: An insight into immune senescence. J Clin Immunol. 2000;20:229–39. doi: 10.1023/a:1006653917314. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 11.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 12.Orrenius S, Zhivotovsky B, Nicotera N. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: Altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–37. [PubMed] [Google Scholar]
- 14.Aggarwal S, Gollapudi S, Gupta S. Increased TNF-α-induced apoptosis in lymphocytes from aged humans: changes in TNF-α receptor expression and activation of caspases. J Immunol. 1999;162:2154–61. [PubMed] [Google Scholar]
- 15.Gupta S. Tumor necrosis factor-α-induced apoptosis in T cells from aged humans: a role of TNFR-I and downstream signaling molecules. Exp Gerontol. 2002;37:293–9. doi: 10.1016/s0531-5565(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Bir Gollapudi S. Central and effector memory CD4+ and CD8+ T cells display differential sensitivity to TNF-α-induced apoptosis. Ann NY Acad Sci. 2005;1050:108–15. doi: 10.1196/annals.1313.012. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S. Molecular mechanisms of apoptosis in the cells of the immune system in human aging. Immunol Rev. 2005;205:114–29. doi: 10.1111/j.0105-2896.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 18.Herndon FJ, Hsu H-C, Mountz JD. Increased apoptosis of CD45RO- T cells with aging. Mech. Ageing Dev. 1997;94:123–34. doi: 10.1016/s0047-6374(97)01882-4. [DOI] [PubMed] [Google Scholar]
- 19.Phelouzat MA, Laforge T, Abrogast A, Quadri RA, Boutet S, Proust JJ. Susceptibility to apoptosis of T lymphocytes from elderly humans is associated with increased in vivo expression of functional fas receptors. Mech Ageing Dev. 1997;96:35–46. doi: 10.1016/s0047-6374(97)01883-6. [DOI] [PubMed] [Google Scholar]
- 20.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in aging humans: Role of PI3Kinase signaling pathway. J Immunol. 2007;178:6912–22. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 24.Lung TL, Saurwein-Teissl M, Parson W, Schonitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–12. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 25.Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–50. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Dang Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in peripheral blood. Hum Immunol. 2009;70:777–84. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–8. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Canaday DH, Amponsah NA, Jones I, Tisch DJ, Hornick TR, Ramchandra L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J Clin Immunol. 2010;30:373–83. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Cabezas B, Naranjo-Gomez M, Fernandez MA, Grifols JR, Pujol-Borrell R, Borras FE. Reduced number of plasmacytoid dendritic cells in aged blood donors. Exp Gerontol. 2007;42:1033–8. doi: 10.1016/j.exger.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–21. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 31.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–63. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 32.Martin M, Schifflerle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of phosphatidylinositol 3 kinase-AKT pathway in the regulation of IL-10 and IL-12 by Pophyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–25. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 33.Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals. The lessons of Centenarians. Immunol Today. 1995;16:12–6. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 34.Weksler ME, Goodhardt M. Do age-associated changes in ‘physiologic’ autoantibodies contribute to infection, atherosclerosis, and Alzheimer's disease? Exp Gerontol. 2002;37:971–9. doi: 10.1016/s0531-5565(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein BE, Meissner A, Lender ES. The mammalian epigenome. Cell. 2007;128:669–81. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Hardin JA. Direct autoimmunity to nucleoprotein particles, the impact of dendritic cells and interferon α in lupus. J Exp Med. 2003;197:681–7. doi: 10.1084/jem.20030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoshan Y, Mevorach D. Accelerated autoimmune disease in MRL/Mpj-Fas(lpr) but not in MRL/Mpj following immunization with high load of syngeneic late apoptotic cells. Autoimmunity. 2004;37:103–9. doi: 10.1080/08916930410001666622. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self antigen, the human DNA. J Immunol. 2009;182:1138–45. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence, and organismal ageing: causal or correlative? Nucleic Acid Res. 2009;35:7417–28. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson BC. Role of DNA methylation in the regulation of cell function: Autoimmunity, aging, and cancer. J Nutr. 2002;132:2401S–5S. doi: 10.1093/jn/132.8.2401S. [DOI] [PubMed] [Google Scholar]
- 41.Issa JP. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109:103–8. doi: 10.1016/s1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher B, Hoeijmakers JH, Garinis GA. Sealing the gap between nuclear DNA damage and longevity. Mol Cell Endocrinol. 2009;299:112–7. doi: 10.1016/j.mce.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 43.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Costissa M, Alt FW. DNA repair, genomic stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 44.Martien S, Abbadie C. Acquisition of oxidative DNA damage during senescence: the first step toward carcinogenesis? Ann NY Acad Sci. 2007;1119:51–63. doi: 10.1196/annals.1404.010. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal A, Tay J, Yaang G-E, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging. 2010;2:93–100. doi: 10.18632/aging.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]


