Abstract
γδ T lymphocytes represent a minor subset of peripheral blood in humans (<10%). γδ T cells expressing Vγ9Vδ2 T cell receptor recognise the endogenous pool of isopentenyl pyrophosphate (IPP) that is overproduced in cancer cells as a result of dysregulated mevalonate pathway. Aminobisphosphonates increase the endogenous pool of IPP in cells by blocking the enzyme farnesyl pyrophosphate synthase (FPPS) of the mevalonate pathway. Activated γδ T cells release copious amounts of interferon (IFN)-γ and tumour necrosis factor (TNF)-α and exhibit potent anti-tumour activity. Combination of γδ T cells with therapeutic monoclonal antibodies can efficiently mediate antibody dependent cellular cytotoxicity against tumours. These features makes γδ T cells attractive mediator of cancer immunotherapy. We review here, the basic properties and importance of γδ T cells in tumour immunity, and highlight the key advances in anti-tumour effector functions of γδ T cells achieved over the last few years and also summarize the results of the clinical trials that have been done till date. Future immunotherapeutic approach utilizing γδ T cells holds considerable promise for treatment of different types of cancer.
Keywords: Aminobisphosphonates, anti-tumor cytotoxicity, clinical trials, immunotherapy, γδ T cells, phosphoantigens
Introduction
The immune system has evolved to protect the host from infections and cancer. Typically, the immune system is divided into two categories- innate immunity and adaptive immunity. The innate immune system comes into play immediately after the appearance of antigen whereas the adaptive immune system provides antigen-specific response. In addition to these defense mechanisms, there are unconventional T cells like the gamma delta (γδ) T lymphocytes and natural killer T (NKT) cells that functionally and phenotypically belong to both the innate and the adaptive immune system and are able to bridge the two1,2,3. In the peripheral circulation of humans, γδ T cells comprise about 1-10 per cent of the circulating T cells, though this percentage can rise to as high as 50 per cent at some mucosal sites4. γδ T cells are involved in combating infectious diseases and have non-redundant capacities in the inhibition of tumour development and progression5,6.
Antigen recognition and activation of γδ T lymphocytes
Unlike αβ T cells, γδ T cells do not require the help of conventional major histocompatibility complex (MHC) class I and class II molecules for recognizing the antigens1. Antigen recognition by γδ T cells is dependent upon the particular variable (V) region of the T cell receptor (TCR) as opposed to the entire rearranged TCR required by αβ T cells. γδ T cells expressing Vδ1 are abundantly found at mucosal sites and these respond to the expression of non-classical MHC molecules on the surface of virally-infected or tumour cells7,8,9. Vδ2+ (Vγ9Vδ2) cells are predominantly present in the peripheral circulation and respond to non-peptide phosphoantigens10,11. Vγ9Vδ2 T cells recognizes self and microbial phosphorylated metabolites generated in the eukaryotic mevalonate pathway and in the microbial 2-C-methyl-D-erythritol 4-phosphate (MEP) or non-mevalonate pathway12. It was observed that during bacterial and protozoan infections, Vγ9Vδ2 T cells expand to high levels which in some individuals represented the majority of circulating T cells13. The first chemically defined antigens for Vγ9Vδ2 were found to be alkyl phosphates14. One natural antigen from mycobacteria was isolated and identified as isopentenyl pyrophosphate (IPP)15. Subsequent characterization of the microbial antigens recognized by human γδ T cells revealed that these are non-proteinaceous in nature and have critical phosphate residues11,16. The Vγ9Vδ2 crystal structure confirmed the presence of a basic, positively charged region in the binding groove that could directly interact with the negatively charged pyrophosphate moiety of the antigen10. These phosphoantigens are generated during the non-mevalonate and mevalonate pathways utilized by prokaryotic and eukaryotic cells, respectively12,17,18. Various compounds like steroid hormones, cholesterol, many types of vitamins, rubber, etc. are derived from this pathway. There are now many synthetic phosphorylated compounds that are capable of stimulating γδ T cells like bromohydrin pyrophosphate (BrHPP), 4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) and mono-ethyl pyrophosphate14,19,20. In addition to phosphoantigens, there are reports on additional ligands for human γδ T cells. Bisphosphonates, especially nitrogen-containing bisphosphonates (NBP) are widely used to treat postmenopausal osteoporosis and skeletal malignancies. NBP like pamidronate, alendronate, zoledronate, etc. inhibit the key enzyme farnesyl pyrophosphate synthase (FPPS) of the mevalonate pathway, thereby upregulating the pool of endogenous IPP. The accumulated IPP activates Vγ9Vδ2 T cells to release inflammatory cytokines interferon (IFN)-γ and tumour necrosis factor (TNF)-α. Another class of molecules that stimulate Vγ9Vδ2 T cells is alkylamines. Alkylamines are secreted by certain commensal bacteria. These are also present in edible plant products such as tea, wine, apples and mushrooms21. Alkylamines act in a manner similar to NBP22. γδ T cells discriminate transformed tumour cells from healthy cells by the upregulation of self-antigens like heat shock proteins (HSP). The expression of these proteins are increased in tumour cells due to higher metabolism and serves as endogenous danger signals6,23. Increased cytotoxicity of γδ T cells was observed against transformed cell lines expressing hsp60/7024. Studies from our laboratory have demonstrated that Vγ9Vδ2 T cells recognize hsp60 on oral tumour cells and have the ability to lyse autologous and allogenic oesophageal tumour targets via recognition of hsp60 and hsp7025,26.
Migration of γδ T cells to the tumour site
The influx of TILs (tumour infiltrating lymphocytes) to the tumour site enhances the potential for anti-tumour immune responses. The numbers and types of lymphocytes present in the infiltrate are related to the chemokines produced by both the tumour cells and tissue stromal cells located at the tumour site. The infiltration of circulating lymphocytes to the tumour is facilitated by these chemokines. For example, breast, cervix and pancreatic tumours as well as ovarian tumour produce CC and CXC chemokines that are important mediators of macrophage and lymphocyte infiltration in those tumours27,28,29. Interestingly, both Vδ1 and Vγ9δ2 T cells display distinct chemokine receptors that bestow these cells the property to migrate to the tumour site. Vδ1 express CCR5 and Vγ9δ2 express both CCR5 and CXCR330. In addition, Vγ9δ2 T cells express NK receptor P1A (NKR-P1A) platelet endothelial cell-adhesion molecules (PECAM) while Vδ1 use NK receptor P1A NKR-P1A for transendothelial migration31. Vδ1 T cell subsets from the peripheral blood utilize a larger array of adhesion molecules, namely LFA-1, VLA-α4, VLA-α5, L-selectin and αEβ7, to bind to squamous cell carcinoma cells compared to the restricted usage of LFA-1, L-selectin and CD44v6 by the Vδ2 T cells32. The mutually exclusive pattern of chemokine receptor expression in both the subsets of γδ T cells indicates independent mechanism of homing to tumour site that might have an important aspect in cancer immunotherapy.
Anti-tumour activity of γδ T lymphocytes
Ability of γδ T lymphocytes to produce abundant proinflammatory cytokines like IFN-γ, potent cytotoxic effector function and MHC-independent recognition of antigens makes it an important player of cancer immunotherapy. γδ T cells kill many different types of tumour cell lines and tumours in vitro, including leukemia, neuroblastoma and various carcinomas33,34,35,36.
Accumulation of mevalonate metabolites in tumour cells is a powerful danger signal that activates the γδ T cells. In normal cells, IPP produced by mevalonate pathway are at a concentration that is insufficient to trigger γδ T cells response. However, dysregulation of mevalonate pathway in certain tumours leads to production of higher concentrations of IPP, which is sensed by γδ TCR as a tumour antigen37,38. It was also shown that mRNA knockdown of IPP-consuming enzyme, FPPS, induced Vγ9Vδ2 T cell stimulation in otherwise non-stimulatory tumour cells39. γδ T cells are able to recognize and kill many different differentiated tumours cells, either spontaneously or after treatment with different bisphosphonates, including zoledronate. It has been shown that human tumour cells can efficiently present aminobisphosphonate and pyrophosphomonoester compounds to γδ T cells, inducing its proliferation and IFN-γ production40.
Combination treatment utilizing Vγ9Vδ2 T cells along with chemotherapeutic agents and zoledronate has been shown to induce an increase in the cytotoxic function of γδ T cells against solid tumour41,42. The ability of γδ T cells to efficiently kill bisphosphonates treated colon cancer stem cells and ovarian cancer stem-like cells has also been reported36,43.
In addition to phosphoantigens, γδ T lymphocytes can also be activated by mitochondrial F1-ATPase-related structure expressed together with apolipoprotein A-I, which are expressed on the surface of some tumour cells44. ATP F1 synthase is an intracellular protein complex involved in ATP generation. F1-ATPase displays characteristic of antigen presentation molecule by binding to the adenylated derivative of IPP and promoting TCR aggregation, cytokine secretion and cytotoxic activity45.
NK receptors and anti-tumour activity of γδ T cells
Natural killer (NK) receptors expressed on γδ T cells play a crucial role in mediating the anti-tumour response of γδ T cells. Natural killer group 2, member D protein (NKG2D) expressed on Vγ9Vδ2 T cells is critical for tumour recognition and provides activation signals upon binding to non-classical MHC molecules of the MHC class I chain-related molecules (MIC) and UL-16 binding protein (ULBP) families expressed on tumour cells46,47,48. This ligand binding to NKG2D can affect the release of TNF-α, interleukin (IL)-2 α receptor (CD25) upregulation and increase cytolytic potential of γδ T cells47. ULBP molecules are involved in Vγ9Vδ2 T cells recognition of leukemias and lymphomas49 and also ovarian and colon carcinomas50. γδ T cells utilizing the Vδ1 chain isolated from tumour-infiltrating lymphocytes can also kill cancer cells. Vδ1 γδ T lymphocytes have been shown to mediate cytolytic activity by recognizing MICA, MICB or ULBP expressed on cancer cells51,52.
γδ T cells resemble NK cells as these also express CD16 (FcγRIII) receptor. Upon recognition of phosphoantigens, a subset of Vγ9Vδ2 T cells upregulates CD1653. It has been reported that CD16 represent activation/memory status of γδ T cells and these CD16high cells have specific phenotypic features that distinguish these from the CD16low subset. These constitutively express several natural killer receptors (NKG2A/CD94) and high amounts of perforin, but express low levels of chemokine receptors (CXCR3, CCR6) and IFN-γ54. CD16/FcγRIII receptor binds to Fc portion of immunoglobulin G (IgG) and engagement of CD16 by γδ T cells leads to antibody-dependent cellular cytotoxicity (ADCC)55. ADCC is a process in which CD16+ effector cells actively lyse tumour cells that have been bound by specific antibodies. Several reports have proven that in vitro γδ T cells respond to activation via CD16 and mediate ADCC against tumour with therapeutic anti-tumour monoclonal antibodies (mAbs) like rituximab, trastuzumab, of atumumab and alemtuzumab35,56,57. It has also been shown that stimulated γδ T cells increase the efficacy of trastuzumab in vivo in Her2+ breast cancer patients58.
Application of γδ T cell immunotherapy in clinics
Given the potent antitumour effector function of γδ T cells and broad reactivity to many different types of tumours has raised a great interest to explore their therapeutic potential. An important feature of γδ T cells is that these favourably kill cancer cells and show low (if any) reactivity towards non-transformed cells which makes these very good candidates for cancer immunotherapy50. The safety and efficacy of γδ T cell-based immunotherapy have been evaluated in several clinical trials59. Presently, two strategies for γδ T cells in tumour immunotherapy have been applied. These are the adoptive cell transfer of in vitro expanded γδ T cells and the in vivo therapeutic application of γδ-stimulating phosphoantigens or aminobisphosphonates together with low-dose recombinant IL2 (rIL2).
Studies carried out in nude mice demonstrated that repeated infusion of γδ T cells leads to tumour growth arrest60. Another study carried out in SCID mice showed the anti-tumour effector functions of NK cells and γδ T lymphocytes against autologous melanoma cells61. In one pilot study, patients with B-cell malignancies that failed conventional therapy were treated with intravenous administration of pamidronate and rIL2 to stimulate Vγ9Vδ2 T cells in vivo62. It was observed that in vivo Vγ9Vδ2 T cells were expanded in five out of nine patients; three out of these five responding patients had partial remissions and one had stable disease. Other trials with adoptive transfer of γδ T cells include patients with advanced cancer like metastatic renal cell carcinoma63 and non-small cell lung carcinoma64 where stable disease was found in 60 and 37 per cent patients, respectively. In these cases, the regimen consisted of ex vivo activation and expansion of autologous Vγ9Vδ2 T cells with either phosphoantigens, such as BrHPP or aminobsphosphnates, like zoledronate or pamidronate or their infusion into the patients. Aminobisphosphonates have also been used in clinical trials to treat metastatic prostate cancer65 and advanced breast cancer66 where partial remissions have been reported. Complete remission of lung metastasis in a patient with renal cell carcinoma has also been reported after adoptive transfer of γδ T cells67. It was shown that the patient was disease free for two years without any additional treatment following in vitro activation and expansion of autologous γδ T cells with HMBPP plus rIL2, combined with the infusion of zoledronate and rIL267. There is also increasing evidence that stimulating γδ effector T cells can enhance monoclonal antibody-induced cytotoxicity and thereby improve the anticancer effects of mAbs. It was found that repeated infusions of phosphoantigens stimulated γδ T cells and trastuzumab increased the efficacy of γδ T cells against HER-2+ breast carcinoma cell lines in vivo58. In addition, a survival advantage to patients with an increased γδ T cells following allogeneic stem cell transplantation (ASCT) has been reported. A long-term survival advantage in a group of high-risk acute leukemia patients who recovered with increased number of circulating γδ T cells following partially mismatched related haematopoietic stem cell transplantation was reported68.
Conclusions
The unique features of human γδ T cells related to antigen recognition, tissue tropism, lack of antigen processing requirement and cytotoxic function make these ideal candidates for cancer immunotherapy. γδ T cells recognize increased pool of endogenous IPP (a consequence of dysregulated mevalonate pathway) in cancer cells, release IFN-γ/TNF-α and mediate cytolyic effector functions. Expression of NKG2D receptors provides a selective advantage to γδ T cells to recognize tumours that express stress induced molecules like MICA/B. This property of γδ T cells can be exploited for immunotherapy as tumours downregulate MHC molecules to evade immune recognition (Fig.). Human γδ T cells show potent cytotoxic effector functions against various types of tumours. One way to exploit γδ T cells for cancer immunotherapy is the use of synthetic phosphoantigens like BrHPP or HMBPP which can act as γδ TCR agonists. Future trials should harness bisphosphonate activated γδ T cells in combination with chemotherapy or monoclonal antibodies for treatment of solid tumours and haematologic malignancies.
Fig.
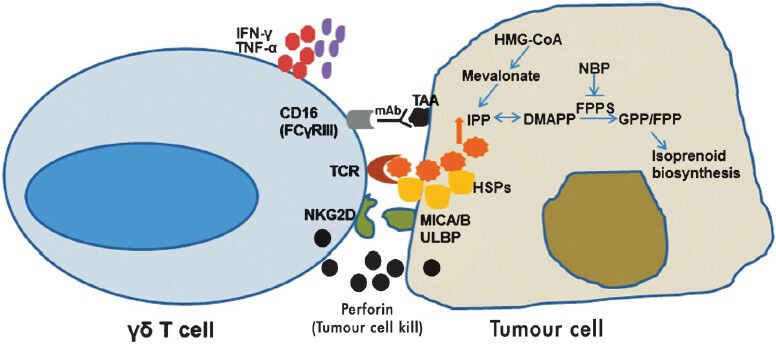
Mechanism underlying γδ T cell killing of tumours: γδ T cell receptor (TCR) interacts with isopentenyl pyrophosphate (IPP) generated through the mevalonate pathway in tumours. Bisphosphonates inhibits farnesyl pyrophosphate synthase (FPPS) leading to increased endogenous pool of IPP and dimethylalleyl pyrophosphate (DMAPP) in tumour cells. γδ T cells recognize heat shock proteins (HSPs) and MHC class I chain-related molecules (MICA/B) or UL-16 binding protein ULBP expressed on tumour cells via their TCR and natural killer group 2, member D protein (NKG2D) receptors, respectively. Perforin released from activated γδ T cells lyse the tumour cell. γδ T cells can also kill tumour cells through antibody dependent cellular cytotoxicity (ADCC). γδ T cells expressing CD16 (FCγRIII) interacts with tumour associated antigens (TAA) via specific monoclonal antibodies and mediate ADCC. Cytokines like interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) released by γδ T cells can recruit other immune cells (bystander effect).
References
- 1.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764–5. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nat Immunol. 2003;4:1164–5. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- 4.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban EM, Chapoval AI, Pauza CD. Repertoire development and the control of cytotoxic/effector function in human gammadelta T cells. Clin Dev Immunol 2010. 2010 doi: 10.1155/2010/732893. 732893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 8.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–78. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–5. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 10.Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411:820–4. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer K, Schoel B, Gulle H, Kaufmann SH, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–9. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 12.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 13.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, et al. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 16.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, et al. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 17.Feurle J, Espinosa E, Eckstein S, Pont F, Kunzmann V, Fournie JJ, et al. Escherichia coli produces phosphoantigens activating human gamma delta T cells. J Biol Chem. 2002;277:148–54. doi: 10.1074/jbc.M106443200. [DOI] [PubMed] [Google Scholar]
- 18.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–24. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belmant C, Espinosa E, Halary F, Tang Y, Peyrat MA, Sicard H, et al. A chemical basis for selective recognition of nonpeptide antigens by human delta T cells. FASEB J. 2000;14:1669–70. doi: 10.1096/fj.99-0909fje. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Zhang Y, Wang H, Raker AM, Sanders JM, Broderick E, et al. Synthesis of chiral phosphoantigens and their activity in gamma delta T cell stimulation. Bioorg Med Chem Lett. 2004;14:4471–7. doi: 10.1016/j.bmcl.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 21.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 22.Thompson K, Rojas-Navea J, Rogers MJ. Alkylamines cause Vgamma9Vdelta2 T-cell activation and proliferation by inhibiting the mevalonate pathway. Blood. 2006;107:651–4. doi: 10.1182/blood-2005-03-1025. [DOI] [PubMed] [Google Scholar]
- 23.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Hu H, Jiang X, He H, Cui L, He W. Membrane HSP70: the molecule triggering gammadelta T cells in the early stage of tumorigenesis. Immunol Invest. 2005;34:453–68. doi: 10.1080/08820130500265349. [DOI] [PubMed] [Google Scholar]
- 25.Laad AD, Thomas ML, Fakih AR, Chiplunkar SV. Human gamma delta T cells recognize heat shock protein-60 on oral tumor cells. Int J Cancer. 1999;80:709–14. doi: 10.1002/(sici)1097-0215(19990301)80:5<709::aid-ijc14>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Thomas ML, Samant UC, Deshpande RK, Chiplunkar SV. gammadelta T cells lyse autologous and allogenic oesophageal tumours: involvement of heat-shock proteins in the tumour cell lysis. Cancer Immunol Immunother. 2000;48:653–9. doi: 10.1007/s002620050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 28.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–2. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 29.Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150:1723–34. [PMC free article] [PubMed] [Google Scholar]
- 30.Kabelitz D, Wesch D. Features and functions of gamma delta T lymphocytes: focus on chemokines and their receptors. Crit Rev Immunol. 2003;23:339–70. doi: 10.1615/critrevimmunol.v23.i56.10. [DOI] [PubMed] [Google Scholar]
- 31.Zocchi MR, Poggi A. Role of gammadelta T lymphocytes in tumor defense. Front Biosci. 2004;9:2588–604. doi: 10.2741/1419. [DOI] [PubMed] [Google Scholar]
- 32.Thomas ML, Badwe RA, Deshpande RK, Samant UC, Chiplunkar SV. Role of adhesion molecules in recruitment of Vdelta1 T cells from the peripheral blood to the tumor tissue of esophageal cancer patients. Cancer Immunol Immunother. 2001;50:218–25. doi: 10.1007/s002620100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chargui J, Combaret V, Scaglione V, Iacono I, Peri V, Valteau-Couanet D, et al. Bromohydrin pyrophosphate-stimulated Vgamma9delta2 T cells expanded ex vivo from patients with poor-prognosis neuroblastoma lyse autologous primary tumor cells. J Immunother. 2010;33:591–8. doi: 10.1097/CJI.0b013e3181dda207. [DOI] [PubMed] [Google Scholar]
- 34.D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–8. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 35.Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–84. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 36.Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–96. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 37.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchida R, Ashihara E, Sato K, Kimura S, Kuroda J, Takeuchi M, et al. Gamma delta T cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem Biophys Res Commun. 2007;354:613–8. doi: 10.1016/j.bbrc.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Herold MJ, Kimmel B, Muller I, Rincon-Orozco B, Kunzmann V, et al. Reduced expression of the mevalonate pathway enzyme farnesyl pyrophosphate synthase unveils recognition of tumor cells by Vgamma9Vdelta2 T cells. J Immunol. 2009;182:8118–24. doi: 10.4049/jimmunol.0900101. [DOI] [PubMed] [Google Scholar]
- 40.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J Immunol. 2001;167:5092–8. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 41.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–97. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhar S, Chiplunkar SV. Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vγ9Vδ2 T cells. Cancer Immun. 2010;10:10. [PMC free article] [PubMed] [Google Scholar]
- 43.Lai D, Wang F, Chen Y, Wang C, Liu S, Lu B, et al. Human ovarian cancer stem-like cells can be efficiently killed by gammadelta T lymphocytes. Cancer Immunol Immunother. 2012;61:979–89. doi: 10.1007/s00262-011-1166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Mookerjee-Basu J, Vantourout P, Martinez LO, Perret B, Collet X, Perigaud C, et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J Immunol. 2010;184:6920–8. doi: 10.4049/jimmunol.0904024. [DOI] [PubMed] [Google Scholar]
- 46.Girlanda S, Fortis C, Belloni D, Ferrero E, Ticozzi P, Sciorati C, et al. MICA expressed by multiple myeloma and monoclonal gammopathy of undetermined significance plasma cells Costimulates pamidronate-activated gammadelta lymphocytes. Cancer Res. 2005;65:7502–8. doi: 10.1158/0008-5472.CAN-05-0731. [DOI] [PubMed] [Google Scholar]
- 47.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–51. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 48.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 49.Lanca T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115:2407–11. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 50.Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood. 2009;114:310–7. doi: 10.1182/blood-2008-12-196287. [DOI] [PubMed] [Google Scholar]
- 51.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64:9172–9. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- 53.Lafont V, Liautard J, Liautard JP, Favero J. Production of TNF-alpha by human V gamma 9V delta 2 T cells via engagement of Fc gamma RIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J Immunol. 2001;166:7190–9. doi: 10.4049/jimmunol.166.12.7190. [DOI] [PubMed] [Google Scholar]
- 54.Angelini DF, Borsellino G, Poupot M, Diamantini A, Poupot R, Bernardi G, et al. FcgammaRIII discriminates between 2 subsets of Vgamma9Vdelta2 effector cells with different responses and activation pathways. Blood. 2004;104:1801–7. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Freedman MS. Correlation of specialized CD16(+) gammadelta T cells with disease course and severity in multiple sclerosis. J Neuroimmunol. 2008;194:147–52. doi: 10.1016/j.jneuroim.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Braza MS, Klein B, Fiol G, Rossi JF. gammadelta T-cell killing of primary follicular lymphoma cells is dramatically potentiated by GA101, a type II glycoengineered anti-CD20 monoclonal antibody. Haematologica. 2011;96:400–7. doi: 10.3324/haematol.2010.029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, So HF, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs - rituximab and trastuzumab. Int J Cancer. 2008;122:2526–34. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 58.Capietto AH, Martinet L, Fournie JJ. Stimulated gammadelta T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol. 2011;187:1031–8. doi: 10.4049/jimmunol.1100681. [DOI] [PubMed] [Google Scholar]
- 59.Chiplunkar S, Dhar S, Wesch D, Kabelitz D. gammadelta T cells in cancer immunotherapy: current status and future prospects. Immunotherapy. 2009;1:663–78. doi: 10.2217/imt.09.27. [DOI] [PubMed] [Google Scholar]
- 60.Zheng BJ, Chan KW, Im S, Chua D, Sham JS, Tin PC, et al. Anti-tumor effects of human peripheral gammadelta T cells in a mouse tumor model. Int J Cancer. 2001;92:421–5. doi: 10.1002/ijc.1198. [DOI] [PubMed] [Google Scholar]
- 61.Lozupone F, Pende D, Burgio VL, Castelli C, Spada M, Venditti M, et al. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res. 2004;64:378–85. doi: 10.1158/0008-5472.can-03-1501. [DOI] [PubMed] [Google Scholar]
- 62.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 63.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yosihda Y, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg. 2010;37:1191–7. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 65.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–7. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–9. [PubMed] [Google Scholar]
- 68.Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39:751–7. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]


